Abstract
Objective
Regionalization may improve critical care delivery, yet stakeholders cite concerns about its feasibility. We sought to determine the operational effects of prehospital regionalization of non-trauma, non-arrest critical illness.
Design
Discrete event simulation study
Patients and setting
All 2006 hospital discharge data from King County, Washington, linked to all adult, eligible patients transported by county EMS agencies.
Methods
We simulated active triage of high-risk patients to designated referral centers using a validated prehospital risk score; we studied three regionalization scenarios: (1) up triage, (2) up & down triage, (3) up & down triage after reducing intensive care unit (ICU) beds by 25%. We determined the effect on patient routing, ICU occupancy at referral and non-referral hospitals, and EMS transport times.
Measurements and Main Results
119,117 patients were hospitalized at 11 non-referral centers and 76,817 patients were hospitalized at three referral centers. Among 20,835 EMS patients, 7,817 (43%) patients were eligible for up triage and 10,242 (57%) patients were eligible for down triage. At baseline mean daily ICU bed occupancy was 61% referral and 47% at non-referral hospitals. Up-triage increased referral ICU occupancy to 68%, up and down triage to 64%, and up and down triage with bed reduction to 74%. Mean daily non-referral ICU occupancy did not exceed 60%. Total EMS transport time increased by less than 3% with up and down triage.
Conclusions
Regionalization based on prehospital triage of the critically ill can allocate high-risk patients to referral hospitals without adversely affecting ICU occupancy or prehospital travel time.
Keywords: regionalization, emergency medical services, intensive care, census, prehospital, occupancy
Under a regionalized system of critical care, selected high-risk patients would be systematically triaged and transferred to specialized referral hospitals capable of caring for a broad range of critically ill patients. As outlined by the Institute of Medicine report and the major United States (US) critical care professional societies, the implementation of regionalized critical care may better match patient needs with resources.(1, 2) These stakeholders cite volume-outcome relationships in the intensive care unit (ICU), economies of scale, and variability in critical care across hospitals as rationales for regionalization.(3-8)
Despite regionalization's potential benefits, key gaps remain in our understanding of how it will impact the delivery of critical care in emergency departments, hospitals, and emergency medical services (EMS) agencies. The implementation of regionalized critical care has the potential to substantially disrupt EMS transport times, EMS provider availability for other calls, hospital census, lengths of stay, and frequency of ICU-related procedures at both referring and referral hospitals. Indeed, physicians cite the potential to overwhelm large hospitals and decrease clinical experience at small hospitals as critical barriers to regionalization.(5) These key barriers to feasibility require investigation prior to widespread implementation of a regionalized system of care.
Our goal was to estimate the effect of tiered prehospital regionalization of the critically ill on patients, hospitals, and EMS. We used discrete-event simulation in a county-level cohort of all hospitalizations linked to EMS transports. We chose county-level EMS transports as our starting point since future implementation of regionalization may occur within these policy relevant regions by EMS personnel.(9, 10) Discrete event simulation is an in silico method of estimating the effects of system-level health care changes, particularly when demonstration projects and clinical trials are infeasible or impractical.(11) By examining a variety of different possible regionalization scenarios, we sought to provide policymakers and clinicians with objective data on routing of patients, hospital census, procedural volume at large and small hospitals, and EMS travel times.
Methods
Model overview
We created a discrete-event simulation model to understand the potential impact of regionalization on critical care delivery. Discrete event simulation is a valuable modeling strategy because, unlike Markov or state transition models, it allows patients to interact and can predict system-level effects resulting from individual-level interactions. We used discrete event simulation to model the experience of adult, non-injured, non-cardiac arrest patients seen by King County Emergency Medical Services (EMS) ground ambulance within the context of all hospitalizations in King County, Washington, both in the observed system and under several regionalization scenarios. We did not explicitly model the regionalization of trauma or cardiac arrest patients as these systems are already exist or are under study in this region.(12, 13) We built the simulation model using Simio (Simio LLC, Sewickley, Pennsylvania).
Data sources
We used a cohort of all acute care hospitalizations in King County, Washington in 2006.(14) First, we linked Washington state hospital discharge records in the Comprehensive Hospital Abstract Reporting System (CHARS) database to patient-level data from the King County EMS administrative database.(14) King County EMS is the primary first response for all medical 9-1-1 calls. King County employs a two-tier system. Emergency medical technicians and fire fighters trained in basic life support provide the first tier, responding to all medical calls. Paramedics trained in advanced life support provide the second tier, responding to selected patients who are more severely ill, based on assessments by both emergency medical dispatchers and first responders. Incident EMS calls were linked at the patient level to 2006 geographic and population data in the US Census, and at the hospital level to the 2006 American Hospital Association Annual Survey (AHA). We used the AHA to define the number of available intensive care unit and ward beds at each hospital.
Model inputs
The discrete event simulation model consisted of both patient-level and hospital-level inputs. For EMS patients, patient-level inputs were prehospital scene arrival date and time, estimated travel time from scene to hospital in minutes, date of hospital admission if applicable, a prehospital critical illness risk score, the identity of the receiving hospital, ward admission (yes/no), ICU admission (yes/no), use of mechanical ventilation during the hospitalization (yes/no), use of hemodialysis during the hospitalization (yes/no), length of stay in ICU and/or ward in days, and hospital mortality (yes/no). For patients admitted to the hospital through means other than EMS, patient-level inputs included all of the above variables except the prehospital risk score and travel time estimates.
We calculated the prehospital risk by using a validated model incorporating age and acute physiology. Specifically, this risk tool use age, gender, and prehospital heart rate, respiratory rate, systolic blood pressure, Glasgow Coma Scale score in categorized variables to populate a score ranging from 0 to 8.(14) First developed in King County EMS, the prehospital risk score is further tested in Southwestern Pennsylvania EMS agencies.(15) We calculated travel time by geolocating each EMS incident and estimating transport intervals using Google Maps and ArcGIS to route paths to destination hospitals.(16) These methods are robust to changes in travel time estimates corresponding to traffic/rush hour.(16) We defined ICU admission using ICU-specific revenue codes.(17) We defined mechanical ventilation and hemodialysis using validated International Classification of Diseases, version 9—clinical modification procedure codes.(18)
Hospital-level inputs included geographic coordinates (geocoded by street address), number of ward beds, number of ICU beds, and referral status (either “referral”, i.e., would act as a destination for high-risk patients under a regionalized system; or “non-referral”, i.e., would not act as a destination for high-risk patients). We categorized hospitals as referral and non-referral by examining a hospital-level plot of ICU and ward beds, selecting hospitals in the greatest quartile of intensive care and ward beds as referral, and designating the remaining hospitals as non-referral.
Model outputs, calibration, and validation
Based on these inputs, the model produced outputs for all patients in the system, including destination hospital, length of stay and procedural requirements. The model also produced estimated outputs for EMS agencies, including total travel time in minutes during the year. After developing the model we calibrated it against observed data by running it for 365 days (January 1, 2006 to December 31, 2006). During calibration, we modified the model to include a one-month warm-up phase prior to January 1, 2006 so that the model outputs would best approximate the observed data on January 1, 2006. We then verified the model using key model outputs. Specifically, we validated the model against true allocation of patients in observed data, mean length of stay, mortality, ward, and intensive care use across hospitals.
Regionalization scenarios
To evaluate the health system impacts of regionalization we created several rules for prehospital triage. These rules were based on: (1) the prehospital critical illness risk score, (2) the estimated travel time to each hospital in the system, and (3) each hospital's census at the time of triage (Figure 1). Patients with a critical illness risk score above a specified threshold were triaged to the nearest referral hospital with an available bed (i.e., “up triage”), and patients with a critical illness score below a certain threshold were triaged to the nearest non-referral hospital with an available bed (i.e. “down-triage”). If the nearest hospital was full, the patient was sent to the next nearest hospital.
Figure 1.
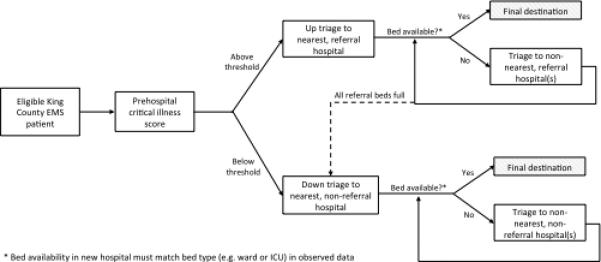
Prehospital triage algorithm in simulated regionalization scenarios.
Once admitted to a hospital, we assumed that patients’ treatment, ICU admission status, and outcomes were similar to their baseline hospital. For example, if a patient was admitted to a ward bed at baseline, the patient was assigned a ward bed at the new hospital. Similarly, observed length of stay, modeled length of stay, mortality, and procedural utilization were not influenced by simulations. We made these assumptions to focus our model on operations and throughput, rather than outcomes, since assessments of the impact on patient outcomes is a secondary concern if the strategy is not feasible. We assumed that the non-EMS patients were admitted to their original hospitals, and we did not limit admissions of non-EMS patients if bed occupancy was high. They still were assigned to their baseline hospital. We did not include interhospital transfers in the simulation, as our goal was to test only tiered regionalization via EMS.
We examined three regionalization scenarios. First, we simulated the up-triage of EMS patients with prehospital risk score ≥ 2 to the nearest referral center without the down-triage of any other EMS patients (“up-triage only”). Second, we simulated both up-triage of EMS patients with risk score ≥ 2 to the nearest referral center and down-triage of patients with score 0 or 1 to the nearest non-referral center (“up and down triage”). Third, we simulated up and down-triage after reducing the number of ICU beds at each hospital by 25%, considering that King County, Washington has ICU bed availability (37.5 per 100,000 capita) that exceeds most US regions which average 20 to 25 ICU beds per 100,000 capita (“up and down triage with ICU bed reduction”).(19)
In a sensitivity analysis, we randomly selected 5% of EMS transports to “decline” up or down triage, so as to model variable patient preferences towards changing destinations and travel times. These patients were assigned to their baseline hospital in all scenarios, irrespective of the prehospital risk score. All regionalization scenarios were tested in this sensitivity analysis.
Analysis
We describe patient and hospital characteristics of referral and non-referral hospitals using mean and standard deviation (SD) for continuous data, median and interquartile range for non-normal data, and proportions for categorical data. We tested differences between referral hospitals and non-referral hospitals using Fisher's exact test and Wilcoxon rank sum-test, as appropriate.
We analyzed the model outputs at baseline and under each regionalization scenario along five domains: patient destinations, hospital admissions and occupancy, resource utilization, procedural volume, and EMS travel time. To analyze hospital destinations, we calculated the number of EMS patients transported to referral and non-referral centers. To analyze hospital admissions and occupancy, we estimated total admissions, ward-only admissions (i.e., the patient was admitted to the ward but not the ICU), ICU admissions, and the daily proportion of occupied beds. We also calculated the days at each hospital when the ward or ICU was over 90% capacity – an extreme threshold above the typical observed range of ICU occupancy.(20) To analyze resource utilization, we calculated the mean ICU and length of stay for referral and non-referral hospitals. To analyze procedural volume, we calculated the observed cases of mechanical ventilation and hemodialysis at each hospital. We chose mechanical ventilation and hemodialysis to illustrate advanced procedures for which a minimum number may be required for clinical competence. Finally, we calculated total EMS travel time (min), the proportion of patients who increased and decreased travel time, and the magnitude of change (min). We also illustrated how regionalization scenarios modified EMS catchment areas by calculating the geographic perimeter around EMS transports for each referral and non-referral hospital. This geographic area corresponds to the furthest distance EMS transported patients for those hospital(s), and is summarized as the area in square miles.
We compared simulation model outputs across scenarios using mean and standard deviation (SD) for normally distributed continuous data, median and interquartile range for non-normal continuous data, and proportions for categorical data. Given the large sample sizes, we evaluated clinical significance of model outputs and trends rather than statistical significance with p values.
Simulation outputs were analyzed using STATA 11.0 SE (Stata Corp, College Station, Texas) and ArcGIS (Redlands, California). The project was approved by the Washington State Department of Health and King County Public Health Review Committees.
Results
Hospitals and patients
We studied a region of 1.9 million people that included 119,117 patients hospitalized at 11 non-referral hospitals and 76,817 hospitalizations at three referral hospitals over the study period (Table 1). At baseline, adult, non-trauma, non-arrest ground EMS patients accounted for 10.4% (N=20,395) of hospitalizations. Of these, 27% (n=5,571) of EMS patients were taken to referral hospitals and 73% (n=14,824) were taken to non-referral hospitals. Adult, non-trauma, non-arrest ground EMS patients comprised 20% of all ICU hospitalizations (6,376 of 30,779). Subjects at referral hospitals were more likely to be male, receive mechanical ventilation, and intensive care. Hospital length of stay, ICU length of stay, and hospital mortality were similar between referral and non-referral hospitals.
Table 1.
Hospital and patient characteristics at baseline. Values are N (%) unless otherwise indicated.
| Variable | Non-referral hospitals | Referral hospitals | P-value |
|---|---|---|---|
| Hospital characteristic, No. | 11 | 3 | |
| Total beds | |||
| <200 | 6 (55) | 1 (33) | 0.06 |
| 200 to 400 | 5 (46) | 2 (66) | |
| > 400 | 0 (0) | 0 (0) | |
| Total ICU beds | |||
| <10 | 2 (18) | 0 (0) | 0.35 |
| 10 to 20 | 3 (27) | 0 (0) | |
| >20 | 6 (55) | 3 (100) | |
| Total admissions, median [IQR] | 9,362 [7471 – 14437] | 18,375 [18,806 – 34,791] | 0.01 |
| Total inpatient days, median [IQR] | 42,161 [25,562-49,168] | 125,189 [118,455 – 155,386] | 0.01 |
| Total operations, median [IQR] | 8,295 [5,327 – 11,445] | 14,692 [12,131-26,962] | 0.06 |
| Resident FTEs, median [IQR] | 4 [0 – 33] | 225 [51 – 433] | 0.02 |
| Critical access hospital | 2 (18) | 0 (0) | 1.0 |
| Patient characteristic, No. | 119,117 | 76,817 | |
| Age, median [IQR], years | 52 [28 – 72] | 44 [27 – 62] | <0.01 |
| Male gender | 46,435 (39) | 33,524 (44) | <0.01 |
| Received eligible prehospital care* | 14,824 (12.4) | 5,571 (7.3) | <0.01 |
| Mechanical ventilation | 2,517 (2.1) | 3,108 (4.1) | <0.01 |
| Hemodialysis | 1,710 (1.4) | 1,000 (1.3) | 0.01 |
| Intensive care | 16,850 (14) | 13,929 (18) | <0.01 |
| Hospital length of stay, median [IQR], days | 2 [1 – 4] | 3 [1 - 5] | <0.01 |
| ICU length of stay, median [IQR], days | 2 [1 - 3] | 2 [1 - 4] | <0.01 |
| In-hospital mortality | 2,229 (1.9) | 1,693 (2.2) | <0.01 |
| In-hospital mortality among ICU patients | 1,235 (7.3) | 1,256 (9.0) | <0.01 |
Abbreviations: FTE = full time equivalent, ICU = intensive care unit , QR = interquartile range
Includes only prehospital transports of age>=18 years, non-cardiac arrest, non-trauma, in whom three or more vital signs were documented.
Patient destinations
Patient destinations at baseline and after each regionalization scenario are shown in Table 2. At baseline, of the 7,817 EMS patients with a prehospital risk score ≥ 2, 29% (N=2,271) were taken to a referral hospital (13% (N=1,041) to the nearest and 16% (N=1,230) to the non-nearest). 71% (N=5,546) were taken to a non-referral hospital (48% (N=3,738) to the nearest and 23% (N=1,808) to the non-nearest). Of the 10,242 EMS patients with risk score <2, 30% (N=3,167) were taken to a referral hospital (13% to the nearest and 17% to the non-nearest) and 70% (N=7,705) were taken to a non-referral hospital (46% to the nearest and 23% to the non-nearest).
Table 2.
Destinations for EMS patients, stratified by prehospital critical illness risk score. Data are presented for both the baseline system and the three regionalization scenarios. Dashes represent destinations that were either not possible (in the baseline data) or not allowed (in the simulation); zeros represent destinations that were allowed but did not occur. Values are N (%).
| Prehospital critical illness score | Destination | Baseline | Regionalization scenario |
||
|---|---|---|---|---|---|
| Up-triage only | Up and down triage | Up and down triage with bed reduction | |||
| Score ≥ 2 (N=7,817) | Referral hospital (any) | 2,271 (29) | 7,817 (100) | 7,817 (100) | 7,817 (100) |
| Non-referral (any) | 5,546 (71) | 0 (0) | 0 (0) | 0 (0) | |
| Referral hospital by type | |||||
| Nearest, same as observed | 1,041 (13) | 909 (12) | 920 (12) | 781 (10) | |
| Nearest, different than observed | -- | 6,741 (86) | 6,785 (87) | 6,115 (78) | |
| Non-nearest, same as observed | 1,230 (16) | 27 (<1) | 22 (<1) | 151 (2) | |
| Non-nearest, different than obs. | -- | 140 (2) | 90 (1) | 770 (10) | |
| Non-referral hospital, by type | |||||
| Nearest, same as observed | 3,738 (43) | 0 (0) | 0 (0) | 0 (0) | |
| Nearest, different as observed | -- | 0 (0) | 0 (0) | 0 (0) | |
| Non-nearest, same as observed | 1,808 (23) | 0 (0) | 0 (0) | 0 (0) | |
| Non-nearest, different than obs. | -- | 0 (0) | 0 (0) | 0 (0) | |
| Score < 2 (N=10.242) | Referral hospital (any) | 3,167 (30) | 3,167 (30) | 0 (0) | 0 (0) |
| Non-referral (any) | 7,075 (70) | 7,075 (70) | 10,242 (100) | 10,242 (100) | |
| Referral hospital by type | |||||
| Nearest, same as observed | 1,381 (13) | 1,381 (13) | 0 (0) | 0 (0) | |
| Nearest, different than observed | -- | -- | 0 (0) | 0 (0) | |
| Non-nearest, same as observed | 1,786 (17) | 1,786 (17) | 0 (0) | 0 (0) | |
| Non-nearest, different than obs. | -- | -- | 0 (0) | 0 (0) | |
| Non-referral hospital, by type | |||||
| Nearest, same as observed | 4,728 (46) | 4,728 (46) | 4,548 (44) | 4,466 (44) | |
| Nearest, different as observed | -- | -- | 5,646 (55) | 5,504 (54) | |
| Non-nearest, same as observed | 2,347 (23) | 2,347 (23) | 21 (<1) | 40 (<1) | |
| Non-nearest, different than obs. | -- | -- | 27 (<1) | 232 (2) | |
In the up-triage only scenario, all high-risk patients were triaged to a referral hospital. Of these, 12% remained at the same referral hospital, 16% changed referral hospital because of proximity, and 66% were newly triaged to a referral hospital. In the up and down triage scenario, all high-risk patients were successfully triaged to a referral hospital in roughly the same distribution. Among low risk patients, 44% were taken to their original non-referral hospital and 55% were taken to a new, non-referral hospital. The results were similar in the up and down triage with bed reduction scenario.
Hospital admissions and occupancy
Hospital admissions and occupancy at baseline and after regionalization are shown in Figure 2. At baseline, mean ICU bed occupancy was 61% at referral hospitals and 47% at non-referral hospitals. Referral hospital ICU occupancy increased to 68% after up-triage, 64% with up and down triage, and 74% under up and down triage with bed reduction. Non-referral hospital ICU occupancy decreased to 41% after up-triage, 45% with up and down triage, and increased to 60% under up and down triage with bed reduction. The percentage of referral ICU days at over 90% capacity was less than 40% in all scenarios. Daily ICU census displayed substantial seasonal and weekday/weekend variability, with most time over 90% capacity on weekdays during the winter months (see Figure E1 in the Supplementary Digital Content).
Figure 2.
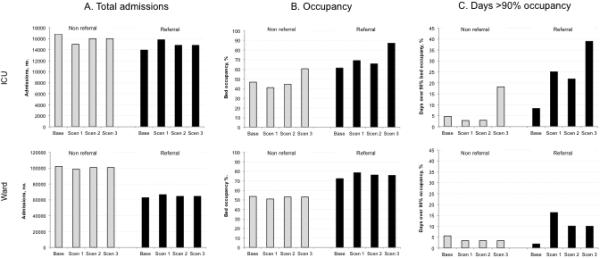
Outcomes for each of four simulation scenarios, including total admissions (Fig 2A), mean daily bed occupancy (Fig 2B), and proportion of days over 90% bed occupied (Fig 2C). Grey bars represent non-referral hospitals, black bars represent referral hospitals. ICU data shown in top row and Ward data shown in lower row.
Hospital resource utilization
At baseline, the mean length of stay at referral ICUs was 4.3 days (SD 7.2 days) and non-referral ICUs was 3.1 (SD 4.2 days). ICU length of stay at referral hospitals was unchanged after up-triage (4.2 days, SD 7.0 days), and remained the same for up and down triage or with ICU bed reduction. Non-referral hospital ICU length of stay was also unchanged with regionalization scenarios.
Hospital procedural volume
At total of 5,625 patients underwent mechanical ventilation and 2,710 received hemodialysis during the study period. The up triage scenario moved 12% (N=697) ventilated patients and 7.2% (N=196) dialyzed patients away from non-referral to referral hospitals. Up-and-down triage moved 10% (N=575) ventilated patients and 3.7% (N=101) dialyzed patients from non-referral to referral hospitals.
EMS travel times
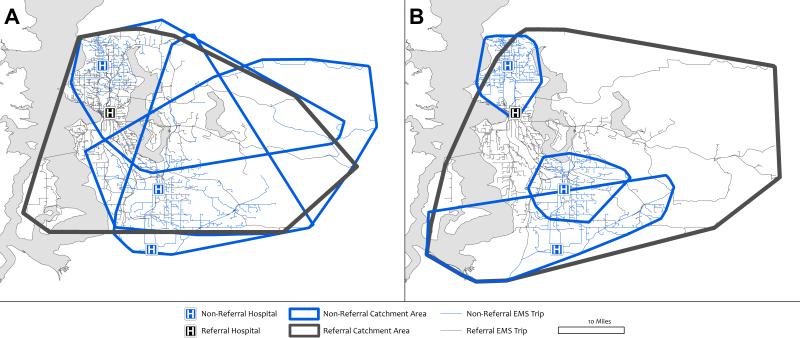
Total EMS travel times increased by 13% with up triage, 2.0% with up and down triage, and 2.9% with up and down triage with bed reduction (Table 3). The latter corresponds to an average change of 19 minutes of EMS travel time per day for the entire County. The absolute changes (either increase or decrease) in EMS travel times were smaller than 12 minutes in the majority of transports. Regionalization concentrated EMS travel distances for non-referral and referral hospitals across the county (Figure 3). Compared to an average EMS catchment of 831 squares miles at baseline, the average EMS catchment per non-referral hospital decreased by 441 sq. miles with up and down triage, while referral hospital catchments would decrease on average by 205 sq. miles.
Table 3.
EMS travel time under different regionalization scenarios.
| Transport characteristic | Up-triage only | Up and down triage | Up and down triage with bed reduction |
|---|---|---|---|
| Total travel time, min. | 271,582 | 245,137 | 247,134 |
| Percentage increase in total travel time, %* | 13.0 | 2.0 | 2.9 |
| Different hospital destination from observed, no. (%) | 6,888 (38) | 12,555 (70) | 12,628 (70) |
| Direction of time change, no. (%)^ | |||
| Increase | 4,137 (60) | 4,918 (39) | 5,175 (41) |
| Decrease | 2,512 (36) | 6,871 (55) | 6,663 (53) |
| No change | 239 (3) | 766 (6) | 790 (6) |
| Magnitude of time increase, % of patientsa | |||
| < 6 minutes | 1,034 (25.0) | 1,753 (35.6) | 1,900 (36.7) |
| 6 to 12 minutes | 1,987 (48.0) | 2,049 (41.7) | 2,120 (41.0) |
| > 12 minutes | 1,116 (27.0) | 1,116 (22.7) | 1,155 (22.3) |
| Magnitude of time decrease, % of patientsb | |||
| < 6 minutes | 2,217 (88.3) | 4,571 (66.5) | 4,390 (65.9) |
| 6 to 12 minutes | 278 (11.1) | 1,750 (25.5) | 1,724 (25.9) |
| > 12 minutes | 17 (0.7) | 550 (8.0) | 549 (8.2) |
Compared to baseline total travel time of 240,278 minutes
Only among those who changed destination from observed data
Only among those with increased travel time
Only among those with decreased travel time
Figure 3.
Examples of EMS catchment areas for one referral (blue) and three non-referral hospitals (black). Panel A shows the baseline data, Panel B shows up and down triage with bed reduction. Catchment areas derived from the EMS transport networks of patients traveling from scene to the hospitals, respectively.
Sensitivity analysis
When a proportion of EMS patients (5%) were randomly selected to decline up or down triage in regionalization scenarios, we observed no relevant changes in output. In particular, the proportion of ICU days at >90% occupancy in referral and non-referral hospitals behaved similar to our primary analysis (Figure e2).
Discussion
Compared to current practice, prehospital regionalization of the critically ill at the county level would reallocate a large proportion of high-risk patients from non-referral to referral hospitals with little change in hospital census in the ICU or ward, procedural volume at smaller hospitals, or EMS transport times. The down triage of lower risk patients to non-referral hospitals also had little impact on daily hospital census in the ICU or ward.
These results provide conceptual support for the regionalization of critical care, suggesting that re-routing of non-arrest, non-trauma critically ill patients is feasible for EMS agencies and hospitals. Except under the most conservative scenario in which we simultaneously altered triage patterns and reduced the number of ICU beds, regionalized care did not lead to substantial hospital strain. Although data conflict,(21) some evidence exists that ICU capacity constraint can increase mortality in critical illness.(22-24) There is a perception among many intensivists that there is little room to increase their patient load.(25) Our results should reassure stakeholders that, under most scenarios, referral hospitals could accept 20% more ICU admissions via EMS transport. Current ICU bed supply and daily occupancy in this County would not be a barrier to the planning of implementation studies and demonstration projects.
We also found that regionalization had only a minor impact on procedural volume at small hospitals. Stakeholders cite the potential reduction in procedures requiring technical skill as a potential barrier to regionalization,(5, 26) expressing concerns that regionalization might actually worsen outcomes at small hospitals, offsetting other gains. However, we show that procedural volume changes at small hospitals may not be as large as anticipated. Previous reports of volume-outcome relationships in mechanical ventilation suggest that such small changes in caseload may not impact the odds of death.(4, 27)
Regionalization of critically ill patients also requires participation by EMS agencies to transport higher risk patients to referral centers. These personnel already participate in triage of trauma patients, which may change prehospital intervals.(28) We show that beyond current practice, re-routing of higher risk, non-trauma patients has minimal impact on average, daily EMS transport times across the County, and may even decrease total EMS transport time. In fact, fewer than one in twenty EMS transports increased time by more than 12 minutes. Although studies suggest that prehospital transport intervals are not associated with outcome in trauma or cardiac arrest,(29, 30) it is unknown how modest changes to prehospital transport intervals could independently impact outcomes of the non-trauma critically ill - or whether increases in transport time are specific to certain triage paths (e.g. non-nearest non-referral hospital to nearest non-referral hospital, etc). Similarly, we do not yet know how patient preferences for referral center treatment in critical illness will balance against these potential trade-offs.
This study also demonstrates that additional ICU beds are not required as the critical care system evolves, at least in the US. The overall number of US ICU beds and the ratio of ICU beds to hospital beds are increasing,(8) yet most ICUs do not face external bed pressure.(20) Such was the case in our simulation, where daily ICU occupancy was often less than 60%. Even more, our ICU bed count was conservative, and did not include “flexible” ICU beds in the operating room, intermediate care, emergency department, or post-acute care units. Such data further supports calls to limit the addition of ICU beds to an already inefficient system.(31, 32)
This study also demonstrates how discrete event simulation can be a valuable tool for health system planning. Many policy changes for the critically ill will span across health systems, payers, regions, and patients – whereby demonstration projects or randomized clinical trials are infeasible or too costly. By leveraging real patient data, discrete event simulation models can predict the impact of various system-level changes – providing key preliminary data for stakeholders.
Our study has several limitations. We modeled single geographic region, which may not generalize to more urban or rural areas, with data from 2006. Yet, King County is one of the 20 most populous counties in the US and is a policy relevant region where regionalization strategies could be implemented.(9, 33) King County does have more ICU beds per capital than most other counties in the US, and our results may underestimate the impact in regions with fewer ICU beds per capita.(34) We did not have data on the emergency room census, availability, throughput, or the presence of intermediate care beds. These data and real-time measures of “divert” status would add greater fidelity to our models – particularly for quantifying overflow beds in times of bed pressure. Our definition of referral centers was empiric and informed by prior studies on volume-outcome relationships in critical care.(27) They may not, in fact, be the highest-performing critical care hospitals. At present there are few agreed-upon metrics for defining critical care referral centers using organizational characteristics, and case-mix adjustment is not yet advanced enough to allow valid identification of critical care centers of excellence. We also defined ICU bed strain using bed occupancy, and acknowledge that other metrics based upon case mix severity, patient flow, clinical staffing, fixed resource availability (e.g. mechanical ventilators) or queuing theory may return different conclusions.(35) We also built a regionalization model that did not alter the destinations of ICU patients who are already regionalized (e.g. trauma) or who do not use prehospital care (e.g. interhospital transfers, direct admissions). The inclusion of EMS data from interhospital transfers, which occur commonly by private ambulances, could add to the scope of the critical care system impacted by regionalization. Finally, we acknowledge that this study focused on the feasibility of regionalization scenarios, and did not address patient-centered outcomes for these system-level changes. By incorporating detailed case-mix adjustment, measures of illness severity derived from electronic health records, and ICU structural and organizational characteristics, future models could more accurately simulate the relationship between system changes and outcome.
Conclusions
This simulation study provides important new insight suggesting that selective prehospital triage can feasibly allocate non-trauma, critically ill patients to referral hospitals without compromising ICU census, procedural volume at smaller hospitals, or EMS travel times. Future work should explore these findings through more complex models that incorporate larger regions and the entire trajectory of critical illness.
Supplementary Material
At a glance commentary.
Scientific knowledge on the subject
Redesign of the critical care system using tiered regionalization may improve patient outcomes, but stakeholders cite major barriers in feasibility and implementation.
What this study adds to the field
In a discrete event simulation, most high-risk, non-trauma critically ill patients transported by EMS can be triaged to referral hospitals, with little impact on ICU occupancy, procedural volume at non-referral hospitals, and prehospital transport times.
Acknowledgments
Funding sources: This work was supported by a Vision Grant from the Society of Critical Care Medicine. Dr. Seymour is supported in part by a grant from the National Institutes of Health (1K23GM104022-01). Dr. Chhatwal is supported in part by a grant from the National Institutes of Health (KL2TR000146). Dr. Wallace is supported in part by a grant from the National Institutes of Health (K12-HL109068).
Footnotes
Copyright form disclosures:
Dr. Seymour received support for article research from the National Institutes of Health (NIH) and consulted for Beckman Coulter. His institution received grant support from the Society of Critical Care Medicine and the NIH. Dr. Wallace received support for article research from the NIH. His institution received grant support from the NHLBI-K12-HL109068. Dr. Chhatwal's institution received grant support from the NIH. Dr. Kahn disclosed non-financial research support from the Cerner Corporation (Kansas City, MO). His institution received grant support from the US National Institutes of Health and the US Health Resources and Services Administration. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This article has an online data supplement, which is accessible from this issue's table of content online at www.atsjournals.org.
References
- 1.Barnato AE, Kahn JM, Rubenfeld GD, et al. Prioritizing the organization and management of intensive care services in the United States: the PrOMIS Conference. Crit Care Med. 2007;35(4):1003–1011. doi: 10.1097/01.CCM.0000259535.06205.B4. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine . Emergency Medical Services at the Crossroads. The National Academies Press; Washington, D.C.: 2007. [Google Scholar]
- 3.Kahn JM. Volume, outcome, and the organization of intensive care. Crit Care. 2007;11(3):129. doi: 10.1186/cc5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn JM, Goss CH, Heagerty PJ, et al. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355(1):41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JM, Asch RJ, Iwashyna TJ, et al. Physician attitudes toward regionalization of adult critical care: A national survey. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181a009d0. [DOI] [PubMed] [Google Scholar]
- 6.Kahn JM, Branas CC, Schwab CW, et al. Regionalization of medical critical care: what can we learn from the trauma experience? Crit Care Med. 2008;36(11):3085–3088. doi: 10.1097/CCM.0b013e31818c37b2. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181(11):1164–1169. doi: 10.1164/rccm.200909-1441CP. [DOI] [PubMed] [Google Scholar]
- 8.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: An analysis of bed numbers, occupancy rates, payer mix, and costs*. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 9.Glickman SW, Kit Delgado M, Hirshon JM, et al. Defining and measuring successful emergency care networks: a research agenda. Acad Emerg Med. 2010;17(12):1297–1305. doi: 10.1111/j.1553-2712.2010.00930.x. [DOI] [PubMed] [Google Scholar]
- 10.Cone DC, Brooke Lerner E, Band RA, et al. Prehospital care and new models of regionalization. Acad Emerg Med. 2010;17(12):1337–1345. doi: 10.1111/j.1553-2712.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 11.Karnon J, Stahl J, Brennan A, et al. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Med Decision Making. 2012;32(5):701–711. doi: 10.1177/0272989X12455462. [DOI] [PubMed] [Google Scholar]
- 12.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation. 121(5):709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 13.Gage AM, Traven N, Rivara FP, et al. Compliance with Centers for Disease Control and Prevention field triage guidelines in an established trauma system. J Am Coll Surg. 2012;215(1):148–154. doi: 10.1016/j.jamcollsurg.2012.02.025. discussion 154-146. [DOI] [PubMed] [Google Scholar]
- 14.Seymour CW, Kahn JM, Cooke CR, et al. Prediction of critical illness during out of-hospital emergency care. JAMA. 2010;304(7):747–754. doi: 10.1001/jama.2010.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobias AZ, Guyette FX, Seymour CW, et al. Pre-resuscitation Lactate and Hospital Mortality in Prehospital Patients. Prehosp Emerg Care. 2014 doi: 10.3109/10903127.2013.869645. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace DJ, Kahn JM, Angus DC, et al. Accuracy of Prehospital Transport Time Estimation. Acad Emerg Med. 2014;21(1):9–16. doi: 10.1111/acem.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwashyna TJ. Critical care use during the course of serious illness. Am J Respir Crit Care Med. 2004;170(9):981–986. doi: 10.1164/rccm.200403-260OC. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(10):2787–2793. e2781–2789. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 20.Wunsch H, Wagner J, Herlim M, et al. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41(12):2712–2719. doi: 10.1097/CCM.0b013e318298a139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashyna TJ, Kramer AA, Kahn JM. Intensive care unit occupancy and patient outcomes. Crit Care Med. 2009;37(5):1545–1557. doi: 10.1097/CCM.0b013e31819fe8f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477–1483. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 23.Gabler NB, Ratcliffe SJ, Wagner J, et al. Mortality among patients admitted to strained intensive care units. Am J Resp Crit Care Med. 2013;188(7):800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stelfox HT, Hemmelgarn BR, Bagshaw SM, et al. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Int Med. 2012;172(6):467–474. doi: 10.1001/archinternmed.2011.2315. [DOI] [PubMed] [Google Scholar]
- 25.Ward NS, Read R, Afessa B, et al. Perceived effects of attending physician workload in academic medical intensive care units: a national survey of training program directors. Crit Care Med. 2012;40(2):400–405. doi: 10.1097/CCM.0b013e318232d997. [DOI] [PubMed] [Google Scholar]
- 26.Kahn JM, Asch RJ, Iwashyna TJ, et al. Perceived barriers to the regionalization of adult critical care in the United States: a qualitative preliminary study. BMC Health Serv Res. 2008;8:239. doi: 10.1186/1472-6963-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn JM. Volume, outcome, and the organization of intensive care. Crit Care. 2007;11(3):129. doi: 10.1186/cc5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampalis JS, Denis R, Lavoie A, et al. Trauma care regionalization: a process-outcome evaluation. J Trauma. 1999;46(4):565–579. doi: 10.1097/00005373-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Spaite DW, Stiell IG, Bobrow BJ, et al. Effect of Transport Interval on Out-of-Hospital Cardiac Arrest Survival in the OPALS Study: Implications for Triaging Patients to Specialized Cardiac Arrest Centers. Ann Emerg Med. 2009 doi: 10.1016/j.annemergmed.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Newgard CD, Schmicker RH, Hedges JR, et al. Emergency medical services intervals and survival in trauma: assessment of the “golden hour” in a North American prospective cohort. Ann Emerg Med. 2010;55(3):235–246. e234. doi: 10.1016/j.annemergmed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seymour CW, Kahn JM. Addressing the growth in intensive care: comment on “Intensive care unit admitting patterns in the Veterans Affairs health care system”. Arch Int Med. 2012;172(16):1226. doi: 10.1001/archinternmed.2012.3773. [DOI] [PubMed] [Google Scholar]
- 32.Gooch RA, Kahn JM. ICU bed supply, utilization, and health care spending: an example of demand elasticity. JAMA. 2014;311(6):567–568. doi: 10.1001/jama.2013.283800. [DOI] [PubMed] [Google Scholar]
- 33.Jollis JG, Roettig ML, Aluko AO, et al. Implementation of a statewide system for coronary reperfusion for ST-segment elevation myocardial infarction. JAMA. 2007;298(20):2371–2380. doi: 10.1001/jama.298.20.joc70124. [DOI] [PubMed] [Google Scholar]
- 34.Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371–1372. doi: 10.1001/jama.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opinion Crit Care. 2011;17(6):648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.