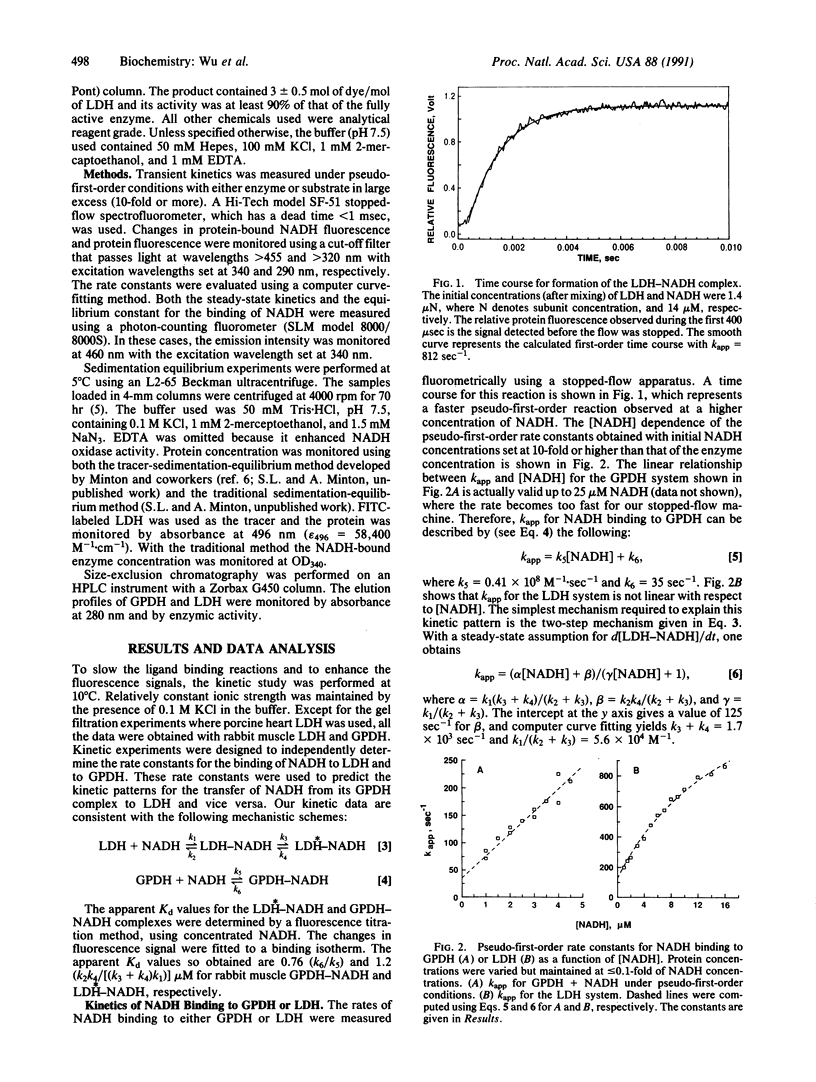
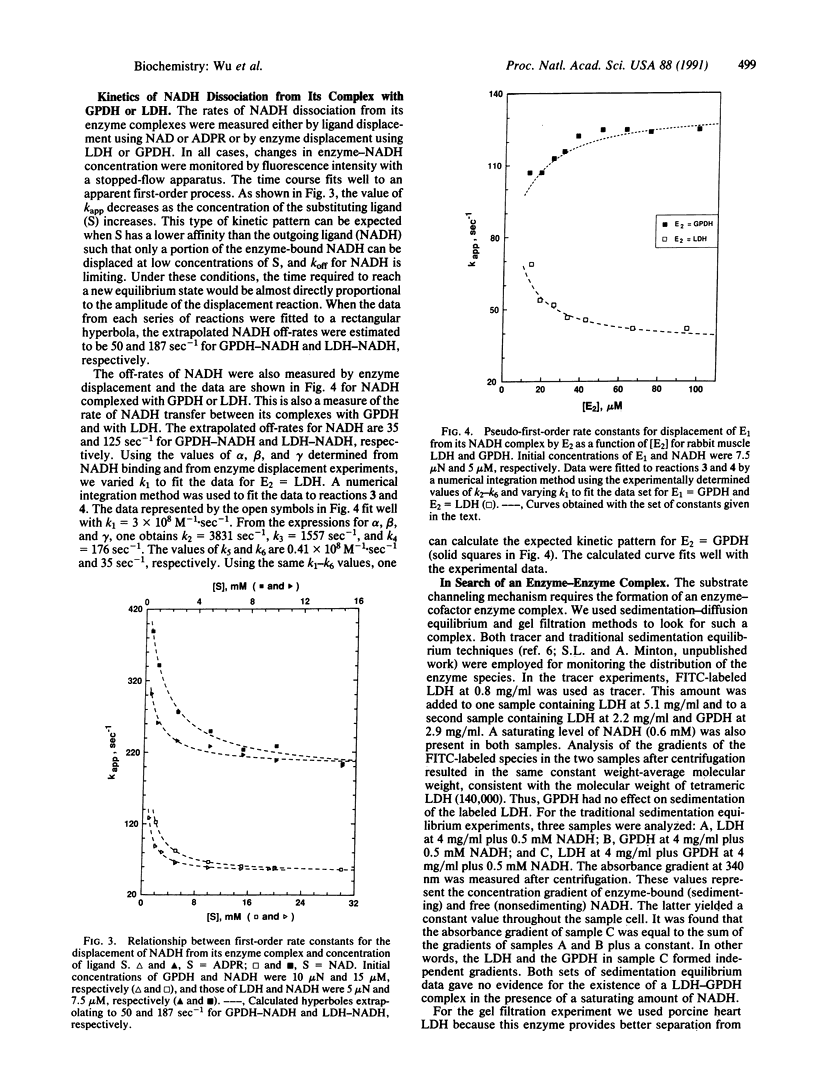
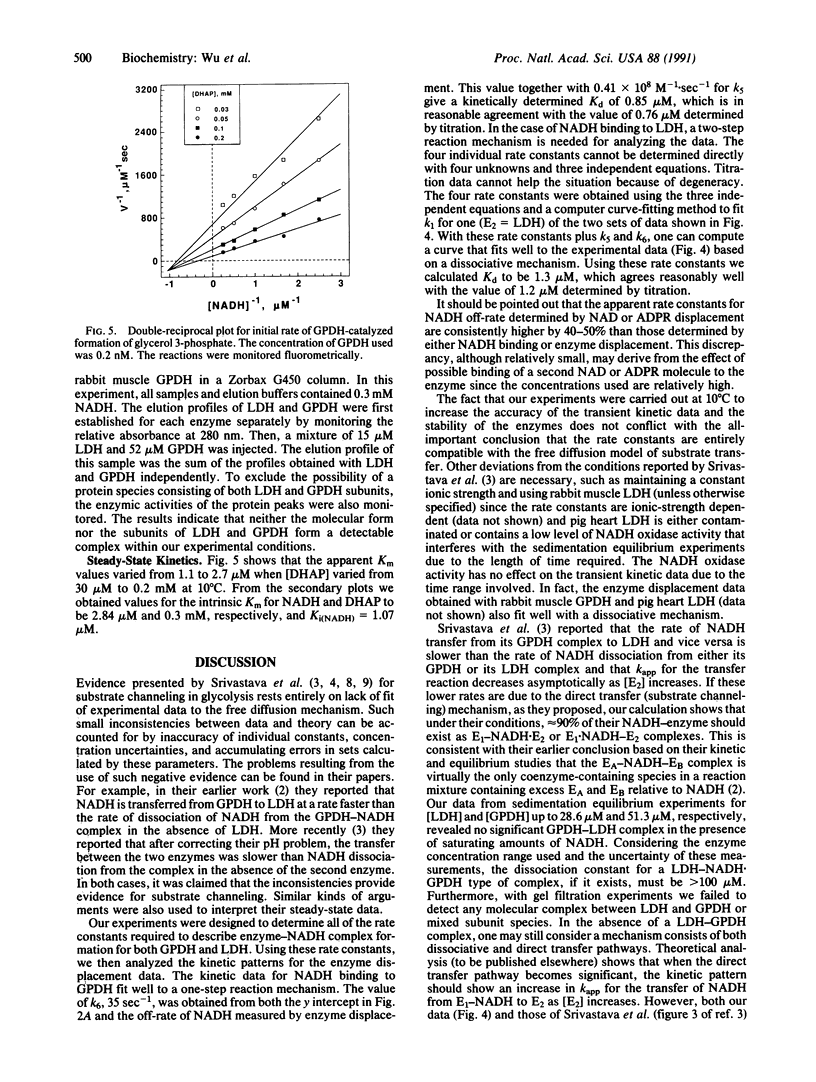
Abstract
It has been proposed that glycolytic enzymes form multienzyme complexes for direct transfer of metabolites from the producing enzyme to the utilizing one. Reexamination of the supporting evidence, which involves the transfer of NADH between its complexes with glycerol-3-phosphate dehydrogenase (alpha-glycerol phosphate dehydrogenase, GPDH; EC 1.1.1.8) and with L-lactate dehydrogenase (LDH; EC 1.1.1.27), has shown that the supporting evidence is based on misinterpretation of the kinetics of ligand exchange. Srivastava et al. have responded with a revision of their own and criticism of our data. To clarify this problem, we have carried out detailed kinetic studies on NADH binding to GPHD and LDH and on the displacement of enzyme-bound NADH by LDH or GPDH. The experiments were conducted at 10 degrees C in 50 mM Hepes, pH 7.5/100 mM KCl/1 mM EDTA/1 mM 2-mercaptoethanol, using rabbit muscle GPDH and LDH. The results show that the kinetic patterns exhibited by the displacement of NADH-bound enzyme by either GPDH or LDH are consistent with a dissociative mechanism but not with a direct transfer mechanism. Theoretical analysis shows that a combined dissociative and direct transfer mechanism can explain the transient kinetic data reported by Srivastava et al. if, and only if, a majority (approximately 90%) of the enzyme present in lower concentration exists as a complex with the second enzyme. However, data from tracer and traditional sedimentation equilibrium and from gel filtration experiments show that LDH and GPDH do not form complexes in the presence of saturating NADH concentration when the enzyme concentrations are ranged between 4 and 50 microM, a concentration equal to or greater than that used by Srivastava et al. Our results demonstrate that GPDH and LDH do not form multienzyme complex and the transfer of NADH between these enzymes proceeds via a dissociative mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attri A. K., Minton A. P. An automated method for determination of the sedimentation coefficient of macromolecules using a preparative centrifuge. Anal Biochem. 1984 Feb;136(2):407–415. doi: 10.1016/0003-2697(84)90236-7. [DOI] [PubMed] [Google Scholar]
- Bentley P., Dickinson F. M. A study of the kinetics and mechanism of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1974 Oct;143(1):19–27. doi: 10.1042/bj1430019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelier R. C. A parameterized overspeeding method for the rapid attainment of low-speed sedimentation equilibrium. Anal Biochem. 1988 Nov 15;175(1):114–119. doi: 10.1016/0003-2697(88)90368-5. [DOI] [PubMed] [Google Scholar]
- Chock P. B., Gutfreund H. Reexamination of the kinetics of the transfer of NADH between its complexes with glycerol-3-phosphate dehydrogenase and with lactate dehydrogenase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8870–8874. doi: 10.1073/pnas.85.23.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvassman J., Pettersson G. Evidence that 1,3-bisphosphoglycerate dissociation from phosphoglycerate kinase is an intrinsically rapid reaction step. Eur J Biochem. 1989 Dec 8;186(1-2):261–264. doi: 10.1111/j.1432-1033.1989.tb15204.x. [DOI] [PubMed] [Google Scholar]
- Kvassman J., Pettersson G. Mechanism of 1,3-bisphosphoglycerate transfer from phosphoglycerate kinase to glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1989 Dec 8;186(1-2):265–272. doi: 10.1111/j.1432-1033.1989.tb15205.x. [DOI] [PubMed] [Google Scholar]
- Kvassman J., Pettersson G., Ryde-Pettersson U. Mechanism of glyceraldehyde-3-phosphate transfer from aldolase to glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1988 Mar 1;172(2):427–431. doi: 10.1111/j.1432-1033.1988.tb13905.x. [DOI] [PubMed] [Google Scholar]
- Ovádi J., Keleti T. Kinetic evidence for interaction between aldolase and D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1978 Apr;85(1):157–161. doi: 10.1111/j.1432-1033.1978.tb12223.x. [DOI] [PubMed] [Google Scholar]
- Ovádi J. Old pathway--new concept: control of glycolysis by metabolite-modulated dynamic enzyme associations. Trends Biochem Sci. 1988 Dec;13(12):486–490. doi: 10.1016/0968-0004(88)90237-x. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Biophysical chemistry of metabolic reaction sequences in concentrated enzyme solution and in the cell. Annu Rev Biophys Biophys Chem. 1987;16:175–204. doi: 10.1146/annurev.bb.16.060187.001135. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Direct transfer of reduced nicotinamide adenine dinucleotide from glyceraldehyde-3-phosphate dehydrogenase to liver alcohol dehydrogenase. Biochemistry. 1984 Sep 25;23(20):4538–4545. doi: 10.1021/bi00315a006. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Enzyme-enzyme interactions and the regulation of metabolic reaction pathways. Curr Top Cell Regul. 1986;28:1–68. doi: 10.1016/b978-0-12-152828-7.50003-2. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Mechanism of transfer of reduced nicotinamide adenine dinucleotide among dehydrogenases. Transfer rates and equilibria with enzyme-enzyme complexes. Biochemistry. 1987 Mar 10;26(5):1240–1246. doi: 10.1021/bi00379a006. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Metabolite transfer via enzyme-enzyme complexes. Science. 1986 Nov 28;234(4780):1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Smolen P., Betts G. F., Fukushima T., Spivey H. O., Bernhard S. A. Direct transfer of NADH between alpha-glycerol phosphate dehydrogenase and lactate dehydrogenase: fact or misinterpretation? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6464–6468. doi: 10.1073/pnas.86.17.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. P., Bernhard S. A. Transfer of 1,3-diphosphoglycerate between glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase via an enzyme-substrate-enzyme complex. Biochemistry. 1982 Aug 17;21(17):4189–4194. doi: 10.1021/bi00260a042. [DOI] [PubMed] [Google Scholar]



