Abstract
One of the major research focuses in the field of gene therapy is the development of clinically applicable, safe, and effective gene-delivery methods. Since the first case of human gene therapy was performed in 1990, a number of gene-delivery methods have been developed, evaluated for efficacy and safety, and modified for human application. To date, viral-vector-mediated deliveries have shown effective therapeutic results. However, the risk of lethal immune response and carcinogenesis have been reported, and it is still controversial to be applied as a standard therapeutic option. On the other hand, delivery methods for nonviral vector systems have been developed, extensively studied, and utilized in in vivo gene-transfer studies. Compared to viral-vector mediated gene transfer, nonviral systems have less risk of biological reactions. However, the lower gene-transfer efficiency was a critical hurdle for applying them to human gene therapy. Among a number of nonviral vector systems, our studies focus on hydrodynamic gene delivery to utilize physical force to deliver naked DNA into the cells in the living animals. This method achieves a high gene-transfer level by DNA solution injections into the tail vein of rodents, especially in the liver. With the development of genome editing methods, in vivo gene-transfer therapy using this method is currently the focus in this research field. This review explains the method principle, efficiency, safety, and procedural modifications to achieve a high level of reproducibility in large-animal models.
Keywords: Gene therapy, Liver, Hydrodynamic gene delivery, Non-viral, Image-guided
Core tip: Among a number of nonviral vector systems, hydrodynamic gene delivery has been used to study human diseases. The major advantage of the method is the simple and easy step to deliver naked DNA into living animal cells by physical force. The original method modification of injecting the DNA solution into a rodent tail vein has made it applicable in large animals. This method of delivering naked DNA can contribute to treat, not only liver disease but also other systemic diseases that can be cured by facilitating/altering gene expression through the liver.
INTRODUCTION
The liver is the largest organ in the body and is the center of numerous metabolic pathways. Therefore, it is involved in various inherited diseases. These diseases are often caused by a critical gene-product deficiency or overproduction in the hepatocytes. Recent advances in diagnostic strategies using molecular biology and genetics have helped establish various therapeutic methods. Moreover, genetic abnormalities are altered by introducing a gene-coding sequence or a nucleic-acid sequence into these cells to inhibit the specific gene overexpression. Thus, liver-targeted gene therapy emerged as a promising therapeutic strategy. Originally, human gene therapy was first performed using a retrovirus-mediated ex vivo gene-delivery method to target adenosine-deaminase deficiency in 1990[1]. However, serious adverse events occurred in the following years, including lethal immune reaction to the adenovirus vector and oncogenesis because of genetic transformation caused by the retrovirus vector[2-4]. Over this time period, viral vectors have been improved toward higher levels of safety and efficiency, yet concerns regarding biological safety, including lethal immune reaction and oncogenesis, have remained. On the other hand, various nonviral gene-delivery methods have also been extensively studied for use in clinical applications. However, the major obstacle of the methods was lower gene-delivery efficiency compared to the viral vectors. Many ongoing studies modify the current strategies to provide a better gene-delivery efficiency while maintaining its safety features. Among these methods, this report focuses on the hydrodynamic gene-delivery (HGD) method for human gene delivery. The liver-targeted HGD efficacy and safety are described, including recent progress of the procedure applied toward clinical application. We hope the information described will help physicians to understand the principles of HGD and lead to new strategies to better treat patient diseases compared to the conventional treatment methods.
NUCLEIC-ACID DELIVERY TO THE LIVER AND THE PRINCIPLE OF HGD
Concerns regarding carcinogenesis and immune reaction because of viral-vector-based gene transfer have inspired the efforts to develop methods of nucleic-acid delivery in its naked form in vivo. The objective of naked nucleic-acid delivery is either: (1) express gene of which the product is missing or low level; (2) gene vaccination; (3) inhibition of specific gene expression; and (4) deliver necessary parts of genome editing; etc. Table 1 summarizes the nonviral methods of nucleic-acid delivery for liver-target gene delivery. The barriers for nucleic-acid delivery to hepatocytes are the plasma membrane and the endothelium in the sinusoidal structure. Therefore, the physical methods of intrahepatic nucleic-acid delivery included needle injection, gene gun, electroporation, sonoporation, and HGD. They were developed to overcome the structural barriers using physical forces of pressure, shock wave, electric pulse, ultrasound wave, and hydrodynamic pressure. Among these methods, HGD to the mouse model was reported as an easy and effective in vivo gene-delivery method by injecting naked DNA solution into the tail vein[5,6]. Various genes were delivered into rodent hepatocytes to analyze their function and to examine the therapeutic effect within the research fields of gastroenterology and hepatology[5,7-15] (Table 2).
Table 1.
Non-viral gene delivery systems toward the liver
| Method | Functional component |
| Lipids | Cationic lipids |
| Polymers | Cationic polymers |
| Proteins | Natural or chemically modified proteins in cationic nature |
| Peptides | Lysine or arginine residues in peptides |
| Needle injection | Mechanic force |
| Gene gun | Pressure |
| Electroporation | Electric pulse |
| Sonoporation | Ultrasound |
| Hydrodynamic delivery | Hydrodynamic pressure |
Table 2.
Summary of the applications of hydrodynamic delivery for functional analysis of therapeutic genes related to the diseases of gastroenterology and hepatology
| Disease | Therapeutic Genes | Ref. |
| Nonalcoholic steatohepatitis | Inducible nitric oxcide synthetase | [8] |
| Hepatitis | HBV knockdown | [5,9] |
| HCV knockdown | ||
| Fulminant hepatitis | NKG2D knockdown | [7,10] |
| osteopontin knockdown | ||
| Liver injury | c-met | [7,11] |
| IL-37 | ||
| caspase knockdown | ||
| Liver fibrosis | platelet-derived growth factor receptor beta knockdown | [12] |
| Liver Regeneration | fibroblast growth factor 7 | [13] |
| Fabry disease | alpha-galactosidase A | [7] |
| Pancreatitis | pancreatitis associated protein 1 | [14] |
| Colon cancer | IL-15 | [15] |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; IL: Interleukin.
The principle of HGD relies on the mechanical force developed using a quick injection of a large amount of plasmid volume. Briefly, a 10% body weight DNA solution is injected within 5-7 s into a 20 g mouse. This force created a transient congestion in their right ventricle allowing the solution to flow back into the hepatic veins. Next, the solution passes through the sinusoidal structure to the portal veins allowing the force to make transient pores in the hepatocyte cell membrane[16-19]. Then, nucleic acids enter into the hepatocytes, move to the nucleus, and finally facilitate targeted gene expression. The transient pores naturally disappears in a short period[18], and the exogenous gene can be expressed in the hepatocytes. Due to the large amount of solution and its rapid flow rate, the blood is transiently cleared away from the vessel, and there is no concern regarding DNA degradation in the blood by DNase. A number of transfected cells are confirmed in the overall targeted area in the liver, although relatively higher gene-delivery efficiency was seen in the local areas highly impacted by the injection flow. The major advantage of the method is a less risk of immune response and oncogenesis. Specifically, naked DNA plasmids and saline do not possess any immunogenicity reagents or the potential of DNA integration, compared to the chemical compounds used for viral or other nonviral gene-delivery methods.
EFFICIENCY AND SAFETY
The efficiency of the original procedure was confirmed, and various genes were examined for therapeutic effects in mouse disease models[5,7-15]. Only one procedure transfected a large number of hepatocytes with a specific protein secreting gene, approximately 40% of hepatocytes in a targeted area, leading to a high level of gene expression in the liver and body[5]. In 2013, we reported that only one delivery method could achieve a high level of FIX expression in rats, which was expected to stop bleeding in patients with hemophilia B[20]. Regarding its safety, the impact of hydrodynamic injection to the liver is known to only elicit slight liver damage. In a microscopic study, destruction of cells and tissue was merely seen in the liver[5,18]. Also, transient abnormal aminotransferase (ALT) increases recover in a short term, and there are no signs of hepatic failure[20,21]. Considering the half-life of ALT and the small number of destroyed hepatocytes, we hypothesize that the ALT increase is derived from leakage out of the newly created transient pores and not from destroyed hepatocytes.
Based on this method’s efficiency and safety shown in rodent studies, recent efforts have safely applied this method for large-animal studies to show the clinical applicability. The essential key was to decrease the injection volume of 10% BW used in rodents, which is 5 L in 50-kg patient, to maintain the gene-delivery efficiency. Several reports showed the modification of the original procedures for this purpose, and we have applied catheter-based, target-organ-specific, and target-site-specific HGD in 2009[22]. By this procedure, the hepatocytes were hydrodynamically delivered genes of interest with < 1% of BW solution in each liver lobe. As a result, the gene-delivery efficiency was maintained showing a therapeutic level of human factor IX expression in dogs (manuscript in preparation). This procedure involves a catheter insertion through the jugular vein to each hepatic lobular vein. This is followed by the hydrodynamic injection of naked DNA plasmid solution with temporally occlusion of blood flow using balloon placed at the tip of the catheter (Figure 1A). By this technique, a sufficient intravascular pressure was provided upon the hydrodynamic injection (Figure 1B), a key of successful gene transfer. In addition, no significant solution leakage with the added pressure is seen within the systemic dynamics, which normally impacts cardiopulmonary function. The safety and impact of HGD in large-animal models were carefully evaluated in previous reports as well[22-24]. Hydrodynamics of the procedure were also validated using CT scans during the injection[25]. These studies confirmed the site specificity of the gene-delivery efficiency, and the target-region-specific impacts caused by the injection. In addition, histochemical analyses of the transiently expanded sinusoidal structure showed the same as the phenomena seen in small animals and recovered within a few days[24]. While the systemic inflammatory cytokines including interferon-α, interleukin (IL)-6, IL-8, IL-18, and IL-4 showed increase in mice after HGD through their tail vein affecting the systemic condition, however, the liver-targeted HGD showed an increase in cytokines related to the myocytes and vascular stretching including tumor necrosis factor-α, IL-10, MCP-1, and Canine KC, but not in systemic inflammatory cytokines[24]. This is probably due to the localized effect of injection pressure, flow, etc. Recently, laparoscopy was used to monitor the change in the lobe of the liver upon injection to confirm the site specificity and overall impact on the lobe (Figure 1C). The findings presented the precise site-specific distribution of the DNA solution upon the liver-targeted, lobe-specific HGD resulted in the site-specific expression of injected transgene (Figure 1D). Using a computer-controlled injection device (Figure 2), HGD was performed to the right lateral lobe with approximately 1.5% BW solution completed within 7.5 s (Figure 1). Although the hydrodynamically injected DNA solution transiently made the liver pale, no destruction nor bleeding were seen.
Figure 1.
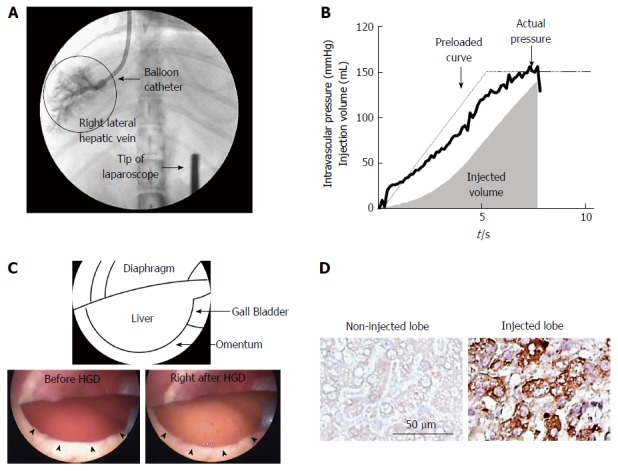
Image-guided, computer-controlled hydrodynamic gene-delivery to the dog liver. The balloon catheter was placed at the appropriate position in the hepatic veins of right lateral lobe and the occlusion of the blood flow by the balloon was confirmed by injecting a small amount of contrast medium into the hepatic vein. Then the hydrodynamic injection of naked DNA solution was performed under the real time monitoring of liver structure by the laparoscope using the computer-controlled injection system (A). B: Time-pressure curve and the volume of injected solution recorded in the injection system. Solid and dotted lines represent actual and preloaded time-pressure curves. A gray area shows cumulative volume of injected saline (mL). C: Laparoscopic findings of the hydrodynamically injected right lateral lobe of the dog. The injected lobe was swollen and the injected DNA solution transiently made the liver pale. No destruction nor bleeding were seen on the surface of the liver (arrowheads). D: The effect of lobe-specific hydrodynamic gene delivery of luciferase expressing plasmid. The immunohistochemical analyses showed positively stained cells in the injected right lateral lobe. No stained cells were found in non-injected left lateral lobe.
Figure 2.
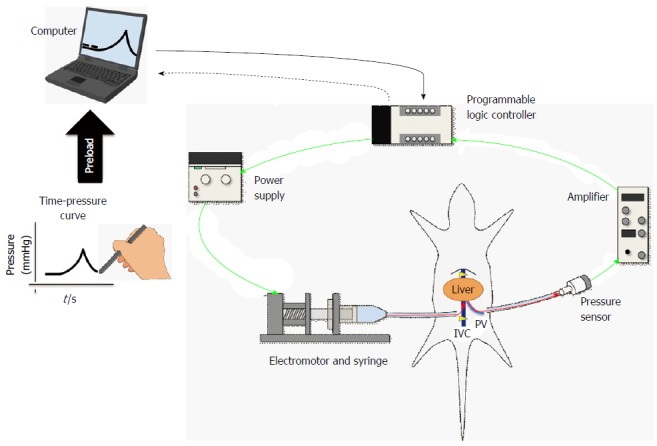
Scheme of computer-controlled injection system. The schema of the newly developed hydrodynamic gene-delivery system. This figure is partly reused and modified with updated information from Figure 1 in Ref [20] with their permission. IVC: Inferior vena cava; PV: Portal vein.
INNOVATION OF INJECTION SYSTEM
Overall, the catheter-based procedure of liver-targeted, lobe-specific HGD showed safe and efficient gene delivery in large-animal models. Therefore, the next step toward clinical application was to ensure the procedure reproducibility using various gene targets. For the liver, the size and elasticity of its tissue can vary between species, age ranges, and even individual subjects. In addition, it is known that many liver diseases show liver fibrosis during their final stage. During HGD, the specific intravascular pressure resulted in various patterns and differences in the gene-delivery efficiency with the same injection pressure (i.e., hydrodynamic pressure). Therefore, the intravascular pressure was used to control the injection speed, and the efforts have been made to develop the computer-controlled HGD system[20,21]. The system was set to control the injection power to reproduce the intravascular pressure during HGD to any target. Specifically, the efficient and safe HGD to the liver is associated with a peak pressure level in the vessel and tissue, duration of the pressure, and concentration of plasmid solution (Figure 2). The reproducibility in gene-delivery efficiency for any target is the advantage of using this system. The system function involves: (1) preload of an arbitrary time-pressure curve to the computer; (2) placement of a sensor to detect an intravascular pressure at the portal vein; (3) insertion of a catheter for a hydrodynamic injection into the inferior vena cava (IVC); (4) beginning of a hydrodynamic injection after occluding the supra- and infra-portions of the hepatic IVC; (5) transmission of the intravascular pressure data to the computer every 50 milliseconds; (6) regulation of the injector power to reproduce the time-pressure curve, which was input before the injection; and (7) repetition of step 5 and 6 until injection completion (Figure 2). Further, the flexibility of the system can control various types of time-pressure curves in vivo[20] so that the safe, efficient, and reproducible injection can be repeatedly performed. Overall, this computer-controlled injection system has the potential of achieving reproducible HGD, even if anyone performs the procedure for any kind of target (Figure 2)[21-24].
Our recent and current studies[26,27] utilizing real-time monitoring of the computer-controlled injection under fluoroscopy and laparoscopy (Figure 1) demonstrated its precise controllability. Therefore, the current ongoing studies are focusing on treating diseased-animal models, such as liver fibrosis with the different liver structure characteristics and various levels of fibrosis. This scenario provides the best disease model to examine the intravascular-pressure-based injection control. For these purposes, we are continuously modifying the injection system for its clinical trial application, in collaboration with GMP-grade engineers, physicians, and industrial companies.
GENE THERAPY THROUGH THE LIVER
While improving the injection system, we are investigating hepatic gene therapy for systemic diseases as well as for the liver diseases. The target diseases include hemophilia, human alpha-1 antitrypsin deficiency, etc. which can be treated by increasing the hepatic expression of normal proteins in the liver and then their secretion into blood plasma. For liver disease gene therapy, Abe et al[28] recently reported that HGD-mediated MMP13 expression in the rat liver prevented liver fibrosis. Surprisingly, rat liver treated by MMP13 gene therapy did not suffer from significant fibrosis after at least 10 wk. Hyaluronic acid levels of MMP13-treated rats with bile-duct ligation were statistically equivalent to those of normal rats. Therefore, MMP13 is a promising candidate for liver fibrosis gene therapy. Further studies focusing on the therapeutic effect in advanced stage liver fibrosis are currently ongoing. In addition, HGD procedure from hepatic artery is also being examined in our lab to treat hepatocellular carcinoma by this method since hepatocellular carcinoma is fed by the hepatic artery. While early study showed transient increase of platelet count injecting large volume of thrombopoietin-expressing plasmids into human hepatic veins[29], which is the only human trial to date, strict adjustment of injection parameters and setting of the system are necessary to apply HGD for human.
CONCLUSION
Nucleic-acid-based medicine is quickly developing with the detailed analyses of disease-related genes. A simple, safe, effective, and reproducible method is essential before applying this strategy to human diseases. The development of HGD-based gene therapy for large animals provides a great milestone to this point, and further studies are necessary to make the procedure clinically applicable.
ACKNOWLEDGMENTS
The authors would like to thank all members at the Niigata city industrial promotion center and for their excellent assistance in producing the system. The authors would also like to thank Yoshihiko Ohba for the supporting of fine-tuning of the system. They also thank Enago for the critical reading of the manuscript and English language review.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no current financial arrangement or affiliation with any organization that may have a direct influence on their work.
Peer-review started: July 6, 2016
First decision: July 29, 2016
Article in press: August 23, 2016
P- Reviewer: Alino PelliIcer SF, Liu HY, Sanal MG, Strom SC, Tomizawa M S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
References
- 1.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 2.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 3.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 4.Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, de Ridder D, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 7.Bonamassa B, Hai L, Liu D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharm Res. 2011;28:694–701. doi: 10.1007/s11095-010-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anavi S, Hahn-Obercyger M, Margalit R, Madar Z, Tirosh O. A novel antihypoglycemic role of inducible nitric oxide synthase in liver inflammatory response induced by dietary cholesterol and endotoxemia. Antioxid Redox Signal. 2013;19:1889–1901. doi: 10.1089/ars.2012.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 10.Huang M, Sun R, Wei H, Tian Z. Simultaneous knockdown of multiple ligands of innate receptor NKG2D prevents natural killer cell-mediated fulminant hepatitis in mice. Hepatology. 2013;57:277–288. doi: 10.1002/hep.25959. [DOI] [PubMed] [Google Scholar]
- 11.Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci USA. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SW, Zhang XR, Wang CZ, Chen WZ, Xie WF, Chen YX. RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008;28:1446–1457. doi: 10.1111/j.1478-3231.2008.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SM, Wang WP. Expression and function of fibroblast growth factor (FGF) 7 during liver regeneration. Cell Physiol Biochem. 2011;27:641–652. doi: 10.1159/000330073. [DOI] [PubMed] [Google Scholar]
- 14.Shigekawa M, Hikita H, Kodama T, Shimizu S, Li W, Uemura A, Miyagi T, Hosui A, Kanto T, Hiramatsu N, et al. Pancreatic STAT3 protects mice against caerulein-induced pancreatitis via PAP1 induction. Am J Pathol. 2012;181:2105–2113. doi: 10.1016/j.ajpath.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Ochoa MC, Fioravanti J, Duitman EH, Medina-Echeverz J, Palazon A, Arina A, Dubrot J, Alfaro C, Morales-Kastresana A, Murillo O, et al. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PLoS One. 2012;7:e52370. doi: 10.1371/journal.pone.0052370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanefuji T, Yokoo T, Suda T, Abe H, Kamimura K, Liu D. Hemodynamics of a hydrodynamic injection. Mol Ther Methods Clin Dev. 2014;1:14029. doi: 10.1038/mtm.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespo A, Peydró A, Dasí F, Benet M, Calvete JJ, Revert F, Aliño SF. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther. 2005;12:927–935. doi: 10.1038/sj.gt.3302469. [DOI] [PubMed] [Google Scholar]
- 18.Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoo T, Kamimura K, Suda T, Kanefuji T, Oda M, Zhang G, Liu D, Aoyagi Y. Novel electric power-driven hydrodynamic injection system for gene delivery: safety and efficacy of human factor IX delivery in rats. Gene Ther. 2013;20:816–823. doi: 10.1038/gt.2013.2. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- 22.Kamimura K, Suda T, Xu W, Zhang G, Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamimura K, Suda T, Zhang G, Aoyagi Y, Liu D. Parameters Affecting Image-guided, Hydrodynamic Gene Delivery to Swine Liver. Mol Ther Nucleic Acids. 2013;2:e128. doi: 10.1038/mtna.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamimura K, Kanefuji T, Yokoo T, Abe H, Suda T, Kobayashi Y, Zhang G, Aoyagi Y, Liu D. Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PLoS One. 2014;9:e107203. doi: 10.1371/journal.pone.0107203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoo T, Kanefuji T, Suda T, Kamimura K, Liu D, Terai S. Site-Specific Impact of a Regional Hydrodynamic Injection: Computed Tomography Study during Hydrodynamic Injection Targeting the Swine Liver. Pharmaceutics. 2015;7:334–343. doi: 10.3390/pharmaceutics7030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshino H, Hashizume K, Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13:1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- 27.Fabre JW, Whitehorne M, Grehan A, Sawyer GJ, Zhang X, Davenport M, Rela M. Critical physiological and surgical considerations for hydrodynamic pressurization of individual segments of the pig liver. Hum Gene Ther. 2011;22:879–887. doi: 10.1089/hum.2010.144. [DOI] [PubMed] [Google Scholar]
- 28.Abe H, Kamimura K, Kobayashi Y, Ohtsuka M, Miura H, Ohashi R, Yokoo T, Kanefuji T, Suda T, Tsuchida M, et al. Effective Prevention of Liver Fibrosis by Liver-targeted Hydrodynamic Gene Delivery of Matrix Metalloproteinase-13 in a Rat Liver Fibrosis Model. Mol Ther Nucleic Acids. 2016;5:e276. doi: 10.1038/mtna.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khorsandi SE, Bachellier P, Weber JC, Greget M, Jaeck D, Zacharoulis D, Rountas C, Helmy S, Helmy A, Al-Waracky M, et al. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Ther. 2008;15:225–230. doi: 10.1038/sj.cgt.7701119. [DOI] [PubMed] [Google Scholar]


