Abstract
Background
Atrial fibrillation, the world’s most common arrhythmia, is a leading risk factor for stroke, a disease striking nearly 1.6 million Indians annually. Early detection and management of atrial fibrillation is a promising opportunity to prevent stroke but widespread screening programs in limited resource settings using conventional methods is difficult and costly.
Objective
The objective of this study is to screen people for atrial fibrillation in rural western India using a US Food and Drug Administration-approved single-lead electrocardiography device, Alivecor.
Methods
Residents from 6 villages in Anand District, Gujarat, India, comprised the base population. After obtaining informed consent, a team of trained research coordinators and community health workers enrolled a total of 354 participants aged 50 years and older and screened them at their residences using Alivecor for 2 minutes on 5 consecutive days over a period of 6 weeks beginning June, 2015.
Results
Almost two-thirds of study participants were 55 years or older, nearly half were female, one-third did not receive any formal education, and more than one-half were from households earning less than US $2 per day. Twelve participants screened positive for atrial fibrillation yielding a sample prevalence of 5.1% (95% CI 2.7-8.7). Only one participant had persistent atrial fibrillation throughout all of the screenings, and 9 screened positive only once.
Conclusions
Our study suggests a prevalence of atrial fibrillation in this Indian region (5.1%) that is markedly higher than has been previously reported in India and similar to the prevalence estimates reported in studies of persons from North America and Europe. Historically low reported burden of atrial fibrillation among individuals from low and middle-income countries may be due to a lack of routine screening. Mobile technologies may help overcome resource limitations for atrial fibrillation screening in underserved and low-resource settings.
Keywords: atrial fibrillation, India, screening, mobile technology, community health workers
Introduction
Atrial fibrillation is the world’s most common cardiac arrhythmia and, if untreated, increases the risk of stroke by upwards of five-fold [1]. Atrial fibrillation–related complications, particularly stroke, have reached epidemic proportions in low and middle-income countries. This is particularly true in India, where approximately 1.6 million persons suffer a stroke annually [2]. A growing number of people in India are affected by risk factors for atrial fibrillation, including hypertension and diabetes mellitus [3], and the contribution of atrial fibrillation to the ongoing stroke epidemic in India is unclear and understudied [4]. In India, where the majority of health care costs are out of pocket [5], routine evaluations using conventional electrocardiography (ECG) to diagnose atrial fibrillation are not standard of care. Therefore, an understanding of the atrial fibrillation epidemiology becomes dependent on systematic screening programs. Single-time, point-of-care screening programs face difficulties of their own because of the paroxysmal and minimally symptomatic nature of the majority of atrial fibrillation cases.
Here we report findings of a study to screen people for atrial fibrillation in rural western India using a US Food and Drug Administration (FDA)-approved single-lead ECG device, Alivecor, to overcome traditional constraints of dysrhythmia screening [6].
Methods
Residents from 6 different villages in Anand District, Gujarat, India, comprised the base population. These 6 villages were randomly selected from a list of 30 villages where our community health workers are present. Trained research coordinators worked with the community health workers who were familiar with the layout of their respective villages and enrolled 60 participants from each village. Villages in India are typically organized by occupation-based colonies (fariyahs), and an equal number of participants were recruited from all fariyahs. The residents of every third house in each fariyah were approached for enrollment through the use of a systematic random sample. After obtaining informed consent, a team of trained research coordinators and community health workers enrolled a total of 355 participants aged 50 years and older to participate in the study.
The study included two components: (1) screening using FDA-approved single-lead ECG device, Alivecor, and (2) collection of pulse data to develop an automated arrhythmia detection mobile app that can be used in low-resource settings [7,8]. Both Alivecor and pulse data were recorded serially for 2 minutes each on 5 consecutive days over a period of 6 weeks beginning June, 2015. During screening, participants sat cross-legged, resting the smartphone (iPhone 4S) in their lap to stabilize the phone and reduce excess motion that could interfere with the recordings (Figure 1). Additionally, on the day of enrollment, participants responded to a questionnaire that collected information related to their demographic characteristics, lifestyle habits, and past medical history.
Figure 1.

Community health worker screening a study participant for atrial fibrillation using a single-lead ECG device.
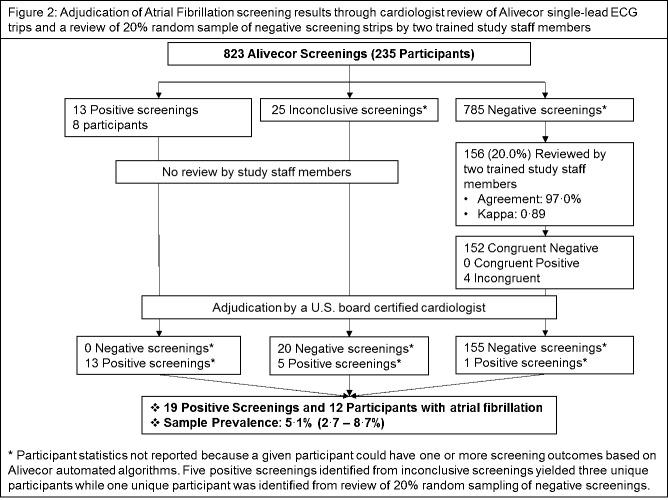
The Alivecor device malfunctioned for two weeks, and therefore 120 participants from two villages were not screened for atrial fibrillation using Alivecor and were excluded from this study. Study staff uploaded ECG and pulse check recordings to a secure, Web-accessible Research Electronic Data Capture study database. Because pulse data were collected with the intention of developing an arrhythmia detection app based on the results of this pilot study, our outcome of atrial fibrillation was determined based solely on the ECG results from the FDA-approved Alivecor device. A board-certified cardiologist reviewed all ECG tracings for participants who had abnormal rhythm findings based on the automated Alivecor algorithm (Figure 2). Any participant found to have atrial fibrillation was referred to a study cardiologist located at a regional academic health center. Due to constraints in our available resources, our research staff did not follow up with participants after screening to assess whether any clinical plan was initiated.
Figure 2.
Adjudication of atrial fibrillation screening results.
A randomly selected 20% subsample of normal ECG tracings were reviewed by two trained study staff members, and discordant readings were adjudicated by the study cardiologist. Thus, a board-certified cardiologist reviewed the ECG tracings of all participants who were determined to have positive screening findings for the presence of atrial fibrillation. The study received institutional review board approval from the University of Massachusetts Medical School and HM Patel Center for Medical Care and Education. Descriptive statistics were utilized to describe the characteristics of study participants. Sociodemographic and comorbid factors were compared across different age groups using Fisher exact tests. Prevalence rates of atrial fibrillation were calculated in a standard manner with accompanying 95% confidence intervals. Given the limited sample size in our pilot investigation and the use of the Alivecor ECG as the source for gold standard measurement, we did not calculate performance measures.
Results
Almost two-thirds of study participants were 55 years or older, nearly half were female, one-third did not receive any formal education, and more than one-half were from households earning less than US $2 per day (Table 1).
Table 1.
Sociodemographic, lifestyle, and health characteristics of 234 participants from rural Gujarat, India, screened for arrhythmias, stratified by age groups.
| Age group (%) | ||||||
| N | 50-55 | 55-65 | 65+ | P valuea | ||
| Femaleb | 140 | 71.4 | 55.0 | 56.3 | .09 | |
| Education | .19 | |||||
| None | 70 | 30.7 | 27.0 | 35.1 | ||
| 10thgrade or less | 129 | 50.0 | 59.6 | 58.4 | ||
| More than 10thgrade | 29 | 19.4 | 13.5 | 6.5 | ||
| Works for pay | 60 | 45.8 | 29.9 | 9.1 | <.001 | |
| Daily household incomec | .03 | |||||
| Less than $1 | 71 | 33.9 | 28.1 | 31.7 | ||
| $1-$2 | 62 | 14.5 | 31.5 | 31.7 | ||
| $2-$4 | 53 | 37.1 | 20.2 | 15.2 | ||
| More than $4 | 44 | 14.5 | 20.2 | 21.5 | ||
| Smoking history | 37 | 7.9 | 14.3 | 11.3 | 0.25 | |
| Chew tobacco | 58 | 33.3 | 22.0 | 21.3 | 0.03 | |
| Hypertension | 87 | 27.0 | 34.1 | 48.8 | 0.02 | |
| Diabetes | 20 | 9.5 | 5.5 | 11.3 | 0.37 | |
| Hypercholesterolemia | 21 | 4.8 | 8.8 | 12.5 | 0.30 | |
aFisher exact test.
bOne participant had completed the screening and thus was included in the analyses but did not respond to the questionnaire.
cBased on a conservative exchange rate of 1 USD = 60 INR for 2015 calendar year.
Twelve participants screened positive for atrial fibrillation yielding a sample prevalence of 5.1% (95% CI 2.7-8.7) (Figure 2); the characteristics of these individuals are shown in Table 2.
Table 2.
Characteristics of 12 atrial fibrillation positive cases identified by a cardiologist review of single-lead ECG recording.
| Gender | Age | Index positivea | # positiveb | Smoking | Hypertension | |
| 1 | Female | 50-55 | 3 | 1/3 | No | No |
| 2 | Female | 55-60 | 1 | 1/3 | No | No |
| 3 | Female | 60-65 | 1 | 5/5 | No | No |
| 4 | Female | 60-65 | 1 | 2/5 | No | Yes |
| 5 | Female | 75-80 | 1 | 1/4 | No | Yes |
| 6 | Female | 80-85 | 1 | 3/4 | No | No |
| 7 | Male | 50-55 | 3 | 1/3 | Yes | Yes |
| 8 | Male | 55-60 | 1 | 1/1 | Yes | No |
| 9 | Male | 60-65 | 1 | 1/1 | No | Yes |
| 10 | Male | 70-75 | 4 | 1/5 | Yes | No |
| 11 | Male | 75-80 | 3 | 1/3 | No | Yes |
| 12 | Male | 75-80 | 4 | 1/5 | No | Yes |
aRefers to the number of screening when atrial fibrillation was first recognized.
bRefers to the total number of positive screenings for a given participant.
Only one participant had persistent atrial fibrillation throughout all of the screenings; 9 screened positive only once. The cumulative prevalence of atrial fibrillation in this population according to increasing number of screenings is presented in Table 3.
Table 3.
Cumulative prevalence of atrial fibrillation by number of screenings.
| Screening number | Cumulative prevalence (95% CI) |
| 1 | 3.0 (1.2-6.1) |
| 2 | 3.0 (1.2-6.1) |
| 3 | 4.3 (2.1-7.7) |
| 4 | 5.1 (2.7-8.7) |
| 5 | 5.1 (2.7-8.7) |
The first screening only identified 7 participants with a positive screen for atrial fibrillation. The remaining 5 participants who screened positive for atrial fibrillation were identified at the fourth screening. A comparison of the 235 participants included in the analyses, with the 120 excluded participants, revealed no meaningful differences between the two groups (see Multimedia Appendix 1 for details).
Discussion
Principal Findings
Our study suggests a prevalence of atrial fibrillation in this Indian region (5.1%) that is markedly higher than has been previously reported in India and similar to the prevalence estimates reported in studies of persons from North America and Europe [1,9,10]. This finding is noteworthy and challenges conventional wisdom that individuals of European descent have higher rates of atrial fibrillation than individuals of Asian descent [1].
Current understanding of the global epidemiology of atrial fibrillation is dependent on robust surveillance systems and high quality community-based studies, but there remains a paucity of such investigations outside of North America and Europe, particularly in countries with less developed health systems [10]. A 2012 meta-analysis of community-based screening studies identified only one study from India [10]. That study was conducted in a tribal Himalayan village and found only one case of atrial fibrillation among 984 screened individuals, a prevalence rate of 0.1% [9]. However, 94% of participants in that study were less than 65 years old and thus not representative of the age profile of typical atrial fibrillation patients. A recently published opportunistic screening study of festival attendees reported a slightly higher but still low prevalence of atrial fibrillation (0.5%) among individuals 50 years of age or older [11]. Reasons for the discrepancies between our results and prior studies may include the shortcomings of opportunistic screening efforts involving younger individuals and the use of a single spot-check for atrial fibrillation. Our approach, in contrast to the two prior studies in India, utilized a randomized home-based serial screening of participants aged 50 years and older in order to detect paroxysmal and persistent atrial fibrillation. The higher yield from multiple rhythm checks versus a single check for the detection of paroxysmal atrial fibrillation in the community has been emphasized by other studies [12] and is made evident by our findings. Namely, we observed that out of the 12 participants who screened positive for atrial fibrillation, only one had persistent atrial fibrillation. Moreover, 5 participants who were ultimately found to have paroxysmal atrial fibrillation did not have atrial fibrillation detected during their first screen.
Recently, there has been increased attention in North America and Europe to leverage mobile technology for the screening of persons with undetected atrial fibrillation [12,13]. The establishment of the National Programme for Prevention and Control of Stroke by the Indian government supports the importance of stroke prevention in India. However, due to the cost of ECG-based screening programs and paucity of trained health professionals in many regions, atrial fibrillation screening has not been possible to date. Our efforts suggest that by engaging community health workers to use novel mobile technologies for arrhythmia monitoring we can screen large numbers of Indians for atrial fibrillation. Our capacity to recruit and serially screen residents of the rural Anand community was strengthened by a long-standing relationship between investigators and community health workers in India.
Limitations
The findings of our study need to be interpreted with appropriate caution given several concerns and limitations. First, this study is based on a relatively small sample size of 235 participants. Therefore, we have presented information about sample sizes and accompanying 95% confidence intervals to demonstrate the range of possible prevalence estimates consistent with the variability observed in our data. Second, we did not perform a gold standard 12-lead ECG to confirm our positive screening findings. It is important to note, however, that Alivecor devices are FDA-approved and are widely used by cardiologists in diverse clinical settings [14]. Lastly, our cross-sectional study design limits our ability to assess any potential outcomes associated with atrial fibrillation or characterize the clinical presentation of atrial fibrillation in more detail. Therefore, future efforts should explore the feasibility and costs associated with replicating our approach in other environments to define the accuracy of the automated algorithms employed in larger and more diverse cohorts, to create referral mechanisms which can accommodate newly identified patients, to more systematically characterize the clinical presentation of atrial fibrillation (eg, valvular diseases, comorbidities, psychosocial impact), and to demonstrate reduced stroke rates through the primary prevention of stroke in screened populations.
Conclusions
In conclusion, our study has two important implications: (1) mobile technologies may help overcome resource limitations for screening adults for atrial fibrillation in underserved and low-resource settings and (2) serial screening for atrial fibrillation enhances the ability to identify persons at risk for atrial fibrillation.
Acknowledgments
We thank Ms Nada Esa, Ms. Dharti Patel, Ms Ami Brahmbhatt, and Mr Utsav Patel for supporting data collection efforts. We are grateful to Dr. Jay Himmelstein and the Office of Health Policy and Technology at the University of Massachusetts Medical School for supporting our research.
AS received support from the National Center for Advancing Translational Sciences (TL1-TR001454), and JA received support from the National Institute on Minority Health and Health Disparities (P60-MD006912-05). DDM’s time was supported by KL2RR031981, 1R15HL121761-01A1, 1UH2TR000921-02, and 1R01HL126911-01A1 from the National Heart, Lung, and Blood Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ECG
electrocardiogram
- FDA
US Food and Drug Administration
Multimedia Appendix 1
Comparison of sociodemographic, lifestyle, and health characteristics of participants that were excluded versus included in the study.
Footnotes
Conflicts of Interest: DDM discloses equity stakes or consulting relationships with Flexcon, Bristol-Myers Squibb, Mobile Sense, ATRIA, and Boston Biomedical Associates. He has also received research funding from Sanofi Aventis, Otsuka Pharmaceuticals, Philips Healthcare, Biotronik, and Pfizer.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y, McAnulty JH, Zheng Z, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014 Feb 25;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=24345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gourie-Devi M. Epidemiology of neurological disorders in India: review of background, prevalence and incidence of epilepsy, stroke, Parkinson's disease and tremors. Neurol India. 2014;62(6):588–598. doi: 10.4103/0028-3886.149365. http://www.neurologyindia.com/article.asp?issn=0028-3886;year=2014;volume=62;issue=6;spage=588;epage=598;aulast=Gourie%2DDevi;type=2. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan 10;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. http://europepmc.org/abstract/MED/25530442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deore R, Vora A. Epidemiology and risk factor for atrial fibrillation in India. J Prev Cardiol. 2014;3(3):507. [Google Scholar]
- 5.Binnendijk E, Koren R, Dror DM. Can the rural poor in India afford to treat non-communicable diseases? Trop Med Int Health. 2012 Nov;17(11):1376–1385. doi: 10.1111/j.1365-3156.2012.03070.x. http://dx.doi.org/10.1111/j.1365-3156.2012.03070.x. [DOI] [PubMed] [Google Scholar]
- 6.Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013 Apr 30;165(1):193–194. doi: 10.1016/j.ijcard.2013.01.220. [DOI] [PubMed] [Google Scholar]
- 7.McManus D, Chong JW, Soni A, Saczynski JS, Esa N, Napolitano C, Darling CE, Boyer E, Rosen RK, Floyd KC, Chon KH. PULSE-SMART: pulse-based arrhythmia discrimination using a novel smartphone application. J Cardiovasc Electrophysiol. 2016 Jan;27(1):51–57. doi: 10.1111/jce.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soni A, Earon A, Handorf A, Fahey N, Allison J, Chon K. Using mobile technologies to detect arrhythmias in rural India. National Institutes of Health Electr Electron Eng Point Care Technol Healthc; 2015; Bethesda, MD. 2015. [Google Scholar]
- 9.Kaushal SS, DasGupta DJ, Prashar BS, Bhardwaj AK. Electrocardiographic manifestations of healthy residents of a tribal Himalayan village. J Assoc Physicians India. 1995 Jan;43(1):15–16. [PubMed] [Google Scholar]
- 10.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012 Dec;142(6):1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 11.Saggu D, Sundar G, Nair S, Bhargava V, Lalukota K, Chennapragada S. Prevalence of atrial fibrillation in an urban population in India: the Nagpur pilot study. Heart Asia. 2016;8:56–59. doi: 10.1136/heartasia-2015-010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: The STROKESTOP study. Circulation. 2015 Jun 23;131(25):2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=25910800. [DOI] [PubMed] [Google Scholar]
- 13.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation: a systematic review. Thromb Haemost. 2013 Aug;110(2):213–222. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen HH, Silva JNA. Use of smartphone technology in cardiology. Trends Cardiovasc Med. 2016;26:376–386. doi: 10.1016/j.tcm.2015.11.002. [DOI] [PubMed] [Google Scholar]