Abstract
Gastric polyps become a major clinical problem because of high prevalence and tendency to malignant transformation of some of them. The development of gastric hyperplastic polyps results from excessive proliferation of foveolar cells accompanied by their increased exfoliation, and they are macroscopically indistinguishable from other polyps with lower or higher malignant potential. Panendoscopy allows detection and differentiation of gastric polyps, usually after obtaining histopathological biopsy specimens. Unremoved gastric hyperplastic polyps may enlarge and sometimes spontaneously undergo a sequential progression to cancer. For this reason, gastric hyperplastic polyps larger than 5 mm in size should be removed in one piece. After excision of polyps with atypical focal lesion, endoscopic surveillance is suggested depending on histopathological diagnosis and possibility of confirming the completeness of endoscopic resection. Because of the risk of cancer development also in gastric mucosa outside the polyp, neighboring fragments of gastric mucosa should undergo microscopic investigations. This procedure allows for identification of patients who can benefit most from oncological endoscopic surveillance. If Helicobacter pylori (H. pylori) infection of the gastric mucosa is confirmed, treatment strategies should include eradication of bacteria, which may prevent progression of intestinal metaplasia. The efficacy of H. pylori eradication should be checked 3-6 mo later.
Keywords: Gastric hyperplastic polyp, Pathophysiology, Gastric cancer, Surveillance
Core tip: The present review is one of only a few papers describing the clinical problem of gastric hyperplastic polyps and their tendency to malignant transformation. For this reason, gastric hyperplastic polyps larger than 5 mm in size should be removed, preferably in one piece. After excision of polyps with dysplasia, careful endoscopic surveillance is needed, both places after polypectomy and surrounding mucosa.
INTRODUCTION
A polyp is a proliferative or neoplastic lesion of the mucous membrane, directed toward the gastrointestinal lumen, projecting from the surrounding mucosa, and having the head and (sometimes) the stalk[1]. Some gastric polyps tend to have malignant transformation to cancer and gastric cancer is the third most common cause of cancer-related death in the world and being still difficult to cure because of advanced disease at the moment of diagnosis.
Gastric polyps are detected during 1%-6% of upper gastrointestinal endoscopies and in 0.1%-0.8% of autopsies[2-4]. Still several years ago, gastric polyps were twice more frequent in the antrum than in the body of the stomach. It seems, however, that their location has changed in the past 10 years; the incidence of polyps has increased in the stomach body (19% vs 32%) and decreased in the antrum (46% vs 24%)[3]. Also, altered age distribution of gastric polyps was observed in the last decade; patients aged 45-59 have currently twice more gastric polyps than 10 years ago, but the inverse relationship is observed for patients aged 60 years and over[5].
According to the macroscopic classification of Yamada and Ichikawa, polyps can be divided into: 1/flat polyps, i.e., slightly elevated and with indistinct margins, height < 2.5 mm (width of closed biopsy forceps), 2/sessile polyps, i.e., elevated with a distinct border at the base, yet without a notch, height exceeds 2.5 mm, 3/semi-pedunculated polyps, i.e., elevated with distinct margins and clear notch at the base, but without peduncle and 4/pedunculated polyps[6]. Gastric epithelial polyps include fundic gland polyps, hyperplastic polyps and adenomatous polyps[7,8].
Gastric hyperplastic polyps (GHPs) can be single (68%-75%) or multiple, they occur sporadically (isolated polyps) or as a component of a rare hyperplastic polyposis syndrome (the presence of 50 or more polyps). Sporadic GHPs are macroscopically and histologically indistinguishable from the syndromic GHPs and the latter are associated with a higher risk of malignant transformation and higher 5-year mortality rate. Solitary GHPs with distinct margin, red color and protuberant shape can be difficult to distinguish from well-differentiated adenocarcinoma. Sometimes GHPs are found to coexist with other types of polyps or tumors (synchronous polyps)[2,9,10]. Geographical differences exist in the prevalence of gastric polyps. In Western countries, the incidence of GHPs decreased[2], while in areas where the prevalence of Helicobacter pylori (H. pylori) is high, GHPs have been reported more frequently[11].
PATHOPHYSIOLOGY
It is believed that GHP development results from excessive proliferation of foveolar cells (mucin-producing epithelial cells lining the gastric surface and the gastric pits) accompanied by their increased exfoliation; glands are usually not involved in the formation of polyps. Stimuli directly responsible for the appearance of GHPs have not been known yet, although it seems that abnormal hyper-regenerative processes triggered in response to nonspecific gastric mucosal injury can be suspected. Two main pathologies lie in the background of GHPs: longstanding H. pylori-associated gastritis and autoimmune metaplastic atrophic gastritis in Addison-Biermer disease; GHPs are less common in other inflammatory conditions, in the vicinity of ulcers, erosions and surgical gastroenterostomy, secondary to prior endoscopic coagulation therapy, in gastric mucosa with slight atrophy or metaplasia, and in cardia in patients with gastrointestinal reflux. GHPs almost never occur in normal gastric mucosa.
The risk of developing GHPs increases with the degree of mucosal atrophy, especially when the stomach body is affected. Over time, GHPs may remain stable, increase in size, or even regress. It was not long ago that GHPs were believed to be benign lesions not associated with the risk of malignant transformation. Today, however, unremoved GHPs are known for their ability to enlarge and sometimes spontaneously undergo a sequential progression and a few-phase neoplastic transformation[12]. This process has been confirmed and well documented in GHPs, although it is much less common than in adenomatous polyps of the stomach.
Although Helicobacter pylori is sometimes present within GHPs[13], the bacterium not induces specifically their growth or malignant transformation[14]. It is estimated that most of GHPs remain stable in time, but 27% may enlarge[15]. It appears that, in addition to age, there are known some clinical factors predicting for the possibility of neoplastic transformation of GHPs, such as polyp size (greater than 1 cm), pedunculated morphology, postgastectomy state, and synchronous neoplastic lesion[16,17].
Early gastric cancer in gastric hyperplastic polyps, by definition, does not infiltrate deeper than the submucosa, irrespective of local lymph node involvement. In the pedunculated polyps, the submucosal layer ascends through the stalk to the head, while in sessile ones, the submucosal layer forms convexity toward the tumor. The depth of penetration can be assess only when the cross-sectional image perpendicular to the lesion and contiguous normal wall are obtained. The prevalence of gastric cancer in GHPs shows a positive correlation with the polyp size, whereas mortality due to GHPs depends on the presence and severity of neoplastic transformation in the polyp[18]. Gastric metastases are relatively rare, but even a case of metastasis to GHP has been recently described to[19].
EPIDEMIOLOGY AND SYMPTOMATOLOGY
Although the existing epidemiological data are not explicit, the multi-center research trial conducted in the United States to assess so far the largest number of panendoscopies (120000) and gastric polyps (5877) suggests that the most common types of gastric polyps are gastric fundic gland polyps (77%) and GHPs (14%); much less common are: polypoid foveolar hyperplasia (2.7%), adenocarcinomas (1.3%), lymphomas (0.9%) and adenomas (0.7%)[2]. A recently published retrospective study which assessed the United States national database of histopathological reports involving approximately 741000 patients, confirmed the highest prevalence of gastric fundic gland polyps and hyperplastic polyps, 7.72% and 1.79% of patients undergoing gastroscopy, respectively[20]; adenomatous polyps of the stomach were much less common. In other clinical studies, depending on the definition and histopathological criteria, the study period and population, GHPs accounted for 25%-28% to 71%-76% of all gastric polyps[4,21-28].
The incidence of GHPs increases with age and although they can also be found in children, GHPs usually affects the 65-75 year-old population[1]. Most studies proved higher incidence of all types of gastric polyps in women than in men[1,3,22]. In a study performed by Cao et al[3] gastric polyps were found in 34% of men and 66% of women (24121 patients). However, it was the result of higher prevalence of gastric fundic gland polyps in women (43% vs 55%), since the prevalence of gastric hyperplastic polyps was similar in both genders (27% vs 29%). Besides, adenomatous polyps, which were much less common, were more often observed in men (15% vs 4%). Although the percentage of all gastric polyps found during panendoscopies has not changed in the last decade, it seems that the relative incidence of GHPs showed a twofold decrease, which was accompanied by a substantial increase in the relative incidence of gastric fundic gland polyps. It is speculated that this phenomenon can be the effect of a common use of proton pump inhibitors[3].
Gastric traditional serrated adenomas (TSA) were described for the first time in 2001. A novel histologic phenotype of gastric adenoma are characterized by protruding glands with lateral saw tooth-like notches due to scalloped epithelial indentations; gastric TSA have emerged as very aggressive, because nearly 75% of them exhibited invasive carcinoma[29].
GHPs are usually asymptomatic and therefore incidentally found during panendoscopies performed for various reasons[2]. Symptoms due to GHPs are nonspecific: dyspepsia, heartburn, bleeding from the upper GI tract (usually latent), and sometimes gastric outlet obstruction. Only sideropenic anemia can be an indirect nonspecific presentation of a large and fragile GHPs. Imaging diagnostic examinations (X-ray with contrast agent, computed tomography) have little significance due to high false-negative rates; they can sometimes reveal only large GHPs. Panendoscopy is the investigation of choice allowing detection and differential diagnosis of gastric polyps, usually after obtaining histopathological biopsy specimens.
MACROSCOPIC AND HISTOPATHOLOGICAL PICTURE
GHPs are usually small, flat or sessile dome-shaped lesions with smooth surface and lobular structure (Figure 1). The proportional prevalence of GHPs according to size is estimated at: 47% (< 0.5 cm), 25% (0.6-0.9 cm), 18% (1-2 cm), 6% (2-3 cm) and 4% (> 3 cm)[30]. Sometimes GHPs may have erosions on their surface and they are often difficult to distinguish from polypoid foveolar hyperplasia or gastric adenomatous polyps[1]. Sometimes GHPs are very big and have aciniform structure. They may reach even 13 cm in size and then they resemble a neoplastic tumor. A large size of gastric hyperplastic polyps and granular structure with visible depression and mucus threads on the surface may suggest their malignant transformation.
Figure 1.
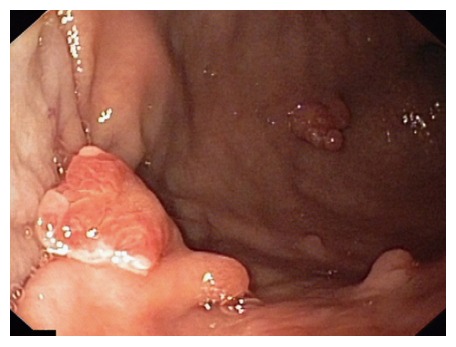
Endoscopic view. Large gastric hyperplastic polyp.
Endoscopy with optic image magnification and NBI allows the assessment of the network of fine blood vessels, which correlates well with histopathological findings and increases the possibility of early differentiation of gastric polyps already during endoscopy; dense distribution of irregular capillaries on the polyp surface is characteristic of GHPs[31].
Contrary to hyperplastic polyps of the colon, GHPs show swelling of the submucosal membrane with pronounced foveolar hyperplasia and infiltration of the lamina propria by inflammatory cells, among which smooth muscle cells derived from thickened and cracked muscle membrane can be seen. Mucin-secreting cells from the foveolar layer of GHPs are enlarged and elongated; they form canals that extend to the stroma, which can enlarge and form marked irregular cysts varying in shape and size. PAS/Alcian blue or mucicarmine stains highlight acidic mucin in goblet cells and can demonstrate the neutral mucin in foveolar epithelium[10].
GHPs have two major and typical microscopic features[10]. The first and salient includes distinctly elongated, dilated, distorted and branched pits of the mucosa, with a folded epithelial lining (differing in height) that does not exfoliate in proper time (Figure 2A-C). This leads to increased mucus secretion and a spiral appearance of the mucosal pits on the horizontal section or serrated and star-like appearance on the cross-section[18]. The foveolar cells mature excessively, contain large amounts of cytoplasm and small nuclei, and exhibit low mitotic activity[21]. The swollen stroma shows a network of randomly arranged, diffused fine bundles of smooth muscles, located in the lamina propria. The glandular epithelium can sometimes occur only in deeper layers of the polyps.
Figure 2.
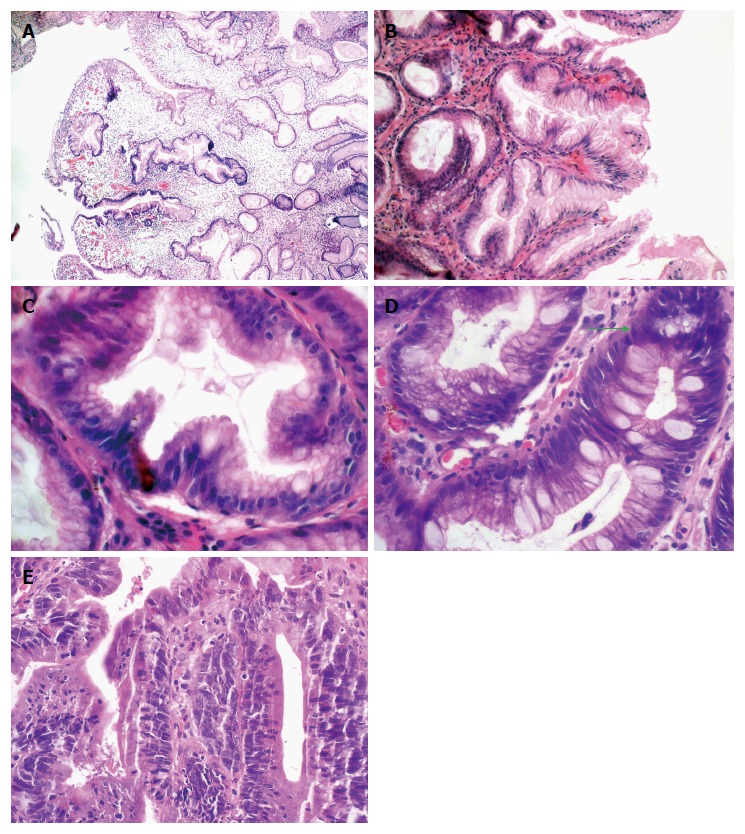
Histopathological findings. A: Gastric hyperplastic polyp with dilated, elongated, branched and foveolar epithelium and edematous end inflamed stroma (original magnification × 10); B: Gastric hyperplastic polyp with well visible elongated foveolar epthelium (original magnification × 20); C: A cross-section of mucosal crypt shows a serrated ligh of the gland and the goblet cells (original magnification × 40); D: The green arrow indicates a regeneration zone in the foveolar epthelium with hyperchromatic nuclei (original magnification × 40); E: Focus of adenocarcinoma in the gastric hyperplastic polyp (original magnification × 40). Hematoxylin-eosin staining.
The second typical microscopic feature is swelling and inflammatory reaction of the stroma of varied intensity, either acute or chronic, i.e., visible infiltration of the lamina propria by numerous neutrophils, plasmatic cells, lymphocytes, eosinophils, mastocytes and macrophages. These regions are strongly vascularized and vascular proliferation resembles granulation. Because of local trauma, the surface of GHPs can be ulcerated and inflamed, with regenerative atypia of epithelial and interstitial cells (Figure 2D). Sometimes the surface of the mucous membrane can also exhibit budding pits having features of pseudo-invasion. For a histopathologist, such lesions are a major diagnostic issue, since hyperplastic polyps may sometimes contain foci of dysplasia and cancer. Abnormal regenerative changes may be difficult to differentiate accurately from dysplastic atypia[32,33].
If dysplasia within GHPs is confirmed by biopsy, it is crucial to determine its grade and boundaries, and assess whether it is limited only to the polyp or is just a fragment of the extensive neoplastic process. If dysplasia develops only in the polyp and its focus is removed radically during polypectomy, both macroscopically and microscopically, the lesion is considered to be cured.
DIFFERENTIAL DIAGNOSTICS
GHPs should be differentiated from other sporadic polyps (fundic gland polyps, adenomatous polyps) and lesions of the mucosa present in familial polyposis syndrome (Ménétrier disease, juvenile polyposis and Cronkhite-Canady syndrome). Earlier clinical studies suggesting high incidence of GHPs launched a debate on diagnostic criteria and the factual incidence rate. It seems that most of the previously described tiny hyperplastic polyps were in fact only the hyperplasia of the foveolar layer of the gastric mucosa. Polypoid foveolar hyperplasia (PFH) is regarded as a precursor of gastric hyperplastic polyps and differs slightly from them in the microscopic structure. Elongated pits of the mucosa but without features of dilatation can be also seen in PFH, and the lamina propria is either normal or only slightly swollen[2]. Differentiation between these two lesions is of crucial clinical significance since malignant transformation affects gastric hyperplastic polyps but not foveolar polypoid hyperplasia[24]. Precise categorization of gastric polyps is being conducted. Multicenter clinical study results published in 2011, revised the previous histopathological assessment of gastric hyperplastic polyps using precise diagnostic criteria; only in 20% of cases, previous diagnosis of GHP was confirmed[24].
CLINICAL SIGNIFICANCE OF INTRAEPITHELIAL NEOPLASIA IN THE MUCOSA SURROUNDING A POLYP
Oncological risk associated with GHPs depends on the risk of cancer development not only in the polyp, but also in gastric mucosa outside the polyp. Thus, also the neighboring fragments of gastric mucosa should undergo endoscopic and microscopic investigations.
The risk of focal gastric cancer is five-fold higher in gastric adenomatous polyps than in the hyperplastic ones (10% vs 2.1%), and 2-fold higher in gastric mucosa surrounding the adenomatous than hyperplastic polyps (13.3% vs 7.1%). Thus the risk of cancer growth in gastric mucosa outside the polyp is probably slightly higher than in the polyp itself[21].
Gastric hyperplastic polyps are frequently associated with inflammatory lesions in the local gastric mucosa. Chronic inflammation of gastric mucosa can be observed in associations with H. pylori infection (25%), autoimmune inflammation (12%), atrophic gastritis, lymphocytic inflammation or CMV infection[30]. In a patient with GHPs the Sydney biopsy protocol recommends collection of five separate specimens: two from the stomach body (greater and lesser curvature), two from the antrum (greater and lesser curvature) and one from the gastric angle. When H. pylori infection of the gastric mucosa is confirmed, treatment of small GHPs should begin from eradication therapy, which in many cases reduces or eliminates the polyps[34]. The efficacy of such treatment should be checked 3-6 mo later.
If the gastric mucosa surrounding a hyperplastic polyp exhibits features of chronic atrophic gastritis, its stage should be examined using the OLGA system (Operative Link on Gastritis Assessments), allowing better stratification of the risk and identification of patients who can benefit most from oncological endoscopic surveillance. It seems that patients with diffused atrophy should be included in such a program and have panendoscopy performed at first each year (OLGA IV), and then every 2 years (OLGA III) or every 5 years (OLGA II)[35].
The mucosa surrounding the hyperplastic polyps frequently shows chronic inflammation and sometimes oncologically hazardous focal intestinal metaplasia (37%) and dysplasia (2%) or even adenocarcinoma (6%)[30].
CLINICAL SIGNIFICANCE OF INTRAEPITHELIAL NEOPLASIA IN THE POLYP
Only a small percentage (< 2%-3%) of GHPs, usually the larger ones (> 1-2 cm), show features of focal intraepithelial neoplasia (IEN) or cancer (Figure 2E). Therefore, large polyps should be removed and as a whole subjected to histopathological analysis. Studies assessing the presence of intraepithelial neoplasia and adenocarcinoma in GHPs are rare[2,21,30,36-38].
The p53 protein, a p53 suppressor gene product, inhibits neoplastic transformation (by prolonging G1 phase of the cellular cycle), which gives the cells enough time to repair damaged DNA threads. If the damage is severe and cannot be quickly repaired, p53 initiates the process of programmed cell death. Mutations of p53 gene result in the synthesis of mutated, i.e., functionally abnormal p53, deprived of the inhibitory function, which promotes transfer of genetic disorders to daughter cells and facilitates neoplastic transformation. The half-life period of normal p53 is approximately 20 min only, whereas pathological p53, being the product of the mutated p53 gene, shows a prolonged half-life, is accumulated in the cell and can be then easily detected.
The assessment of cell proliferation is a useful marker in the diagnosis of neoplastic transformation. Ki-67 antigen observed during all active phases of the cell cycle but absent from the G0 phase is presented as the percent of marked cell nuclei. It is a widely accepted marker of proliferation; the higher the expression of Ki-67, the higher malignancy grade.
In the histopathological material including 497 GHPs collected from 412 patients during an 11-year-period, the prevalence of intestinal metaplasia, dysplasia (intraepithelial neoplasia) and cancer within GHPs was estimated at 5%, 10% and 2.2%, respectively[38]. Positive expression of p53 and high proliferation index (mitotic index) of Ki-67 were observed in cases of focal intraepithelial neoplasia (41%) and cancer (50%) within GHPs, as compared to the hyperplastic regions and metaplastic foci[33,38]. The foci of intraepithelial neoplasia were always found close to the foci of adenocarcinoma. The research seems to confirm the theory of neoplastic transformation in hyperplastic polyps of the hyperplasia-dysplasia-adenocarcinoma type. The expression of certain membrane proteins called claudins (Cld) that are found in tight intracellular junctions and are responsible for cell membrane integrity is one of the markers of malignant transformation within hyperplastic polyps of the stomach, since the expression of Cld-3 has been demonstrated only within the foci of intraepithelial neoplasia and cancer[16].
Orlowska et al[21] estimated the prevalence of metaplasia, intraepithelial neoplasia (dysplasia) and cancer in GHPs to be 5.6%, 3.3% and 2.1%. In their research[21] and in a study conducted by Abraham et al[30], the percentage of intraepithelial neoplasia in hyperplastic polyps of the stomach is 10 times higher (3.3%-4% vs 0.4%) than in the study by Carmack et al[2], and in the study by Terada[38] even 25 times higher (10% vs 0.4%); perhaps, these authors were dealing with specially selected patients.
The risk of developing cancer in GHPs increases with the polyp size and is believed to be higher for GHPs > 2 cm, although cases of cancer in 5-10 mm polyps have also been described. Neoplastic transformation usually starts from a small focus of intraepithelial neoplasia, which grows and acquires features of invasiveness. Intraepithelial neoplasia involves cytological and architectonic disorders within the cell: changes in the nucleus-cytoplasm ratio in favor of the nuclei, increased number of mitoses in enlarged nuclei, excessive number of epithelial cells with their build-up and loss of nuclear polarization (hyperchromatic cell nuclei lose their parallel arrangement). Mild and severe intraepithelial neoplasia can be distinguished, depending on impairment severity.
TREATMENT
The management of gastric polyps depends on the clinical condition of the patient, malignant potential of detected polyps and at which stage of malignant transformation polyps have been found. Endoscopic removal of adenomatous or hyperplastic polyps, symptomatic or with dysplastic foci, is recommended if it is possible and safe. Studies comparing biopsy findings with histopathological assessment of radically removed polyps have shown approximately 90% compatibility. When the polyp is removed in one piece with a diathermic loop it is less probable that some advanced dysplastic and neoplastic lesions can be missed and more likely that total removal is accomplished; piecemeal polypectomy technique does not ensure radical removal. Biopsy of gastric mucosa outside the polyp and examination for H. pylori infection and its eradication are additionally recommended, with a single endoscopic check-up one year later[7]. At present, repeat endoscopic examinations of GHPs negative for intraepithelial neoplasia are not recommended (Table 1).
Table 1.
The proposed management decisions and oncologic surveillance program regarding gastric hyperplastic polyps
| Before endoscopic resection of GHPs |
| GHP without dysplasia or cancer, asymptomatic and small (< 5 mm) - surveillance not recommended |
| GHP symptomatic or larger than 5 mm - endoscopic resection recommended |
| GHP with dysplasia or cancer - endoscopic or surgical resection recommended |
| GHP not classified for removal due to the risk of postsurgical complications - periodic gastroscopies with representative biopsies every 1-2 yr |
| GHP in patients with high risk of gastric cancer1 - gastroscopies every 1-2 yr |
| GHP with dysplasia outside the polyp - consider subtotal gastrectomy and gastroscopies every 1-3 yr |
| After endoscopic resection of GHPs |
| After complete resection of GHP with dysplasia - gastroscopy 1 yr later, and then depending on the clinical situation |
| After complete resection of GHP with early gastric cancer - gastroscopy 1 yr after and then 3 yr after |
| After incomplete resection of GHP with gastric cancer - consider gastrectomy with lymphadenectomy |
Family history of gastric cancer or OLGA 3-4 on histopathological examination. GHP: Gastric hyperplastic polyp.
Polypectomy is indicated for all gastric polyps > 10 mm, to eliminate sampling error by missing any neoplastic foci and prevent neoplastic transformation. Periodic biopsies of polyps that are not classified for removal due to their size, number and the risk of postsurgical complications should be performed[25]. When multiple polyps occur, it is recommended to obtain bioptates or remove the largest polyp as well as obtain biopsy specimens from the remaining polyps. And then, decision for polypectomy should be made based on histopathology findings. Most of GHPs can be detected and treated using endoscopy alone. According to current recommendations, GHPs > 5 mm should be removed whole[8,17,18,39], especially the pedunculated ones[17].
Some researchers, however, considering the risk of the procedure (bleeding, perforation), suggest the removal of only large GHPs, in which the probability of intraepithelial neoplasia and cancer is the highest. In the case of multiple hyperplastic polyps without foci of intraepithelial neoplasia, conservative management and follow up endoscopy seems to be safer strategy than numerous polypectomies, although there are no reliable studies to support this suggestion.
Oncological surveillance of patients with hyperplastic polyps containing foci of dysplasia and cancer should be patient-tailored, since there are no generally accepted guidelines. It seems that when cancer was detected early in an endoscopically radically resected polyp, the oncological surveillance should involve repeated endoscopy, at the same frequency than for adenomatous gastric polyps, i.e., first one year after and then 3 years after the procedure[17,39].
Endoscopic treatment of the polyp containing cancer is considered sufficient if it has been completely resected according to the endoscopist (macroscopic radicality) and histopathologist (microscopic radicality). If the cancer does not exceed the gastric mucosa, the excision margin free of cancer cells is greater than 2 mm in the microscopic investigation, differentiation degree of the cancer is high or moderate and no angioinvasion is observed, the resection is approved oncologically radical. The percentage of cancer relapse after radical resection of gastric hyperplastic polyps containing focal cancer is unknown, although it is certainly lower than that after the endoscopic resection of nonpolypoid early gastric cancer (1.2%)[40].
If incomplete resection of hyperplastic polyp containing early gastric cancer is evidently confirmed or when effective endoscopic treatment is impossible, we have to consider gastrectomy with local lymphadenectomy[41]. In surgical treatment of early gastric cancer, conventional open laparotomy is increasingly more often replaced by low-invasive surgical techniques.
The surveillance of malignant GHPs following endoscopic removal is difficult because of the possibility of residual neoplastic cells within the stomach wall. It is commonly believed that the patient with diagnosed cancer or high grade dysplasia in the polyp should be treated by a multi-specialist team dealing with the diagnosis of the upper GI tract. The diagnosis should be established by two pathologists, with at least one specializing in gastrointestinal diseases. The strategy of management and therapy should be discussed with the patient and experienced endoscopists should perform surveillance panendoscopy.
When the resected GHPs are free of dysplasia and cancer, the management of patients should depend on the risk of developing cancer assessed on the basis of the presence of chronic atrophic gastritis and/or other risk factors. Last data (405211 patients) predict that about 1 in 256 people with normal gastric mucosa, 1 in 85 with gastritis, 1 in 50 with atrophic gastritis, 1 in 39 with intestinal metaplasia, and 1 in 19 with dysplasia will develop gastric cancer within 20 years after gastroscopy[42].
In patients with high risk of gastric cancer (OLGA 3-4), with moderate or high grade diffuse atrophy of the mucosa, usually with enhanced metaplasia, in patients with a family history of gastric cancer, gastroscopies are recommended regularly, every year or every two years. Patients with low risk of gastric cancer (OLGA 1-2) should have at least one gastroscopy within 3-6 mo after the procedure, to confirm eradication of H. pylori and exclude the presence of new or residual polyps that would have to be removed.
When dysplasia is present in the mucous membrane of the stomach outside the polyp, especially in diffuse lesions, subtotal gastrectomy should be considered with postsurgical endoscopic surveillance, during which numerous bioptates are collected from the mucosa of the stomach stump at 1-3 year-intervals to exclude multifocal lesions[17,40].
CONCLUSION
Recent studies have confirmed that cancer may arise within GHP, and a malignant lesion is likely to take a hyperplasia-dysplasia-adenocarcinoma course. Polypectomy of GHPs > 5 mm is recommended, with histopathological diagnosis, and periodic biopsies of the polyps which are not qualified for removal should be obtained. Additionally, biopsy of gastric mucosa outside the polyp is indicated as well as H. pylori eradication in the case of confirmed infection. Endoscopic check-up is suggested a year after removal of dysplasia-free GHP. The surveillance of patients after polypectomy of GHP containing foci of dysplasia and cancer should be more intensive and individual; precise guidelines do not exist.
Extensive knowledge concerning the mechanisms of origin, malignancy potential, diagnostic possibilities and GHP management may increase the efficacy of treatment of gastric polyps in everyday clinical practice.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There is no conflict of interest among the authors of this study.
Peer-review started: May 30, 2016
First decision: July 13, 2016
Article in press: August 23, 2016
P- Reviewer: Chung JW, Dhalla SS, Garcia-Olmo D, Porumb V S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Park DY, Lauwers GY. Gastric polyps: classification and management. Arch Pathol Lab Med. 2008;132:633–640. doi: 10.5858/2008-132-633-GPCAM. [DOI] [PubMed] [Google Scholar]
- 2.Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524–1532. doi: 10.1038/ajg.2009.139. [DOI] [PubMed] [Google Scholar]
- 3.Cao H, Wang B, Zhang Z, Zhang H, Qu R. Distribution trends of gastric polyps: an endoscopy database analysis of 24 121 northern Chinese patients. J Gastroenterol Hepatol. 2012;27:1175–1180. doi: 10.1111/j.1440-1746.2012.07116.x. [DOI] [PubMed] [Google Scholar]
- 4.Archimandritis A, Spiliadis C, Tzivras M, Vamvakousis B, Davaris P, Manika Z, Scandalis N. Gastric epithelial polyps: a retrospective endoscopic study of 12974 symptomatic patients. Ital J Gastroenterol. 1996;28:387–390. [PubMed] [Google Scholar]
- 5.Fan NN, Yang J, Sun G, Lu ZS, Ling Hu EQ, Wang XD, Yang YS. Changes in the spectrum of gastric polyps in the Chinese population. World J Gastroenterol. 2015;21:9758–9764. doi: 10.3748/wjg.v21.i33.9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada T, Ichikawa H. X-ray diagnosis of elevated lesions of the stomach. Radiology. 1974;110:79–83. doi: 10.1148/110.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B. The management of gastric polyps. Gut. 2010;59:1270–1276. doi: 10.1136/gut.2009.182089. [DOI] [PubMed] [Google Scholar]
- 8.Sharaf RN, Shergill AK, Odze RD, Krinsky ML, Fukami N, Jain R, Appalaneni V, Anderson MA, Ben-Menachem T, Chandrasekhara V, et al. Endoscopic mucosal tissue sampling. Gastrointest Endosc. 2013;78:216–224. doi: 10.1016/j.gie.2013.04.167. [DOI] [PubMed] [Google Scholar]
- 9.Sung HY, Cheung DY, Cho SH, Kim JI, Park SH, Han JY, Park GS, Kim JK, Chung IS. Polyps in the gastrointestinal tract: discrepancy between endoscopic forceps biopsies and resected specimens. Eur J Gastroenterol Hepatol. 2009;21:190–195. doi: 10.1097/MEG.0b013e3283140ebd. [DOI] [PubMed] [Google Scholar]
- 10.Baishali B. Non-Neoplastic Disorders of the Stomach. Gastric hyperplastic polyps. In: Lacobuzio-Donahue CA, Montgomery E, editors. Gastrointestinal and liver pathology. Philadelphia: Elsevier Inc; 2012. pp. 145–173. [Google Scholar]
- 11.Nayudu SK, Niazi M, Balar B, Kumbum K. A rare complication of hyperplastic gastric polyp. Case Rep Gastrointest Med. 2013;2013:631975. doi: 10.1155/2013/631975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowski AR, Guzinska-Ustymowicz K. Gastric hyperplastic polyp with focal cancer. Gastroenterol Rep (Oxf) 2016;4:158–161. doi: 10.1093/gastro/gou077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath B, Pai RK. Prevalence of Helicobacter pylori in Gastric Hyperplastic Polyps. Int J Surg Pathol. 2016 doi: 10.1177/1066896916648380. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Genta RM, Lash RH. Helicobacter pylori and Gastrointestinal Polyps. In: Backert S, Yamaoka Y. Helicobacter pylori Research: From Bench to Bedside. Japan: Springer, 2016: 387-402 In: Backert S, Yamaoka Y, editors. [Google Scholar]
- 15.Turner JR, Odze RD. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. Philadelphia: Saunders, 2015: 540-578 In: Odze RD, Goldblum JR, editors. [Google Scholar]
- 16.Imura J, Hayashi S, Ichikawa K, Miwa S, Nakajima T, Nomoto K, Tsuneyama K, Nogami T, Saitoh H, Fujimori T. Malignant transformation of hyperplastic gastric polyps: An immunohistochemical and pathological study of the changes of neoplastic phenotype. Oncol Lett. 2014;7:1459–1463. doi: 10.3892/ol.2014.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82:1–8. doi: 10.1016/j.gie.2015.03.1967. [DOI] [PubMed] [Google Scholar]
- 18.Han AR, Sung CO, Kim KM, Park CK, Min BH, Lee JH, Kim JY, Chang DK, Kim YH, Rhee PL, et al. The clinicopathological features of gastric hyperplastic polyps with neoplastic transformations: a suggestion of indication for endoscopic polypectomy. Gut Liver. 2009;3:271–275. doi: 10.5009/gnl.2009.3.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groisman GM, Depsames R, Ovadia B, Meir A. Metastatic carcinoma occurring in a gastric hyperplastic polyp mimicking primary gastric cancer: the first reported case. Case Rep Pathol. 2014;2014:781318. doi: 10.1155/2014/781318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg A, Genta RM. Prevalence of benign gastric polyps in a large pathology database. Dig Liver Dis. 2015;47:164–169. doi: 10.1016/j.dld.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Orlowska J, Jarosz D, Pachlewski J, Butruk E. Malignant transformation of benign epithelial gastric polyps. Am J Gastroenterol. 1995;90:2152–2159. [PubMed] [Google Scholar]
- 22.García-Alonso FJ, Martín-Mateos RM, González Martín JA, Foruny JR, Vázquez-Sequeiros E, Boixeda de Miquel D. Gastric polyps: analysis of endoscopic and histological features in our center. Rev Esp Enferm Dig. 2011;103:416–420. doi: 10.4321/s1130-01082011000800005. [DOI] [PubMed] [Google Scholar]
- 23.Kekilli M, Beyazit Y, Karaman K, Sayilir A, Kurt M, Onal IK, Yesil Y, Akdogan M, Sasmaz N. Endoscopic and pathological aspects of gastric polyps: a Turkish referral center study. Hepatogastroenterology. 2012;59:1147–1149. doi: 10.5754/hge10785. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Obeso E, Fujita H, Deshpande V, Ogawa F, Lisovsky M, Genevay M, Grzyb K, Brugge W, Lennerz JK, Shimizu M, et al. Gastric hyperplastic polyps: a heterogeneous clinicopathologic group including a distinct subset best categorized as mucosal prolapse polyp. Am J Surg Pathol. 2011;35:670–677. doi: 10.1097/PAS.0b013e3182127d2b. [DOI] [PubMed] [Google Scholar]
- 25.Muehldorfer SM, Stolte M, Martus P, Hahn EG, Ell C. Diagnostic accuracy of forceps biopsy versus polypectomy for gastric polyps: a prospective multicentre study. Gut. 2002;50:465–470. doi: 10.1136/gut.50.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roseau G, Ducreux M, Molas G, Ponsot P, Amouyal P, Palazzo L, Amouyal G, Paolaggi JA. [Epithelial gastric polyps in a series of 13000 gastroscopies] Presse Med. 1990;19:650–654. [PubMed] [Google Scholar]
- 27.Morais DJ, Yamanaka A, Zeitune JM, Andreollo NA. Gastric polyps: a retrospective analysis of 26,000 digestive endoscopies. Arq Gastroenterol. 2007;44:14–17. doi: 10.1590/s0004-28032007000100004. [DOI] [PubMed] [Google Scholar]
- 28.Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659–665. doi: 10.1055/s-2007-1009061. [DOI] [PubMed] [Google Scholar]
- 29.Rubio CA. Traditional serrated adenomas of the upper digestive tract. J Clin Pathol. 2016;69:1–5. doi: 10.1136/jclinpath-2015-203258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500–507. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Omori T, Kamiya Y, Tahara T, Shibata T, Nakamura M, Yonemura J, Okubo M, Yoshioka D, Ishizuka T, Maruyama N, et al. Correlation between magnifying narrow band imaging and histopathology in gastric protruding/or polypoid lesions: a pilot feasibility trial. BMC Gastroenterol. 2012;12:17. doi: 10.1186/1471-230X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueira AM, Carneiro F, Seruca R, Cirnes L, Veiga I, Machado JC, Sobrinho-Simões M. Microsatellite instability in hyperplastic and adenomatous polyps of the stomach. Cancer. 1999;86:1649–1656. [PubMed] [Google Scholar]
- 33.Murakami K, Mitomi H, Yamashita K, Tanabe S, Saigenji K, Okayasu I. p53, but not c-Ki-ras, mutation and down-regulation of p21WAF1/CIP1 and cyclin D1 are associated with malignant transformation in gastric hyperplastic polyps. Am J Clin Pathol. 2001;115:224–234. doi: 10.1309/VLF5-UCNH-XQM2-X410. [DOI] [PubMed] [Google Scholar]
- 34.Ji F, Wang ZW, Ning JW, Wang QY, Chen JY, Li YM. Effect of drug treatment on hyperplastic gastric polyps infected with Helicobacter pylori: a randomized, controlled trial. World J Gastroenterol. 2006;12:1770–1773. doi: 10.3748/wjg.v12.i11.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45:1–8. doi: 10.1007/s00535-009-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daibo M, Itabashi M, Hirota T. Malignant transformation of gastric hyperplastic polyps. Am J Gastroenterol. 1987;82:1016–1025. [PubMed] [Google Scholar]
- 37.Zea-Iriarte WL, Sekine I, Itsuno M, Makiyama K, Naito S, Nakayama T, Nishisawa-Takano JE, Hattori T. Carcinoma in gastric hyperplastic polyps. A phenotypic study. Dig Dis Sci. 1996;41:377–386. doi: 10.1007/BF02093832. [DOI] [PubMed] [Google Scholar]
- 38.Terada T. Malignant transformation of foveolar hyperplastic polyp of the stomach: a histopathological study. Med Oncol. 2011;28:941–944. doi: 10.1007/s12032-010-9556-6. [DOI] [PubMed] [Google Scholar]
- 39.Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim DH, Lee JH, Kim MY, Kim BS, Oh ST, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–948. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Yada T, Yokoi C, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc. 2013;2013:241320. doi: 10.1155/2013/241320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. doi: 10.1136/bmj.h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]


