Abstract
Objectives:
Dental radiography may involve situations where the patient is known to be pregnant or the pregnancy is noticed after the X-ray procedure. In such cases, the radiation dose to the foetus, though low, needs to be estimated. Uniform and widely used guidance on dental X-ray procedures during pregnancy are presently lacking, the usefulness of lead shields is unclear and practices vary.
Methods:
Upper estimates of radiation doses to the foetus and breasts of the pregnant patient were estimated with an anthropomorphic female phantom in intraoral, panoramic, cephalometric and CBCT dental modalities with and without lead shields.
Results:
The upper estimates of foetal doses varied from 0.009 to 6.9 μGy, and doses at the breast level varied from 0.602 to 75.4 μGy. With lead shields, the foetal doses varied from 0.005 to 2.1 μGy, and breast doses varied from 0.002 to 10.4 μGy.
Conclusions:
The foetal dose levels without lead shielding were <1% of the annual dose limit of 1 mSv for a member of the public. Albeit the relative shielding effect, the exposure-induced increase in the risk of breast cancer death for the pregnant patient (based on the breast dose only) and the exposure-induced increase in the risk of childhood cancer death for the unborn child are minimal, and therefore, need for foetal and breast lead shielding was considered irrelevant. Most important is that pregnancy is never a reason to avoid or to postpone a clinically justified dental radiographic examination.
Keywords: dentistry, radiation protection, phantoms, imaging, radiography, panoramic, tomography, CBCT
Introduction
The number of dental radiographs taken annually in Finland is approximately 2.7 million. In 2014, the annual number of intraoral, panoramic, cephalometric and CBCT examinations in Finland was 2.35 million, 300,000, 35,000 and 7500, respectively (T Helasvuo, June 11, 2015, personal communication).1 In 2004, the most dental radiographs were taken in Germany (22.5 million), Sweden (15 million) and the UK (12.5 million), whereas in Finland, the annual number of dental radiographs was 1.5 million.2 As in other areas of radiography, digital dental radiography is also an existing practice. Digital dental radiography offers many advantages, especially in image manipulation, time and storage, over film imaging, as well as potentially lower radiation doses.3–5 CBCT has become a common radiographic tool in dentistry for diagnosis and treatment planning.6 Patient doses from CBCT are significantly higher than from conventional dental radiography techniques.7–10
The dose absorbed by the uterus has been used as a surrogate for the dose absorbed by the embryo and foetus in medical radiation dosimetry.11 Initially, uterine exposure to radiation during dental X-ray examinations has been determined by, for example, Weber et al12 and Orsini et al.12,13 The mean organ dose in the uterus for the most common examination procedures was 0.4 μSv per radiograph; a protective apron reduced this dose by a factor of two. According to the results of Weber et al,12 dental radiography involves the least radiation risk to the foetus of all diagnostic radiography procedures. Orsini et al13 also showed that X-ray shields for the uterus were unnecessary.
Based on the literature review, the number of studies of doses to the breasts in dental radiography are very limited, although the radiation sensitivity of the breast tissue is relatively high; the tissue weighting factor for breasts has increased from 0.05 to 0.12 in International Commission of Radiological Protection Publications 60 and 103.14,15 In addition, children and teenagers (7–16 years) often undergo panoramic and cephalometric dental examinations.1,16 Table 1 shows a few studies of uterus and breast doses in different dental modalities. Many studies of dosimetry in dental examinations focus on the effective dose and organ doses in the head and neck region. Some studies24–26 also report that the dose to non-shielded breasts is negligible or, presumably, zero.
Table 1.
Uterus and breast doses from studies with different dental modalities and head CT for comparison
| Modality | Dental device | Uterus dose (μGy) | Breast dose (μGy) | Reference |
|---|---|---|---|---|
| Bitewing (film, rect. coll.) | 0.55 (0.01) | Ludlow17,a | ||
| FM intraoral (film, rect. coll.) | 0.21 (0.09) | Ludlow17,a | ||
| FM intraoral (film, round cone coll.) | 1.04–2.37 (0.43–0.97) | Ludlow17,a | ||
| Intraoral (film) | Siemens Heliodent | 0.5–1.7b | Lecomber and Faulkner18 | |
| Intraoral (film) | Siemens Heliodent | 0.3–0.7c | Lecomber and Faulkner18 | |
| Intraoral (film) | Siemens Heliodent | 2.66 (2.36) | Buch et al19 | |
| Intraoral (digital) | Gendex | 2.4 (2.23) | Buch et al19 | |
| Panoramic (digital) | SCANORA® 3D | 31.8–39.0 (35.9–53.8) | 2.3–27.4 (5.1–36.6) | Rottke et al20 |
| Panoramic (digital) | ProMax® 3D | 19.8–75.6 (17.3–85.4) | 7.1–84.1 (3.8–92.8) | Rottke et al20 |
| Panoramic | Instrumentarium | 7.97 (2.24) | Buch et al19 | |
| Panoramic | 1.77 (0.04) | Ludlow17,a | ||
| PA cephalometric | 0.56 (0.01) | Ludlow17,a | ||
| Lateral cephalometric | 0.62 (0.01) | Ludlow17,a | ||
| CBCT | 3D Accuitomo | 0.01–0.03 | Okano et al21 | |
| CBCT | CB MercuRay® | 0.16d | Okano et al21 | |
| CBCT | 3D Accuitomo | 0.05–0.16 | 9.10–32.25 | Okano et al21 |
| CBCT | CB MercuRay | 1.46d | 145.91d | Okano et al21 |
| CBCT (small FOV) | 8.1–53.7 (0.2–1.2) | Ludlow17,a | ||
| CBCT (medium FOV) | 7.6–61.6 (0.2–1.4) | Ludlow17,a | ||
| CBCT (large FOV) | 7.5–62.6 (0.2–1.4) | Ludlow17 | ||
| CBCT | Alphard VEGA | 0.1,e 0.2–0.4,f 1g,h | 13,e 16–21,f 34,g 27h | Okano et al22 |
| CBCT | 3DX multi-image micro CT | 3–6i | Okano et al22 | |
| Head CT | Toshiba Xpress | 320 (75) | Beaconsfield et al23 |
CBVI, cone-beam volumetric imaging; FM, full mouth; FOV, field of view; PA, posteroanterior; rect.coll., rectangular collimator.
Doses reduced with a lead shield, when available, appear in parenthesis
Values are presented with the accuracy of the original publications.
Dental device manufacturers are as follows: 3D Accuitomo (J Morita Mfg. Corp., Kyoto, Japan); 3DX multi-image micro CT (J Morita Mfg. Corp.); Alphard VEGA (Asahi Roentgen Ind., Co., Ltd, Kyoto, Japan); CB MercuRay (Hitachi Medical Corp., Tokyo, Japan); Gendex (Gendex Dental Systems, Hatfield, PA); Instrumentarium (Instrumentarium Dental, Tuusula, Finland); ProMax 3D (Planmeca Oy, Helsinki, Finland); SCANORA 3D (Soredex, Tuusula, Finland); Siemens Heliodent (Siemens, Erlangen, Germany); Toshiba Xpress (Toshiba Electron Tubes & Devices Co., Ltd, Tochigi, Japan).
Absorbed doses calculated from the effective doses given by Ludlow17 using percentages of effective dose contribution of indirectly irradiated tissues. For the dental devices, see Ludlow.17
Bisecting angle technique.
Paralleling technique.
Implant mode (FOV 102 × 102 mm2).
Dental mode (FOV 51 × 51 mm2).
Special implant mode (FOV 102 × 60 mm2), implant mode (FOV 102 × 102 mm2).
Panoramic mode (FOV 154 × 154 mm2).
Cephalo mode (FOV 200 × 179 mm2).
FOV 40 × 30 mm2.
The usefulness of lead shields in dental radiography during pregnancy is ambiguous, and practices vary. Some studies27,28 recommend using protective lead aprons and thyroid collars to minimize foetal exposure. European guidelines on radiation protection in dental radiology2 state that there is no contraindication preventing females who are or may be pregnant from undergoing dental radiography when clinically justified. Moreover, the European guidelines also state that there is no need to use a lead protective apron in dental radiography.2 The national authority in Finland states that shields are recommended if they can minimize the patient's radiation exposure. However, the extent of a reasonable dose reduction remains uncertain. Thyroid shields are used in intraoral and cephalometric radiography, but not in panoramic radiography, as the shield may interfere with the primary beam. In CBCT, the need for thyroid shielding requires local evaluation. There is no evidence to justify routine use of abdominal shielding in conventional dental radiography or CBCT examinations.2,29
Practices and opinions regarding dental radiography during pregnancy vary. Pina and Douglass30 found that the majority of dentists in Connecticut favoured providing dental treatment during the second trimester of pregnancy. However, even though 97% of the respondents had treated pregnant patients, only 45% felt very comfortable doing so.30 Different interpretations of the use of shielding confuse users, who aim to follow good practices and to adhere to the ALARA principle (As Low As Reasonably Achievable). Although the dose to the uterus from scattered radiation during a routine dental diagnostic radiograph is minimal, it is usually recommended avoiding radiographs during pregnancy or postponing them until after delivery.27 Miller,27 however, recommended taking radiographs when necessary to diagnose and to treat a dental emergency at any time during pregnancy. Dental radiography is unique in that the number of radiographs taken is so large that it will always include those patients who are unaware of their pregnancy. After learning of the pregnancy, they may become concerned about the effects of the X-ray procedure on their unborn child. If practices were consistent, patients would feel reassured and not resort to unjust accusations should the child be born unhealthy.
The aim of this study was to determine the upper estimate of radiation exposure to the foetus and the breasts of a pregnant patient from different dental X-ray examinations both with and without lead shielding. The results will serve as the basis of guidelines for good practices in dental radiography during pregnancy. For a directional estimation of the dose to the foetus and breasts, dose conversion factors are provided as doses per the dose–area product (DAP) values of the dental examinations. Moreover, this study should provoke a discussion about the usefulness of radiation shields in dental radiography and practices of enquiring pregnancy from a female patient prior to the dental X-ray examination in the field of radiation protection.
Methods and materials
Dental modalities
Intraoral
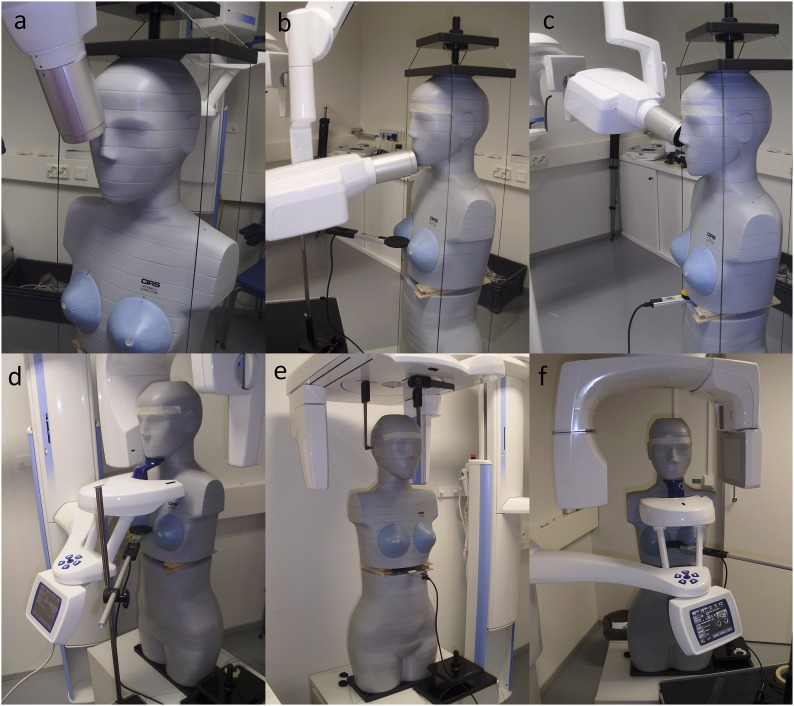
The tube output of the intraoral round cone device (Planmeca ProX™; Planmeca®, Helsinki, Finland) was measured with a RaySafe Xi unit using an 8202031-J Xi R/F & MAM detector (Unfors RaySafe AB, Billdal, Sweden) attached directly to the X-ray tube. For comparison purposes, the DAP was calculated based on the tube output. The focus-to-detector distance was 30 cm. The projections in the Planmeca ProX intraoral round cone device (Planmeca) were as follows: upper occlusal from a +65° vertical angle (worst case scenario), mandibular incisor from a −5° vertical angle and maxillary premolar (right) from a +17° vertical angle. The exposure parameters were those recommended by the scanner manufacturer and appear in Table 2; the measurement settings appear in Figure 1a–c.
Table 2.
Exposure parameters in intraoral, panoramic and cephalometric modalities
| Modality | Tube voltage (kV) | Filtration (mmAl) | Tube current (mA) | Time (s) | FOV (cm × cm) | DAP (mGy cm2) | FSD/FID (cm) | HVL (mmAl) |
|---|---|---|---|---|---|---|---|---|
| Intraoral | ||||||||
| Upper occlusal | 70 | min. 2.5 | 6 | 0.2 | 6 (diam.) | 21 | 30 | 2.78 |
| Mandibular incisor | 60 | min. 2.5 | 7 | 0.1 | 6 (diam.) | 9 | 30 | 2.37 |
| Maxillary premolar (right) | 63 | min. 2.5 | 6 | 0.2 | 6 (diam.) | 17 | 30 | 2.50 |
| Panoramic | 66 | min. 2.5 | 8 | 15.8 | 14 × 30 | 71 | 50 | |
| Cephalometric | 66 | min. 2.5 | 10 | 6.4 | 24 × 18 | 16 | 170 | |
DAP, dose–area product; diam., diameter; FID, focus-to-image distance; FOV, field of view; FSD, focus-to-skin distance; HVL, half value layer; min., minimum; mmAL, millimetres of aluminium.
Figure 1.
Measurement settings in three intraoral projections: upper occlusal (a), mandibular incisor (b) and right maxilla premolar (c) and in panoramic (d), cephalometric (e) and CBCT (f) exposures. The dose measurements were performed inside the phantom at the slice 21 position (gap by wooden spacers) and at the breast level outside the phantom. Both measurement positions were used for all modalities.
Panoramic and lateral cephalometric
A Planmeca ProMax® 2D S2 (Planmeca) was used in panoramic examinations. A Planmeca ProMax cephalostat (Planmeca) was used in the lateral cephalometric examinations. The exposure parameters for panoramic and lateral cephalometric examinations were those recommended by the scanner manufacturer and appear in Table 2; the measurement settings appear in Figure 1d and Figure 1e, respectively.
CBCT
A Planmeca ProMax 3D Mid (Planmeca) was used in the CBCT scans. Small, medium, large and extralarge fields of view were used in the CBCT of the mandibular molar (left). The exposure parameters were those recommended by the scanner manufacturer and appear in Table 3; the measurement settings appear in Figure 1f. Clinical practice often uses the manufacturer's exposure parameters, but lower values can be achieved in optimized scan protocols. The dose distribution of different manufacturers' CBCT devices may also vary owing to different cone-beam geometries and collimations.
Table 3.
Exposure parameters in CBCT with different field of views (FOVs)
| Modality | Tube voltage (kV) | Filtration (mmAl + mmCu) | Tube current (mA) | Time (s) | FOV (cm × cm) | DAP (mGy cm2) | FID (cm) | CTDI (mGy) |
|---|---|---|---|---|---|---|---|---|
| CBCT | ||||||||
| Small FOV | 90 | min. 2.5 + 0.5 | 10 | 12 | 4 × 5 | 557 | 60 | 8.2 |
| Medium FOV | 90 | min. 2.5 + 0.5 | 10 | 12 | 8 × 5 | 820 | 60 | 11.2 |
| Large FOV | 90 | min. 2.5 + 0.5 | 10 | 12 | 8 × 8 | 1093 | 60 | 9.6 |
| Extra large FOV | 90 | min. 2.5 + 0.5 | 10 | 27 | 20 × 18 | 2491 | 60 | 5.4 |
CTDI, CT dose index; DAP, dose–area product; FID, focus-to-image distance; min., minimum; mmAL, millimetres of aluminium; mmCu, millimetres of copper.
Phantom and measurement set-up
An anthropomorphic adult female phantom (ATOM®, Model 702-D; CIRS, Norfolk, VA) with small breasts was used in all modalities. Scattered radiation doses were measured as air kerma with a RaySafe Xi unit using 8202062-C Xi Survey Detector (Unfors RaySafe AB). The detector and phantom positioning were the same in all foetal dose and all breast dose measurements.
Xi Survey detector is a solid state detector with 154 silicon diodes, 77 on each side of the circuit board. The angular dependency of the detector provided by the manufacturer (Unfors RaySafe AB) showed relatively constant (±10%) response over the front axial range of 150°. The minimum response time of Xi Survey detector is 0.5 s, and the maximum resolution is 0.001 μSv. Thus, that the detector was suitable for cumulative scatter dose measurements inside the phantom. Moreover, the detector has high sensitivity and requires no temperature or pressure corrections.
Foetal doses
For the foetal dose measurements, slice 21 of the phantom was removed and replaced with wooden spacers as separators to provide a gap (2.5 cm) for the dose measurement (Figure 1c,e). The distance from the centre of the detector to the surface of the phantom was 6 cm. The dose was measured inside the phantom at the slice 21 position, which represented the liver level and the apex of the foetal coverage in late pregnancy. Thus, the dose measurement location is assumed to be the point of the foetus closest to the scattered radiation source (the pregnant patient's mandibular and maxillofacial region), thereby providing an upper estimate for the foetal dose in the dental X-ray exposures. The average dose at this point was used as an upper estimate for the radiation dose to the foetus, referred to in this study as the foetal dose. The number of repeated cumulative measurements for the upper occlusal projection in the intraoral modality was five, and for the other examination types in the intraoral modality and other three modalities, it was three. Foetal dose conversion factors were calculated as the average dose divided by the DAP value of the examination.
Breast doses
Scatter doses were also measured at the breast level outside the phantom by attaching the detector between the breasts (Figure 1b,d,f). The distance from the detector to the breastbone was 3.25 cm. The average dose at the breast level was used as an upper estimate for the breast dose. The number of repeated cumulative measurements for the intraoral modality was five, and for other three modalities, it was three. Breast dose conversion factors were calculated as the average dose divided by the DAP value of the examination.
Lead shields
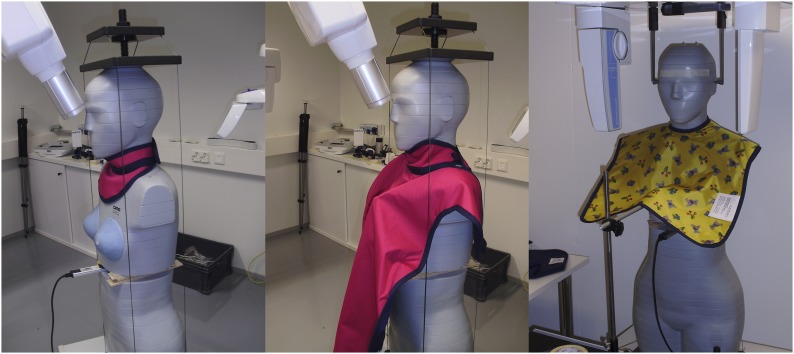
Doses were measured both with and without lead shields. The lead shield for thyroid only (0.5 mmPb, Shield 1, model RA 615; MAVIG); the lead shield for thyroid, breasts and abdominopelvic region (model RD 642-99, 0.5 mmPb, Shield 2; MAVIG); and the lead apron for breasts and upper abdomen (model 642, 0.5 mmPb, Shield 3; MAVIG) were used according to clinical practice. In the intraoral modality, Shields 1 and 2 were used, and in the other modalities, Shield 3 was used. Additional coverage for the lower abdomen and back of the trunk was considered unnecessary owing to the distribution of radiosensitive organs on the anterior side of the trunk. Scattered radiation in the lower trunk was considered insignificant. The shields are shown in Figure 2.
Figure 2.
Lead shield for thyroid only (Shield 1, left); lead shield for thyroid, breasts and abdominopelvic region (Shield 2, middle); and lead apron for breasts and upper abdomen (Shield 3, right).
Results
Tables 4 and 5 show the results of the foetal and breast dose measurements. The effect of the lead shield is presented as a relative dose reduction compared with the non-shielded case. The repeatability of the measurements is presented as a relative variation of the maximum and minimum doses compared with the average dose. The dose conversion factors are calculated as the foetal and breast doses divided by the corresponding DAP value.
Table 4.
Intraoral foetal and breast doses with effect of the lead shield expressed as a relative dose reduction in percentages. Dose conversion factors are calculated as the foetal or breast dose divided by the corresponding dose–area product value
| Modality | Foetal dose |
Breast dose |
||||
|---|---|---|---|---|---|---|
| No shield | Lead thyroid shield (Shield 1) | Lead apron and thyroid shield (Shield 2) | No shield | Lead thyroid shield (Shield 1) | Lead apron and thyroid shield (Shield 2) | |
| Intraoral (μGy) | ||||||
| Upper occlusal | 0.553 | 0.022 | 0.017 | 1.882 | 0.994 | 0.016 |
| Dose reduction (%) | 96 | 97 | 47 | 99 | ||
| Conversion factor (μGy Gy cm−2) | 27.0 | 1.1 | 0.8 | 91.7 | 48.5 | 0.8 |
| Mandibular incisor | 0.009 | 0.006 | – | 0.602 | 0.283 | 0.002 |
| Dose reduction (%) | 39 | 53 | >99 | |||
| Conversion factor (μGy Gy cm−2) | 1.0 | 0.6 | 67.7 | 31.8 | 0.3 | |
| Maxillary premolar (right) | 0.012 | 0.005 | – | 0.659 | 0.512 | 0.004 |
| Dose reduction (%) | 57 | 22 | 99 | |||
| Conversion factor (μGy Gy cm−2) | 0.7 | 0.3 | 39.3 | 30.5 | 0.2 | |
Table 5.
Panoramic, cephalometric and CBCT foetal and breast doses with effect of the lead shield expressed as a relative dose reduction in percentages. Dose conversion factors are calculated as the foetal or breast dose divided by the corresponding dose–area product value
| Modality | Foetal dose |
Breast dose |
||
|---|---|---|---|---|
| No shield | Lead apron (Shield 3) | No shield | Lead apron (Shield 3) | |
| Panoramic dose (μGy) | 0.11 | 0.04 | 3.57 | 0.61 |
| Dose reduction (%) | 61 | 83 | ||
| Conversion factor (μGy Gy cm−2) | 1.5 | 0.6 | 50.3 | 8.6 |
| Cephalometric dose (μGy) | 0.71 | 0.69 | 4.33 | 0.08 |
| Dose reduction (%) | 3 | 98 | ||
| Conversion factor (μGy Gy cm−2) | 44.4 | 43.1 | 270.8 | 5.2 |
| CBCT dose (μGy) | ||||
| Small FOV | 2.64 | 0.80 | 43.20 | 5.74 |
| Dose reduction (%) | 70 | 87 | ||
| Conversion factor (μGy Gy cm−2) | 4.7 | 1.4 | 77.6 | 10.3 |
| Medium FOV | 3.75 | 1.10 | 61.9 | 9.09 |
| Dose reduction (%) | 71 | 85 | ||
| Conversion factor (μGy Gy cm−2) | 4.6 | 1.3 | 75.5 | 11.1 |
| Large FOV | 4.52 | 1.28 | 75.26 | 10.36 |
| Dose reduction (%) | 72 | 86 | ||
| Conversion factor (μGy Gy cm−2) | 4.1 | 1.2 | 68.9 | 9.5 |
| Extralarge FOV | 6.93 | 2.11 | 75.38 | 8.24 |
| Dose reduction (%) | 69 | 89 | ||
| Conversion factor (μGy Gy cm−2) | 2.8 | 0.8 | 30.3 | 3.3 |
FOV, field of view.
The repeatability of the measurements was used as an estimate of the measurement uncertainty. The measurement uncertainty was the highest at the lowest measured dose level, 0.002 μGy, for which it was 45%. At the highest measured dose level, 75.4 μGy, the measurement uncertainty was 0.5%. The measurement uncertainty was in each case lower than the relative dose reduction.
Discussion
The aim of this study was to determine the upper estimate of radiation exposure to the foetus and the breasts of a pregnant patient from different dental X-ray examinations both with and without lead shielding. The results will serve as the basis of guidance for good practices in dental radiography during pregnancy. The European guidelines on radiation protection in dental radiology2 indicate that national practices may vary. It is therefore essential to establish consistent practices to improve patient safety and to put the need for radiation shields into perspective with actual radiation doses in dental radiography.
In this study, the upper estimates of foetal doses varied from 0.009 to 6.9 μGy without lead shielding and from 0.005 to 2.1 μGy with lead shielding. The foetal doses with or without the lead shields were far below the level associated with any practical radiation detriment to the foetus. However, exposure situations can vary considerably depending on the imaging techniques applied. The cephalometric projection, for example, was directed laterally, which explains the minimal reduction in the foetal dose with the frontal shielding, since the dose accumulates almost completely as internal scatter. In panoramic radiography and CBCT, the rotating scan geometry causes more frontal exposure without the shields, so these cases usually have relatively higher shielding efficiency (Table 5). However, the focus of this study was on pregnant patients, and cephalometric examinations are mostly performed for (non-pregnant) paediatric patients.31 The highest relative shielding occurs in upper occlusal projection, as one would expect with the downward frontal direction of the exposure geometry (Figure 1a). It should be noted, however, that in clinical practice, implementation of the shielding geometry and coverage inevitably varies considerably owing to individual working methods and competence, patient morphology and other specific factors involved in physical examinations. This also leads to considerable variance in the effectiveness of the shields.
The doses at the breast level varied from 0.602 to 75.4 μGy without lead shielding and from 0.002 to 10.4 μGy with lead shielding. The breast dose reduction in the cephalometric projection was one of the highest, being at the same level with the intraoral dose reductions with much more covering shield. This can be explained by the fact that breasts were more shielded by the frontal shield than the estimated foetal position. For patients with frequent dental examinations, especially in orthodontic radiography in paediatric female patients, the accumulated dose to the breasts can be a notable addition to the personal radiation burden in relative terms.
The use of lead shields reduced the foetal dose by 39–97% and reduced the breast dose by 22%–99%. However, the absolute foetal dose was negligible even without shielding. According to the European Council Directive (2013/59/Euratom),32 it is correct to enquire whether the patient is pregnant or breastfeeding unless it can be ruled out for obvious reasons or it is not relevant for the radiological procedure. Furthermore, the directive also states that if pregnancy cannot be ruled out, special attention shall be given to the justification and optimization of the radiological procedure, in particular if abdominal and pelvic regions are involved. The International Atomic Energy Agency Basic Safety Standards33 state that ascertaining the pregnancy status shall be ensured when significant foetal doses are expected. The oral region is far from the abdominopelvic region, and the foetal dose is originated from scattered radiation. The doses measured in this study are minimal, and they are many orders of magnitude lower than foetal doses from radiological procedures of the abdominopelvic region. Compared with the annual dose limit of 1 mSv for a member of the public or to the embryo/foetus of a declared pregnant worker,15 the order of magnitude of the radiation exposure to the foetus from a single dental examination without lead shielding is <1%. The natural background radiation dose rate is 0.09–0.14 μSv h−1 in Finland34 and 5 μSv h−1 in an airplane at normal cruising altitude.35 Therefore, the accumulated dose within two days or a 2-h flight would result in a radiation exposure of the same order of magnitude as the upper estimate for foetal dose in dental examinations. From this perspective, the use of shielding to reduce the foetal dose could be considered irrelevant. Based on this study, the practice of enquiring pregnancy from a female patient could be discarded in dental radiology.
Compared with other studies on uterine doses in dental examinations, the doses in this study were smaller than the doses measured by Buch et al19 in intraoral and panoramic examinations, but larger than those measured in CBCT by Okano et al.22 It must be noted that the foetal doses in this study represent the upper estimate of the foetal dose, as measured at the liver level. The studies by Buch et al19 and Okano et al21,22 include dose measurement in the normal uterus at one to three measurement points. Consequently, the doses measured at those points should be much less than the upper estimates of the foetal dose in the present study, as measured at the liver level. In the study by Okano et al,21 the uterine doses in CBCT were lower than the CBCT doses in the present study, and closer to the doses in intraoral, panoramic and cephalometric examinations.
The doses to the breast calculated from the effective doses reported by Ludlow17 in CBCT examinations corresponded well to the results of the present study. In intraoral and panoramic examinations, the dose level in the present study was about two to three times the level by Ludlow,17 and in cephalometric examinations, the dose level was sevenfold. This may be due to high uncertainties in Ludlow's17 effective dose calculations from low doses, since many organs receive doses close to zero. The maximum breast doses in CBCT measured by Okano et al21,22 were about half the maximum breast doses in CBCT of the present study, excluding the implant mode in CBCT, for which the dose was double. An exception in the otherwise uniform exposure parameters was in the study by Okano et al;21 the tube voltage in implant mode was 120 kV instead of the more typical dental values of 65–90 kV common in the other studies (Table 1).
The guidelines of the European Academy of DentoMaxilloFacial Radiology29 emphasize the lack of evidence supporting the routine use of lead apron shielding. Recently, Rottke et al20 found no statistically significant differences between absorbed organ doses in panoramic examinations with or without the use of lead apron shielding. The opposite was found by Buch et al,19 who showed that the use of a lead apron in panoramic examinations significantly (72%) reduced the dose to the uterus. However, in intraoral full-mouth examinations, the dose reduction effect found by Buch et al19 was small (7%). Ludlow17 stated that of indirectly irradiated tissues, the breasts receive the largest proportion of the dose. According to his results, the use of a lap apron may reduce the potential dose to the breasts by an order of magnitude or more, and thereby rendering the dose to the breasts negligible. Beaconsfield et al23 found that wearing a 5-mm lead-equivalent bib in conjunction with two thyroid collars reduced the breast dose from 320 to 75 μGy (76%) and, therefore, strongly recommended the use of lead shields as a simple precaution, especially for paediatric patients and young adults.
Despite the fact that the breasts receive higher radiation doses in dental examinations than the foetus, the exposure-induced increase in the risk of breast cancer death for the pregnant patient (based on the breast dose only) and the exposure-induced increase in the risk of childhood cancer death for the unborn child are of the same order of magnitude, 10−5%.36–38 To increase the risk of childhood cancer death by 0.06%,36 the dose level to the foetus must be >1000 times higher than the highest dental dose level measured in the present study. At present, there is no dose or risk limit to define the level of protection needed for patients. However, risk estimation is essential and the decision about the use of shields should be made based on the risk. From this point of view, the use of shields to reduce foetal and breast doses in dental radiography is irrelevant. Acute dental pain or other circumstances that require the use of dental X-ray examinations are always justified regardless of pregnancy. Pregnancy may affect dental health and, compared with the negligible radiation risk to the foetus from dental X-rays, failure to provide the patient proper care and diagnostics for possible dental problems is much more harmful to the foetus. Noticing the pregnancy only after the radiograph is taken should raise no concerns about radiation detriment for the foetus.
The limitations of this study are related to the measurement geometry and the phantom dimensions. The presence and accurate positioning of the Xi Survey detector inside the phantom and between the breasts did not allow the lead shield to be as close to the phantom as it would be in patient exposures. The phantom represents only one patient size in early pregnancy, and it had no added material in the abdominal region to simulate later pregnancy. Also, one slice was removed to allow scatter dose measurements inside the phantom. Scattered dose coming from the back of the Xi Survey detector could not be taken into account. The dose conversion factors provide a directional estimate of the foetal and breast doses in dental examinations given that the radiation quality does not change much and that the exposure geometry is the same. Furthermore, the dose conversion factors for CBCT are dependent on the cone-beam geometry and collimation. The effect of different CBCT collimations at the same geometry of image acquisition on the effective dose and organs in the head and the neck regions has been shown to be considerable.39 However, the effect of collimation far from the primary beam (at foetal and breast positions) may not be as large as it is for all organs in the head and the neck regions.
Current practices in dental radiography should reflect that fact that lead aprons are unnecessary to protect the foetus and the breasts based on the minimal increase in the risk of exposure-induced cancer death. The use of shields causes extra costs and requires training of the dental staff. When shields are not used, there is no need to enquire pregnancy and document the answer, which is time saving. Most important is that pregnancy is never a reason to avoid or to postpone a clinically justified dental radiographic examination.
Conclusions
Based on the results of this study, the foetal dose levels without lead shielding was <1% of the annual dose limit of 1 mSv for a member of the public. Foetal doses in intraoral, panoramic and cephalometric examinations without lead shields reached 0.1–10% of the maximum foetal doses in CBCT. The breast doses in CBCT were at most tenfold compared with the maximum foetal doses. The exposure-induced increase in the risk of breast cancer death for the pregnant patient (based on the breast dose only) and the exposure-induced increase in the risk of childhood cancer death for the unborn child are minimal and therefore, need for foetal and breast lead shielding was considered irrelevant. At present, there is no dose or risk limit to define the level of protection needed for patients. Discussion about such a limit would be highly beneficial.
Acknowledgments
Acknowledgments
The authors are grateful to sales manager Jari Outavaara of Planmeca for letting us use the dental devices in Planmeca, and to product manager Erkki Hiltunen of Planmeca for helping us to use them.
References
- 1.Helasvuo T, ed. Number of radiological examinations in Finland in 2011. STUK-B 161. Helsinki, Finland: STUK; 2013. [Google Scholar]
- 2.European Commission. Radiation protection no. 136. European guidelines on radiation protection in dental radiology. The safe use of radiographs in dental practice. Luxembourg: EC; 2004. Available from: ec.europa.eu/energy/sites/ener/files/documents/136.pdf [Google Scholar]
- 3.Brennan J. An introduction to digital radiography in dentistry. J Orthod 2002; 29: 66–9. doi: 10.1093/ortho/29.1.66 [DOI] [PubMed] [Google Scholar]
- 4.Wenzel A, Møystad A. Work flow with digital intraoral radiography: a systematic review. Acta Odontol Scand 2010; 68: 106–14. doi: 10.3109/00016350903514426 [DOI] [PubMed] [Google Scholar]
- 5.Berkhout E, Sanderink G, van der Stelt P. Digital intra-oral radiography in dentistry. Diagnostic efficacy and dose considerations. Oral Radiol 2003; 19: 1–13. doi: 10.1007/BF02493286 [DOI] [Google Scholar]
- 6.Pauwels R, Beinsberger J, Collaert B, Theodorakou C, Rogers J, Walker A, et al. ; SEDENTEXCT Project Consortium. Effective dose range for dental cone beam computed tomography scanners. Eur J Radiol 2012; 81: 267–71. doi: 10.1016/j.ejrad.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 7.Roberts JA, Drage NA, Davies J, Thomas DW. Effective dose from cone beam CT examinations in dentistry. Br J Radiol 2009; 82: 35–40. doi: 10.1259/bjr/31419627 [DOI] [PubMed] [Google Scholar]
- 8.Ludlow JB, Davies-Ludlow LE, Brooks SL. Dosimetry of two extraoral direct digital imaging devices: NewTom cone beam CT and Orthophos Plus DS panoramic unit. Dentomaxillofac Radiol 2003; 32: 229–34. doi: 10.1259/dmfr/26310390 [DOI] [PubMed] [Google Scholar]
- 9.Ludlow JB, Davies-Ludlow LE, Brooks SL, Howerton WB. Dosimetry of 3 CBCT devices for oral and maxillofacial radiology: CB Mercuray, NewTom 3G and i-CAT. Dentomaxillofac Radiol 2006; 35: 219–26. doi: 10.1259/dmfr/14340323 [DOI] [PubMed] [Google Scholar]
- 10.Li G. Patient radiation dose and protection from cone-beam computed tomography. Imaging Sci Dent 2013; 43: 63–9. doi: 10.5624/isd.2013.43.2.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Radiology. ACR practice guideline for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation: resolution 26. Reston, VA: American College of Radiology; 2008. [Google Scholar]
- 12.Weber J, Ewen K, Schubel F. Determining organ doses in the uterus during dental x-ray examinations. [In German.] Dtsch Zahnarztl Z 1989; 44: 340–3. [PubMed] [Google Scholar]
- 13.Orsini S, Campoleoni M, Rozza M, Conti U, Landini A, Eulisse G, et al. The doses absorbed by the patients and the exposure of the operators in dental radiodiagnosis. [In Italian.] Radiol Med 1992; 83: 101–5. [PubMed] [Google Scholar]
- 14.International Commission on Radiological Protection. ICRP publication 60; 1990 recommendations of the ICRP. Annals of the ICRP. Amsterdam, Netherlands: Elsevier; 1990. [Google Scholar]
- 15.International Commission on Radiological Protection. ICRP publication 103: the 2007 recommendations of the ICRP. Annals of the ICRP, volume 37. Amsterdam, Netherlands: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 16.Tenkanen-Rautakoski P, ed. Number of radiological examinations in Finland in 2008. STUK-B 121. Helsinki, Finland: STUK-B 121; 2010. [Google Scholar]
- 17.Ludlow JB. Dose and risk in dental diagnostic imaging: with emphasis on dosimetry of CBCT. Korean J Oral Maxillofac Radiol 2009; 39: 175–84. [Google Scholar]
- 18.Lecomber AR, Faulkner K. Organ absorbed doses in intraoral dental radiography. Br J Radiol 1993; 66: 1035–41. doi: 10.1259/0007-1285-66-791-1035 [DOI] [PubMed] [Google Scholar]
- 19.Buch B, Fensham R, Maritz MP. An assessment of the relative safety of dental x-ray equipment. SADJ 2009; 64: 348–50. [PubMed] [Google Scholar]
- 20.Rottke D, Grossekettler L, Sawada K, Poxleitner P, Schulze D. Influence of lead apron shielding on absorbed doses from panoramic radiography. Dentomaxillofac Radiol 2013; 42: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okano T, Harata Y, Sugihara Y, Sakaino R, Tsuchida R, Iwai K, et al. Absorbed and effective doses from cone beam volumetric imaging for implant planning. Dentomaxillofac Radiol 2009; 38: 79–85. doi: 10.1259/dmfr/14769929 [DOI] [PubMed] [Google Scholar]
- 22.Okano T, Matsuo A, Gotoh K, Yokoi M, Hirukawa A, Okumura S, et al. Comparison of absorbed and effective dose from two dental cone beam computed tomography scanners. [In Japanese.] Nihon Hoshasen Gijutsu Gakkai Zasshi 2012; 68: 216–25. doi: 10.6009/jjrt.2012_JSRT_68.3.216 [DOI] [PubMed] [Google Scholar]
- 23.Beaconsfield T, Nicholson R, Thornton A, Al-Kutoubi A. Would thyroid and breast shielding be beneficial in CT of the head? Eur Radiol 1998; 8: 664–7. doi: 10.1007/s003300050456 [DOI] [PubMed] [Google Scholar]
- 24.Morant JJ, Salvadó M, Hernández-Girón I, Casanovas R, Ortega R, Calzado A. Dosimetry of a cone beam CT device for oral and maxillofacial radiology using Monte Carlo techniques and ICRP adult reference computational phantoms. Dentomaxillofac Radiol 2013; 42: 1–9. doi: 10.1259/dmfr/92555893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gijbels F, Sanderink G, Wyatt J, Van Dam J, Nowak B, Jacobs R. Radiation doses of collimated vs non-collimated cephalometric exposures. Dentomaxillofac Radiol 2003; 32: 128–33. doi: 10.1259/dmfr/33233723 [DOI] [PubMed] [Google Scholar]
- 26.Tsiklakis K, Donta C, Gavala S, Karayianni K, Kamenopoulou V, Hourdakis CJ. Dose reduction in maxillofacial imaging using low dose Cone Beam CT. Eur J Radiol 2005; 56: 413–17. doi: 10.1016/j.ejrad.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 27.Miller MC. The pregnant dental patient. Oral Health 1996; 86: 17–25. [Google Scholar]
- 28.Sandley E, Emmerling H. Dental radiography. RDH 2011; 69–73. [Google Scholar]
- 29.European Commission. Radiation protection no. 172. Cone beam CT for dental and maxillofacial radiology. Evidence based guidelines. Luxembourg: EC; 2012. ec.europa.eu/energy/nuclear_protection/doc/publication/172.pdf [Google Scholar]
- 30.Pina PM, Douglass J. Practices and opinions of Connecticut general dentists regarding dental treatment during pregnancy. Gen Dent 2011; 59: e25–31. [PubMed] [Google Scholar]
- 31.Esmaeili EP, Ekholm M, Haukka J, Waltimo-Sirén J. Quality assessment of orthodontic radiography in children. Eur J Orthodont 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Council Directive 2013/59/Euratom. Official J Eur Union 2014; L 013. ec.europa.eu/energy/sites/ener/files/documents/CELEX-32013L0059-EN-TXT.pdf [Google Scholar]
- 33.International Atomic Energy Agency. IAEA safety standards for protecting people and the environment. Radiation protection and safety of radiation sources: international basic safety standards. General safety requirements part 3, 2014. Vienna, Austria: IAEA.
- 34.STUK. Natural background radiation. [cited 8 September 2015.] Available from: http://www.stuk.fi/web/en/topics/environmental-radiation/natural-background-radiation
- 35.European Radiation Dosimetry Group. Exposure of air crew to cosmic radiation. A report of EURADOS working Group 11, EURADOS report 1996.01. In: McAulay IR, Bartlett DT, Dietze G, Menzel HG, Schnuer K, Schrewe UJ, eds. European Commission report radiation protection 85. Luxembourg: European Communities; 1996. [Google Scholar]
- 36.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol 1997; 70: 130–9. doi: 10.1259/bjr.70.830.9135438 [DOI] [PubMed] [Google Scholar]
- 37.Tapiovaara M, Siiskonen T. PCXMC—A Monte Carlo program for calculating patient doses in medical x-ray examinations. STUK-A231. Helsinki, Finland: STUK; 2008. [Google Scholar]
- 38.Biological Effects of Ionizing Radiation. Committee to assess health risks from exposure to low levels of ionizing radiation. Health risks from exposure to low levels of ionizing radiation. BEIR VII. Washington, DC: National Academy of Sciences; 2006. [Google Scholar]
- 39.Lorenzoni DC, Bolognese AM, Garbi DG, Guedes FR, Sant'anna EF. Cone-beam computed tomography and radiographs in dentistry: aspects related to radiation dose. Int J Dent 2012; 2012: 813768. doi: 10.1155/2012/813768 [DOI] [PMC free article] [PubMed] [Google Scholar]