Abstract
Objective
Human serum paraoxonase (PON1) prevents lipids from peroxidation and functions as an antioxidant mechanism. Malonyldialdehyde (MDA) is the final product of lipid peroxidation and can be used as an indicator of oxidative stress. The aim of this study was to investigate PON1, MDA, and arylesterase (ARY) levels in schizophrenic patients who are taking typical, atypical, or combined (typical and atypical) antipsychotic drug treatment, with respect to those of healthy controls.
Methods
We evaluated 41 patients (11 taking typical antipsychotics, 19 taking atypical antipsychotics, 11 taking combined anti-psychotics) and 43 healthy controls.
Results
MDA levels were higher in schizophrenic patients taking typical antipsychotics compared with healthy controls (p=0.001). ARY levels were higher in patients taking atypical antipsychotics compared with healthy controls (p=0.005). PON1 activity was similar in all groups.
Conclusion
Our results indicate that treatment with typical antipsychotic drugs could be related to increased MDA levels; and antipsychotic medication may increase PON1 levels in schizophrenic patients.
Keywords: Paraoxonase, Arylesterase, Malondialdehyde, Schizophrenia, Antipsychotics, Treatment
INTRODUCTION
Schizophrenia is a devastating disorder, usually starting in adolescence or early adulthood and continuing throughout a patient’s life with relapses and remissions. One percent of the general population is affected by schizophrenia, and the expected life span is shorter among these patients than for those who do not suffer from this disease.1,2) Both typical and atypical antipsychotic drugs are used to treat schizophrenia.3) Many neuroimaging, biochemical, and genetic research studies done in attempts to understand the etiology and neurobiology of schizophrenia.4–6) These studies include the investigation of peripheral blood, an easily accessible, and thus feasible and useful, tissue to examine; genetic markers like microRNAs; and biochemical studies, which include oxidative stress levels and antioxidant capacity assays. Prolidase levels and inflammatory cytokines also have been topics of interest in this context.7–12)
Human serum paraoxonase (PON1) is a serum esterase, synthesized by liver, which has both paraoxonase and arylesterase (ARY) activity, and takes an important place in preventing low-density lipoprotein from peroxidation.13,14) PON1 is attached to the exterior surface of the cell and transported to high-density lipoprotein (HDL) by lipoproteins. After this transportation, PON1 sets a strict conjugation with HDL,15) and because of reported antioxidant effects, PON1 activity has been investigated in schizophrenic patients. To date, most studies have considered the effects of atypical antipsychotics like olanzapine, quetiapine, and risperidone on PON1.16–18) As far as we know, atypical antipsychotics usually increase PON1 levels.
ARY is a form of paraoxonase that doesn’t represent any polymorphism of activity. Thus, it can be evaluated as an identifier of actual protein concentration. PON1 activity is independent from age and sex, but is influenced by smoking, pregnancy, diet, and acute phase reactants.19,20) To date, completed studies did not include patients taking typical antipsychotics in terms of PON1 and ARY. In our study, we evaluated patients taking both typical and combined antipsychotic treatment, as well as atypical antipsychotics.
Free oxygen radicals are of importance in oxidative processes, and their increase will result in excessive lipid peroxidation, which is responsible for tissue damage.21) Malonyldialdehyde (MDA) can be used as an indicator of oxidative stress because it is the final product of lipid peroxidation.22) In this context, numerous studies have investigated MDA levels in schizophrenic patients and, despite controversial results, conclusions from previous data consistently find that increased MDA is present in schizophrenics. Earlier studies of MDA have also included atypical antipsychotics like olanzapine and quetiapine.23)
There are several factors that form a basis for brain vulnerability to oxidative stress. For instance, the brain consumes oxygen at high levels; therefore, increased oxygen consumption may induce an increase in free radicals. Additionally, brain tissue contains a high amount of lipids, which is a potential substrate of peroxidation. Finally, the brain has modest antioxidant mechanisms, which may be inadequate in the presence of excessive oxidation, and the imbalance of disrupted oxidative and antioxidant processes has been implicated in schizophrenia.24)
In this study, our aim is to investigate three parameters in schizophrenic patients: PON1, ARY, and MDA. PON1 is an important enzyme that prevents lipids from peroxidation, in contrast to MDA, which is a substrate of lipid peroxidation and can be a useful marker to determine oxidative stress levels. Investigating ARY is important because it represents actual protein concentration. Finally, an additional important feature of this research is the inclusion of patients who are taking typical, atypical, or combined antipsychotic treatments.
METHODS
Subjects
Dicle University Ethical Committee approved our study (approval no. 45). Written informed consent was obtained from all patients according to the Helsinki Declaration. Patients who were admitted to Dicle University Faculty of Medicine Psychiatry Department were included. We evaluated patients with the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impressions Scale (CGI). The control group consisted of individuals without any psychiatric or medical condition. Schizophrenic patients between 18 and 65 years of age were diagnosed, without any comorbid psychiatric or medical condition, according to the Diagnostic and Statistical Manual of Mental Disorders 4th edition, text revision (DSM-IV-TR) in the Department of Psychiatry at Dicle University Hospital. Only patients using antipsychotic drugs for schizophrenia were included in this study. Our study included 41 patients and 43 healthy controls who met these parameters.
Blood Sampling
Blood samples were obtained via the antecubital vein. Samples were then transferred into heparinized tubes and centrifuged at 3.000 rpm to separate the sera, and then transferred to storage at −20°C within six hours.
Measurement of Paraoxonase and Arylesterase Activity
We used paraoxon and phenyl acetate substrates to measure paraoxonase and ARY activities. We measured the rate of paraoxon hydrolysis (diethyl-p-nitrophenyl phosphate) by monitoring an increase of absorbance at 412 nm at 37°C. The amount of generated p-nitrophenol was calculated from the molar absorptivity coefficient at pH 8, which was 17,000 M−1 cm−1. To express paraoxonase activity we used U/L serum. ARY activity was measured with phenyl acetate. The molar absorptivity coefficient of the produced phenol used to calculate enzyme activity was 1,310 M−1 cm−1. One unit of ARY activity was defined as 1 μmol phenol generated/min under the above conditions and expressed as U/L serum.25)
Measurement of Malondialdehyde Levels
The method of Draper et al.,26) which is based on the reaction of MDA with thiobarbituric acid (TBA) at 95°C, was used to determine MDA levels. MDA with TBA reacts to form a pink pigment with an absorption maximum at 532 nm, and was the basis of TBA test reaction. We performed reaction testing at pH 2–3 at 95°C for 15 minutes. For protein precipitation, the sample was mixed with 2.5 volumes of 10% (w/v) trichloroacetic acid. The precipitate was pelleted by centrifugation and an aliquot of the supernatant was allowed to react with an equal volume of 0.67% TBA in a boiling water bath for 15 minutes. The absorbance was read at 532 nm, following cooling. Arbitrary values obtained were compared with a series of standard solutions (1, 1, 3, 3 tetramethoxypropane). Results were expressed as nmol/ml.26)
Statistical Analysis
Statistical analyses were performed using SPSS software, version 11.5 (SPSS Inc., Chicago, IL, USA). The variables were investigated with Kolmogorov-Smirnov/Shapiro-Wilk’s tests to determine whether or not they were normally distributed. To compare means and parametric variables, the Student’s t-test was performed, and was also used to compare ARY levels between groups. Comparisons of non-parametric variables were done with the Mann-Whitney U-test, as well as to compare PON1 and MDA levels. A p value of less than 0.05 was considered significant. Either the chi-square test or Fisher’s exact test, when chi-square test-assumptions do not hold due to low expected cell counts, were used to compare data where appropriate. We used the Pearson test to define correlation coefficients and their significance when both parameters were normally distributed. To detect significance between multiple groups, we used ANOVA for parametric variables, and Mann-Whitney U-test for non-parametric variables. Finally, we employed the Tamhane T2 test as a post hoc test to compare parametric variables between groups, and the Bonferroni correction was applied to detect significance, while comparing non-parametric variables in multiple groups. p<0.0125 was accepted as significant after correction.
RESULTS
Patient and control groups were similar in terms of age, gender, smoking status, and where each placed on the body mass index (Table 1). Shown in Figures 1 and 2, are the overall mean plasma PON1, MDA, and ARY levels of both schizophrenic patients and the healthy controls.
Table 1.
Sociodemographical characteristics of patients
| Characteristic | Patient (n=41) | Control (n=43) | p value |
|---|---|---|---|
| Age (yr) | 35.5±9.5 | 35.4±8.8 | 0.672 |
| Gender, male/female | 35/6 | 36/7 | 0.835 |
| Smokers | 27 (65.9) | 34 (79.1) | 0.175 |
| Smoking (pack/day) | 31.8 | 29.9 | |
| Body mass index (kg/m2) | 27.8±5.8 | 26.6±3.2 | 0.269 |
Values are presented as mean±standard deviation, number only, or number (%).
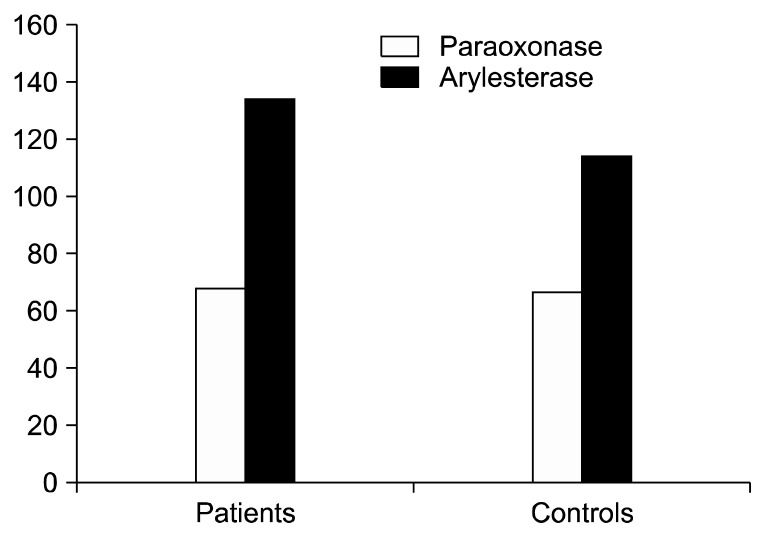
Fig. 1.
Plasma paraoxonase levels of overall schizophrenia patients and controls were 68.6±3.8 U/L and 66.9±5.2 U/L respectively, p=0.159. Plasma arylesterase levels of overall schizophrenia patients and controls were 134.9±24 U/L and 114.5±24.9 U/L respectively, p<0.001.
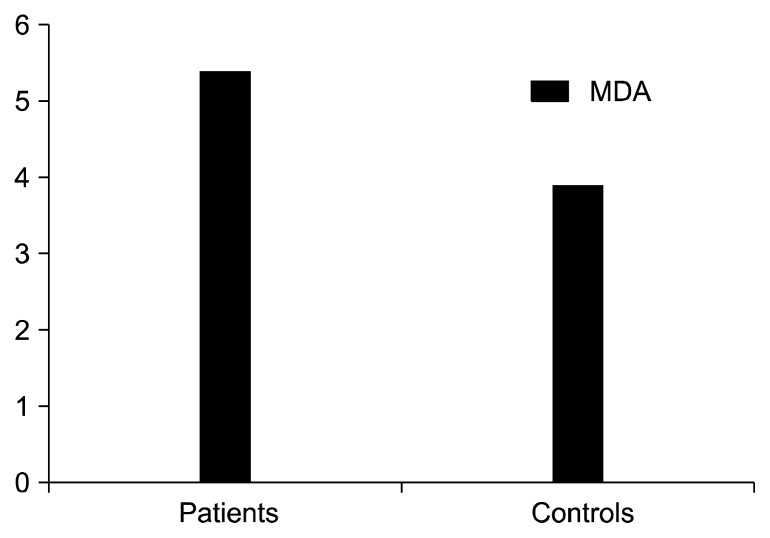
Fig. 2.
Plasma malondialdehyde (MDA) levels of overall schizophrenia patients and controls were 5.4±2.7 nmol/mL and 3.9±1.8 nmol/ml, respectively; p=0.001.
We also took into consideration that 18 of the 41 patients had a family history of schizophrenia, and 24 of the 41 patients fell within the paranoid subtype. Mean and standard deviations for PANSS-P, PANSS-N, PANSS-G, PANSS-overall, and CGI were 21.7±8.2, 22.5±11.1, 46.4±16.7, 90.9±33.4, and 3.6±1.2, respectively.
To determine more specific data, we performed post hoc analyses of patients using typical, atypical, and combined (both atypical and typical) antipsychotics, as well as of the healthy controls. We compared each group with other groups separately to detect the origin of significant differences in ARY and MDA levels. For ARY, we performed ANOVA, because it presented normal distribution. For MDA, we performed the Mann-Whitney U-test separately between groups, and accepted a p value of <0.0125 after the Bonferroni correction. Table 2 presents the mean values of MDA, PON1, and ARY for each group. For ARY, statistical differences originated from comparison of patients using atypical antipsychotics with healthy controls, p=0.005. There weren’t any statistical differences for other comparisons. After applying the Mann-Whitney U-test between each group, MDA levels were significantly higher in patients taking typical anti-psychotics than were those of the healthy controls (p=0.001).
Table 2.
Comparison of MDA, paraoxonase, and arylesterase levels in the patients taking each treatment
| Typical antipsychotics (n=11) | Atypical antipsychotics (n=19) | Combined antipsychotics (n=11) | Healthy controls (n=43) | Comparison | p value | |
|---|---|---|---|---|---|---|
| MDA (nmol/mL) | 6.6±3.4 | 4.5±1.7 | 5.6±3.0 | 3.9±1.8 | 1–4 | 0.001 |
| 2–4 | NS | |||||
| 3–4 | NS | |||||
| Paraoxonase (U/L) | 67.8±4.1 | 68.7±3.4 | 69.1±4.4 | 66.9±5.2 | 1–4 | NS |
| 2–4 | NS | |||||
| 3–4 | NS | |||||
| Arylesterase (U/L) | 131.0±22.4 | 138.1±24.3 | 133.0±26.3 | 114.5±24.9 | 1–4 | NS |
| 2–4 | 0.005 | |||||
| 3–4 | NS |
Values are presented as mean±standard deviation.
MDA, malondialdehyde; PON1, paraoxonase; ARY, arylesterase; NS, not significant.
ARY levels were significantly increased only in patients using atypical antipsychotics p=0.005. MDA was significantly increased in patients on typical antipsychotic medication, p=0.001. Other comparisons between groups were statistically not significant (all p values were above 0.05).
DISCUSSION
This study compared MDA, PON1, and ARY levels in schizophrenic patients with those in the healthy control group. First, when we compared overall schizophrenic patients with the control group, we found that overall ARY levels were significantly higher in the patient group (p<0.001). After performing multiple tests to narrow these findings down to more specific results, we found that of these patients’ groups, the higher levels originated from the group taking atypical antipsychotics (p=0.005). As well, we found that when compared with the control group, overall MDA levels were statistically higher in the patient group. Narrowed down further, we found the higher MDA levels were significant for patients on typical antipsychotic drug treatment (p=0.001).
MDA has been investigated in schizophrenic patients extensively and, as with our study, there are other studies that have found significantly increased MDA levels in this population. For instance, in one study, Kuloglu et al.27) found plasma MDA levels higher in schizophrenic patients than in controls (4.76±0.79 and 2.710±0.50, respectively). In contrast, there are reports indicating no significant differences relating to lipid peroxidation among schizophrenic patients.28) A meta-analysis of 17 studies, which included 761 patients and 458 controls, revealed that increased MDA levels seem to be a consistent, but not a universal, finding in schizophrenic patients.23) One possible explanation for increased MDA levels in patients could be that the heightened level is a disease trait of schizophrenia.
The remarkable finding of our study is the significantly higher MDA levels found in patients who were taking typical antipsychotics than in those on atypical or combined drug therapies. This finding could be interpreted in two ways: one, typical antipsychotics may cause increased oxidation in patients; or two, typical antipsychotics could be inadequate in terms of decreasing oxidative stress in schizophrenic patients. Consistent with the first statement, the typical antipsychotic, haloperidol, was found to be associated with increased cell death secondary to necrosis. This devastating side effect of haloperidol reversed with the administration of vitamin E, a free radical scavenger.29)
Likewise, there is evidence supporting the inadequacy of haloperidol on decreasing oxidative stress. Park et al.30) demonstrated that atypical antipsychotics like olanzapine, aripiprazole, and ziprasidone could be helpful with regard to decreasing oxidative stress by modulating reactive oxygen species and superoxide dismutase activity. In this study, haloperidol lacked the ability to decrease oxidative stress, which is compatible with our second conjecture. Furthermore, supporting our two conjectures, haloperidol treatment at both 45 and 90 days resulted in decreased antioxidant enzyme activities like superoxide dismutase and catalase, while increasing hydroxyalkenals, which are markers for lipid peroxidation.31)
We didn’t find any differences regarding PON1 activity among any of the groups and is probably due to the anti-psychotic medication itself. Several studies indicate that PON1 activity reaches a similar level with subjects after antipsychotic drug treatment as the PON1 activity in the control group. For instance, Sarandol et al.17) compared PON1 activity in schizophrenic patients who were taking atypical antipsychotics to healthy controls. Although they found a significant difference in PON1 activity before treatment, the difference disappeared after atypical anti-psychotic medication. Another study by Unsal et al.16) showed significantly decreased PON1 activity in schizophrenic patients taking olanzapine but not quetiapine. A recent study, which includes 51 drug-naive schizophrenic patients, investigated the effects of risperidone on PON1 activity and found that patients had significantly lower PON1 activity before treatment. However, after risperidone treatment, PON1 activity increased significantly, reaching a similar level to that of the healthy controls.18) Mounting data suggest that atypical antipsychotics increase PON1 activity in schizophrenic patients, which, upon evaluating our results, support our findings on the lack of differences in PON1 activity among groups, and is probably due to antipsychotic drug treatment.
We found ARY levels were higher in patients regardless of the antipsychotic drug type; however, there were significant differences in patients who were taking atypical antipsychotics. Increased ARY levels may be due to anti-psychotic treatment, or may be a result of compensatory increase secondary to lipid peroxidation. Sarandol et al.17) found that antipsychotic treatment increased ARY levels in schizophrenic patients, supporting our first suggestion. Selek et al.32) suggests that increased antioxidant levels in obsessive-compulsive disorder patients may be a rebound response to oxidation. However, they also stated that this could be a result of the chronicity of this disorder. The above-mentioned mechanisms are consistent with our hypothesis that antipsychotic drug treatment increases ARY levels.
Limitations of our study include the cross sectional design, lack of a drug-naïve patient group, and its small sample size. Our study is significant in that it’s the first study to compare and evaluate patients suffering from schizophrenia, and who are on typical, atypical, or combined (typical and atypical) antipsychotic drug treatment.
Briefly and in summation, we found patients taking typical antipsychotics demonstrated higher MDA levels than other groups, suggesting a drug-related secondary increase in oxidative parameters or an inability of the drug to decrease oxidation. PON1 activity was similar in all groups, suggesting that antipsychotic drugs may increase PON1 activity. And finally, because ARY levels were higher in schizophrenic patients, the mechanism in effect may be a compensatory response to increased lipid peroxidation.
REFERENCES
- 1.Roh D, Chang JG, Yoon S, Kim CH. Antipsychotic prescribing patterns in first-episode schizophrenia: A five-year comparison. Clin Psychopharmacol Neurosci. 2015;13:275–282. doi: 10.9758/cpn.2015.13.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatasubramanian G. Understanding schizophrenia as a disorder of consciousness: biological correlates and translational implications from quantum theory perspectives. Clin Psychopharmacol Neurosci. 2015;13:36–47. doi: 10.9758/cpn.2015.13.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LT, Chen KC, Chang WH, Chen PS, Lee IH, Yang YK. Holistic consideration of patients with schizophrenia to improve medication adherence and outcomes. Clin Psychopharmacol Neurosci. 2015;13:138–143. doi: 10.9758/cpn.2015.13.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SJ, Shim JC, Kong BG, Kang JW, Moon JJ, Jeon DW, et al. The relationship between language ability and cognitive function in patients with schizophrenia. Clin Psychopharmacol Neurosci. 2015;13:288–295. doi: 10.9758/cpn.2015.13.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayakumari AA, John JP, Halahalli HN, Paul P, Thirunavukkarasu P, Purushottam M, et al. Effect of polymorphisms of three genes mediating monoamine signalling on brain morphometry in schizophrenia and healthy subjects. Clin Psychopharmacol Neurosci. 2015;13:68–82. doi: 10.9758/cpn.2015.13.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatasubramanian G, Debnath M. Neuroimmunological aberrations and cerebral asymmetry abnormalities in schizophrenia: Select perspectives on pathogenesis. Clin Psychopharmacol Neurosci. 2014;12:8–18. doi: 10.9758/cpn.2014.12.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camkurt MA, Acar Ş, Coşkun S, Güneş M, Güneş S, Yılmaz MF, et al. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatr Res. 2015;69:67–71. doi: 10.1016/j.jpsychires.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Hsu JW, Lirng JF, Wang SJ, Lin CL, Yang KC, Liao MH, et al. Association of thalamic serotonin transporter and interleukin-10 in bipolar I disorder: a SPECT study. Bipolar Disord. 2014;16:241–248. doi: 10.1111/bdi.12164. [DOI] [PubMed] [Google Scholar]
- 9.Camkurt MA, Karababa F, Erdal ME, Bayazıt H, Kandemir SB, Ay ME, et al. Investigation of dysregulation of several microRNAs in peripheral blood of schizophrenia patients. Clin Psychopharmacol Neurosci. 2016;14:256–260. doi: 10.9758/cpn.2016.14.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelamekam S, Nurjono M, Lee J. Regulation of interleukin-6 and leptin in schizophrenia patients: a preliminary analysis. Clin Psychopharmacol Neurosci. 2014;12:209–214. doi: 10.9758/cpn.2014.12.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camkurt MA, Fındıklı E, İzci F, Kurutaş EB, Tuman TC. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls. Psychiatr Res. 2016;238:81–85. doi: 10.1016/j.psychres.2016.01.075. [DOI] [PubMed] [Google Scholar]
- 12.Güneş M, Bulut M, Demir S, İbiloğlu AO, Kaya MC, Atlı A, et al. Diagnostic performance of increased prolidase activity in schizophrenia. Neurosci Lett. 2016;613:36–40. doi: 10.1016/j.neulet.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–106. [PubMed] [Google Scholar]
- 14.Juretić D, Tadijanović M, Rekić B, Simeon-Rudolf V, Reiner E, Baricić M. Serum paraoxonase activities in hemodialyzed uremic patients: cohort study. Croat Med J. 2001;42:146–150. [PubMed] [Google Scholar]
- 15.Précourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 16.Unsal C, Albayrak Y, Albayrak N, Kuloglu M, Hashimoto K. Reduced serum paraoxonase 1 (PON1) activity in patients with schizophrenia treated with olanzapine but not quetiapine. Neuropsychiatr Dis Treat. 2013;9:1545–1552. doi: 10.2147/NDT.S52463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarandol A, Kirli S, Akkaya C, Ocak N, Eroz E, Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J Psychopharmacol. 2007;21:857–863. doi: 10.1177/0269881107077609. [DOI] [PubMed] [Google Scholar]
- 18.Noto C, Ota VK, Gadelha A, Noto MN, Barbosa DS, Bonifácio KL, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210–216. doi: 10.1016/j.jpsychires.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Mackness MI, Arrol S, Mackness B, Durrington PN. Alloenzymes of paraoxonase and effectiveness of high-density lipoproteins in protecting low-density lipoprotein against lipid peroxidation. Lancet. 1997;349:851–852. doi: 10.1016/S0140-6736(05)61755-2. [DOI] [PubMed] [Google Scholar]
- 20.Mackness B, Durrington PN, Mackness MI. Human serum paraoxonase. Gen Pharmacol. 1998;31:329–336. doi: 10.1016/S0306-3623(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 21.Herken H, Uz E, Ozyurt H, Söğüt S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6:66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- 22.Bulut M, Selek S, Gergerlioglu HS, Savas HA, Yilmaz HR, Yuce M, et al. Malondialdehyde levels in adult attention-deficit hyperactivity disorder. J Psychiatry Neurosci. 2007;32:435–438. [PMC free article] [PubMed] [Google Scholar]
- 23.Grignon S, Chianetta JM. Assessment of malondialdehyde levels in schizophrenia: a meta-analysis and some methodological considerations. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:365–369. doi: 10.1016/j.pnpbp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Akyol O, Herken H, Uz E, Fadillioğlu E, Unal S, Söğüt S, et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:995–1005. doi: 10.1016/S0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 25.Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–1138. [PMC free article] [PubMed] [Google Scholar]
- 26.Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993;15:353–363. doi: 10.1016/0891-5849(93)90035-S. [DOI] [PubMed] [Google Scholar]
- 27.Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–175. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 28.Scottish Schizophrenia Research Group. Smoking habits and plasma lipid peroxide and vitamin E levels in never-treated first-episode patients with schizophrenia. Br J Psychiatry. 2000;176:290–293. doi: 10.1192/bjp.176.3.290. [DOI] [PubMed] [Google Scholar]
- 29.Behl C, Rupprecht R, Skutella T, Holsboer F. Haloperidol-induced cell death--mechanism and protection with vitamin E in vitro. Neuroreport. 1995;7:360–364. doi: 10.1097/00001756-199512000-00085. [DOI] [PubMed] [Google Scholar]
- 30.Park SW, Lee CH, Lee JG, Kim LW, Shin BS, Lee BJ, et al. Protective effects of atypical antipsychotic drugs against MPP(+)-induced oxidative stress in PC12 cells. Neurosci Res. 2011;69:283–290. doi: 10.1016/j.neures.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37:43–51. doi: 10.1016/S0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- 32.Selek S, Herken H, Bulut M, Ceylan MF, Celik H, Savas HA, et al. Oxidative imbalance in obsessive compulsive disorder patients: a total evaluation of oxidant-antioxidant status. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:487–491. doi: 10.1016/j.pnpbp.2007.10.002. [DOI] [PubMed] [Google Scholar]