Abstract
The burden of type 2 diabetes and its major complication cardiovascular disease is rapidly increasing worldwide. Understanding the underlying pathogenic mechanisms of these diseases is crucial to develop novel therapeutics. Recent work using genetic and biochemical methods in mouse models and human samples have identified disturbed calcium signalling and endoplasmic reticulum stress as emerging factors involved in the pathogenesis of many metabolic diseases. In this review, we will highlight the specific roles of calcium signalling and endoplasmic reticulum stress response in the development of insulin resistance and atherosclerosis.
Keywords: atherosclerosis, calcium, diabetes mellitus, insulin resistance, metabolic syndrome
Introduction
Calcium is an important second messenger in signal transduction pathways that regulate a wide variety of processes, including gene expression, protein synthesis, secretion, muscle contraction, metabolism and apoptosis [1]. Maintenance of calcium homoeostasis is of crucial importance in the proper functioning of cells, and its dysfunction is associated with many pathological conditions including metabolic disorders, neurological and heart diseases, and ageing [2–4]. Endoplasmic reticulum (ER) is the major calcium storage organelle, and its calcium content is essential for proper protein folding and transport. Protein folding in the ER is sensitive to various conditions, including increase in protein synthesis rate, altered luminal calcium and redox balance. These changes in equilibrium can lead to disrupted protein folding and cause accumulation of misfolded proteins in the ER, which is referred to as ER stress [5]. To cope with ER stress, cells have developed an adaptive signalling pathway termed the unfolded protein response (UPR) or ER stress response. The UPR is initially protective and serves to re-establish homoeostasis and alleviate ER stress through decreasing protein load and activating genes to increase folding capacity. However, under conditions of prolonged and severe ER stress, the UPR can trigger programmed cell death, or apoptosis [6].
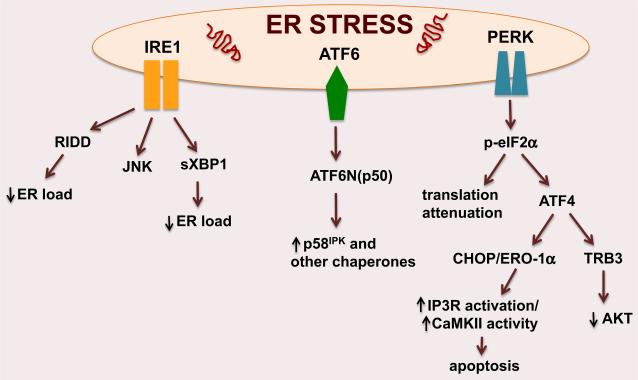
The UPR is triggered by three ER localized proteins, protein kinase RNA-like ER kinase (PERK), inositol requiring protein-1 (IRE1) and activating transcription factor 6 (ATF6) (Fig. 1). Upon sensing unfolded proteins in the ER lumen, IRE1 is activated through transautophosphorylation and then splices the mRNA encoding the transcriptional factor X-box-binding protein-1 (XBP1) which regulates ER-associated degradation (ERAD) and bio-genesis of ER in highly active secretory cells. IRE1 also limits client load of the ER through degrading certain mRNAs other than XBP1, a process known as regulated IRE1-dependent decay (RIDD) [7]. Additionally, through recruiting the adaptor protein TNF receptor-associated factor 2 (TRAF2), IRE1 can also activate JNK, which integrates the UPR with inflammatory and stress signalling [8]. Similar to IRE1, PERK undergoes autophosphorylation upon sensing unfolded proteins. Activated PERK phosphorylates eukaryotic translation initiation factor 2 alpha (eIF2α), which results in global translational attenuation and reduced ER protein load. Of note, phosphorylated eIF2α actually increases the translation of activating transcription factor 4 (ATF4), which induces the UPR effectors C/EBPα-homolog protein [(CHOP), also known as GADD153] and Tribbles homolog 3 (TRB3), an inhibitor of AKT [9]. Prolonged CHOP expression triggers apoptosis through its effects on intracellular calcium metabolism via ER oxidase-1α and by alterations in Bcl family members, whereas TRB3 links UPR with the ER stress-induced inhibition of insulin action and the resultant insulin resistance [10, 11]. Activation of ATF6 leads to its translocation to the Golgi, where it is cleaved by site 1 (S1P) and site 2 (S2P) proteases. The resultant N-terminal domain migrates to the nucleus and upregulates many ER chaperones including p58IPK [12, 13]. Collectively, these UPR branches act at the transcriptional, post-transcriptional and post-translational levels to resolve ER stress. However, chronic and persistent activation of the ER stress pathway leads to tissue dysfunction and contributes to the pathogenesis of many diseases, including insulin resistance and atherosclerosis [14].
Fig. 1.
Endoplasmic reticulum (ER) stress response pathways. Perturbations in ER homoeostasis activate three ER membrane localized proteins: IRE1, ATF6 and PERK. These ER stress transducers lower ER load through regulating several transcription factors such as XBP1s, p58IPK and CHOP. PERK, protein kinase RNA-like ER kinase; IRE1, inositol requiring protein-1; ATF6, activating transcription factor 6; RIDD, regulated IRE1-dependent decay; JNK, c-Jun N-terminal kinase; XBP1, X-box-binding protein-1; p58IPK, double-stranded RNA-activated protein kinase inhibitor; p-eIF2α, phosphorylatedeukaryotic translation initiation factor 2α; ATF4, activating transcription factor 4; CHOP, C/EBP homolog protein; ERO-1α, ER oxidase-1α; IP3R, inositol triphosphate receptor; CaMKII; calcium/calmodulin-dependent protein kinase II; Trb3, Tribbles homolog 3.
Insulin resistance
Type 2 diabetes and its complications constitute significant public health problems worldwide and are important causes of morbidity and mortality [15]. Whilst current treatment options for type 2 diabetes have beneficial impact on the disease and some of its complications, there is a great need for novel therapeutic approaches to improve long-term glycemic control and diabetes-related conditions [16]. A key factor in type 2 diabetes is an inappropriate increase in hepatic glucose production, which results from hepatic insulin resistance together with impaired suppression of glucagon signalling [17, 18]. Defective insulin signalling in hepatocytes, as well as in other cells, is a major feature of hepatic insulin resistance in human obesity [19, 20]. In hepatocytes, this defect disables the pathway that normally suppresses hepatic glucose production [21], and the resulting systemic hyperinsulinaemia excessively stimulates a nonresistant branch of the insulin signalling pathway that promotes hepatic lipid synthesis and storage, fatty liver and dyslipidaemia [22]. In addition to the metabolic disturbances in type 2 diabetes, there is a two- to fourfold increase in the lifetime risk of developing cardiovascular diseases, due in large part to atherogenic dyslipidaemia resulting from disturbances in hepatic lipid metabolism [23].
Work over the past decade has identified chronic ER stress and activation of UPR as a major mechanism contributing to the pathogenesis of obesity-induced insulin resistance in insulin-sensitive tissues and in pancreatic β-cells. Studies using obese mice showed an increase in the activation of the PERK–eIF2α–ATF4 and IRE1–JNK arms of ER stress, whereas XBP1 and ATF6 activation were suppressed in hepatocytes and β-cells of obese/diabetic mice, suggesting differential regulation of the UPR branches and impaired ER function in obesity [24–27]. In this context, mice with defective activation of the eIF2α-dependent arm of ER stress or mice overexpressing XBP1 and ATF6 in the liver were shown to be protected from the development of obesity-induced insulin resistance [24, 28]. In line with these findings, obese or type 2 diabetic mice overexpressing the ER chaperones glucose-regulated protein (Grp78) or oxygen-regulated protein (ORP150) were reported to be more insulin sensitive [29, 30]. Conversely, mice heterozygous for XBP1 or deficient in ATF6 were more glucose intolerant compared to their control littermates when challenged with a high-fat diet [27]. Moreover, weight loss and alleviation of ER stress by chemical chaperones or small-molecule compounds that improve ER protein-folding capacity have been shown to lower ER stress parameters and improve glucose homoeostasis in animal models and obese humans, demonstrating the importance of ER function in glucose homoeostasis [31–34].
During the progression of type 2 diabetes, β-cells adapt their secretory capacity to maintain normoglycaemia and compensate peripheral insulin resistance by hyperinsulinaemia which places a continuous demand on their ER for proper protein synthesis, folding and secretion. However, when the folding capacity of the ER is exceeded, β-cells undergo apoptosis that leads to impaired insulin secretion and marked hyperglycaemia. In this context, recent work has shown that the decline in the expression of ATF6 in the β-cells of experimental mouse models and human samples correlates with a diabetic phenotype [26]. In line with these findings, mice deficient in β-cell XBP1 displayed hyperglycaemia and glucose intolerance resulting from decreased insulin secretion from β-cells, indicating that a decline in β-cell ER function underlies the progression of type 2 diabetes [35]. Moreover, in mouse models of type 2 diabetes, ablation of the proapoptotic Chop gene was shown to result in expanded β-cell mass through protecting against oxidative stress in response to ER stress and improved glycemic control [36]. Thus, ER stress also contributes to development of type 2 diabetes although its effects on β-cells.
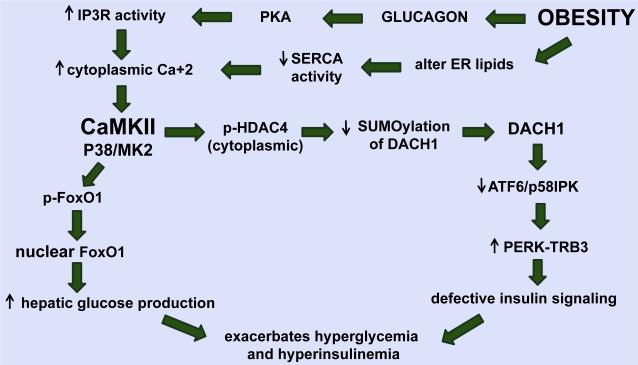
We have recently shown that aberrant glucagon action in hepatocytes in obesity, through protein kinase A-mediated phosphorylation and activation of the ER calcium channel, inositol 1,4,5-trisphosphate receptor (IP3R), promotes excessive calcium release into the cytoplasm [37]. Altered phospholipid content of the ER membrane resulting in dysfunctional sarco(endo)plasmic reticulum calcium-adenosine triphosphatase (SERCA) likely amplifies these events [38, 39]. The resultant increase in cytoplasmic calcium promotes activation of a calcium-sensitive kinase, calcium/calmodulin-dependent protein kinase II (CaMKII) [40] (Fig. 2). CaMKII is a ubiquitously expressed serine/threonine kinase, which is an important mediator of calcium signalling in cells. Our work showed that in response to fasting, CaMKII activates p38 and MAPKAPK2 (MK2) in hepatocytes, which leads to nuclear translocation of FoxO1. FoxO1 induces key genes that increase hepatic glucose production, G6pc (glucose-6-phosphatase) and Pck1 (phosphoenolpyruvate carboxykinase) [41]. Intriguingly, the same CaMKII–FoxO1 pathway is also conserved in Caenorhabditis elegans, where the CaMKII ortholog UNC-43 phosphorylates the FoxO homolog DAF-16 and promotes its nuclear localization and transcriptional activity [42]. In addition to regulating hepatic glucose production, the same CaMKII–p38–MK2 pathway also perturbs hepatocyte insulin receptor signalling and systemic insulin sensitivity [43]. As such, genetic targeting of hepatic CaMKII greatly improves insulin-induced Akt phosphorylation and increases insulin sensitivity. This improvement is not associated with increased tyrosine phosphorylation and activation of the insulin receptor (IR) or insulin receptor substrate (IRS) proteins. Rather, the improvement begins with induction of the ATF6 branch of ER stress, and the ATF6 target p58IPK, a chaperone that suppresses the PERK–p-eIF2α–ATF4 branch. The result is a decrease in the ATF4 target, TRB3, an inhibitor of insulin-induced p-Akt, leading to enhanced insulin receptor signalling. Additionally, in line with an improvement in hyperinsulinaemia, genetic targeting of CaMKII suppresses fatty liver formation and lowers plasma triglyceride levels. Finally, drug-mediated inhibition of the above CaMKII–p38–MK2 pathway improves metabolism in obese, insulin-resistant mice in a manner that is mechanistically ‘on target’ with regard to the above pathway and, importantly, is additive with the current leading type 2 diabetes drug, metformin [44].
Fig. 2.
Illustration showing how the CaMKII–MK2 pathway may exacerbate hyperglycaemia and hyperinsulinaemia in obesity. Obesity-induced activation of CaMKII increases hepatic glucose production through regulating the nuclear localization of FoxO1. Activated CaMKII also alters hepatic insulin signalling through HDAC4–DACH1-mediated activation of the ATF4–TRB3 pathway which further exacerbates hyperglycaemia and hyperinsulinaemia. PKA, protein kinase A; IP3R, inositol triphosphate receptor; SERCA, sarco(endo)plasmic reticulum calcium-adenosine triphosphatase; CaMKII, calcium/calmodulin-dependent protein kinase II; p38, p38 mitogen-activated protein kinase; MK2, mitogen-activated protein kinase-activated protein kinase 2; FoxO1, forkhead box protein O1; p-HDAC4, phosphorylated-histone deacetylase 4; DACH1, dachshund homolog 1; ATF6, activating transcription factor 6; p58IPK, double-stranded RNA-activated protein kinase inhibitor; protein kinase RNA-like ER kinase; Trb3, Tribbles homolog 3.
ATF6 can act as a homoeostatic regulator by inducing the chaperone p58IPK. Both total and nuclear (cleaved) ATF6 levels are decreased in obese mouse liver, and ATF6 cleavage is inversely related to HOMA-IR in human liver, consistent with a protective role of ATF6 in insulin resistance [25, 45]. As with p58IPK induction and TRB3 suppression, ATF6 activation may have additional and independent beneficial effects in obesity and type 2 diabetes. In particular, Montminy and colleagues have provided evidence that ATF6 could suppress hepatic glucose production through disruption of the CREB–CRTC2 interaction and that restoration of ATF6 levels specifically in the livers of obese mice to WT levels improves glucose tolerance [25]. Moreover, ATF6 overexpression in cell culture models was shown to improve insulin signalling, although the underlying mechanisms have not been fully explored [46]. In addition, two independent groups reported an association between variants of the ATF6 gene and disturbed glucose homoeostasis and type 2 diabetes in Pima Indians and Dutch Caucasians [47, 48]. In this context, we recently found in hepatocytes of obese mice that CaMKII phosphorylates and blocks nuclear translocation of histone deacetylase 4 (HDAC4), which results in decreased SUMOylation and degradation of the corepressor Dachshund homolog 1 (DACH1) (Fig. 2). The resulting elevation of nuclear DACH1 represses Atf6, which activates the PERK–ATF4–TRB3 branch of the ER stress response, leading to defective insulin signalling as explained above [49]. Specifically, we showed that livers from obese mice had higher levels of p-Ser632- and p-Ser467 HDAC4 compared with livers from lean mice, and nuclear HDAC4 levels were significantly lower in the livers of obese mice. In this regard, a recent study using an unbiased proteomics approach showed that obesity in humans is associated with a decrease in HDAC4, which improves with physical exercise, suggesting the possibility that nuclear HDAC4 is protective in obesity [50]. As a direct link to CaMKII, we found that the livers of CaMKII-deficient obese mice had lower p-HDAC4 and higher nuclear HDAC4 levels, and siRNA-mediated silencing of HDAC4 decreased Atf6 mRNA levels and abrogated the improvement in insulin signalling in CaMKII-deficient hepatocytes. Moreover, treatment of primary hepatocytes and obese mice with an adenovirus encoding a phosphorylation-defective, constitutively nuclear HDAC4 mutant, HDAC4S3A (246A/S467A/S632A) [51], enhanced insulin-stimulated Akt phosphorylation and lowered fasting blood glucose and plasma insulin levels. These collective data demonstrate a causal link between the proposed upstream role of HDAC4 and the key functional end-point of the CaMKII pathway, hepatocyte insulin signalling. In search for the mechanism, we made the observation that HDAC4 decreases the SUMOylation and degradation of the corepressor DACH1. DACH1 protein levels were increased in the livers of both genetic and diet-induced obese mice compared with lean control mice, and the increase in DACH1 levels in liver of obese mice was significantly suppressed when CaMKII was deleted or when obese mice were treated with adeno-HDAC4S3A to enforce nuclear HDAC4. The ability of DACH1 to repress Atf6 requires its interaction with nuclear receptor corepressor 1 (NCOR), as NCOR-silenced obese mice showed increased Atf6 mRNA levels in liver. Importantly, DACH1 protein level was increased by palmitate treatment of primary human hepatocytes, and analysis of human liver biopsy specimens from subjects with body mass index (BMI) values ranging from 19 to 62 kg m−2 showed a robust correlation between BMI and DACH1 protein levels. These findings reveal a new role for DACH1 in obesity-induced glucose intolerance and insulin resistance in mice, with a striking correlation between liver DACH1 level and obesity in humans.
Atherosclerosis
Atherothrombotic cardiovascular disease is the leading cause of death in the industrialized world, particularly in patients with type 2 diabetes. Whilst potent low-density lipoprotein (LDL) -lowering drugs reduce the risk for cardiovascular disease, the increasing age of the world population and the ongoing epidemic of obesity and type 2 diabetes have offset their benefits [23]. On the other hand, recent regulations require that new medications developed for type 2 diabetes are not associated with adverse cardiovascular effects [52]. Therefore, ideal therapies would achieve euglycaemia and reduce cardiovascular complications.
Studies over the past decade identified subendothelial retention of lipoproteins and a nonresolving inflammatory response as the key contributors to atherogenesis [53]. The major immune cell type in atherosclerotic lesions, the macrophage, is considered to play a key inflammatory role during atherogenesis. As lesions progress, dead and dying macrophages contribute to the development of a necrotic core, which further promotes inflammation, plaque instability and thrombosis in advanced atherosclerotic lesions [54]. Necrotic core development in late lesions is thought to occur through a combination of postapoptotic necrosis and inefficient efferocytosis of apoptotic macrophages, which is a defect known to occur in advanced human lesions [55, 56]. Necrotic cores, which are larger in diabetic plaques, promote plaque instability, probably because they are a reservoir of inflammatory mediators, matrix pro-teases and thrombotic molecules [57]. Therefore, macrophage apoptosis is a key event in converting benign lesions to an unstable phenotype by promoting necrotic core formation.
ER stress has recently been identified as an emerging factor that is relevant to many systemic and arterial wall factors involved in atherosclerosis [58]. Atherosclerotic lesional cells in general and macrophages in particular were shown to display markers of ER stress during the progression of lesions [59, 60]. Causation studies using atherosclerosis-prone mice lacking CHOP, or restoration of ER function although chemical chaperones, showed suppression of macrophage apoptosis and plaque necrosis, demonstrating the link between macrophage ER stress and advanced atheroma [61–65]. Likewise, in human coronary atherosclerotic lesions, there is a strong correlation amongst CHOP expression, apoptosis and advanced plaque stage [66].
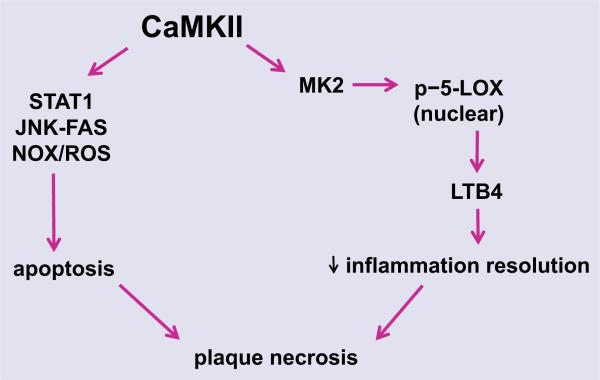
Another consequence of heightened CHOP expression in macrophages is increased cytosolic calcium. This process is initiated by CHOP-mediated induction of the ER oxidase ERO1α, which then activates the ER calcium release channel IP3R [10]. Increased cytosolic calcium is likely augmented by lipid-mediated SERCA inactivation [67]. The released calcium activates CaMKII, which in turn triggers downstream apoptosis pathways, including activation of proapoptotic signal transducer and activator of transcription-1 (STAT1), JNK-mediated induction of the FAS death receptor, permeabilization of the outer mitochondrial membrane and release of cytochrome c, and induction of NADPH oxidase-mediated ROS, which also amplifies CHOP through the upstream kinase PKR [68–70] (Fig. 3). Moreover, in view of the evidence for defective inflammation resolution in atherosclerosis [71], we recently reported that CaMKII promotes MK2-mediated 5-LOX phosphorylation and nuclear localization, which in turn leads to the synthesis of proinflammatory leukotriene B4 production [72]. This is particularly important because therapies that enhance resolution in atherosclerosis have the potential to suppress inflammation and promote tissue repair without being immunosuppressive [53]. In this regard, mice lacking CaMKII in macrophages have less plaque necrosis, suggesting that the benefit of CaMKII inhibition may, in part, be mediated by enhanced inflammation resolution (A. Doran, L. Ozcan, et al. unpublished data).
Fig. 3.
Pathways through which macrophage CaMKII promotes plaque necrosis. Activation of CaMKII in macrophages regulates apoptosis through inducing STAT1, JNK–FAS and NOX/ROS pathways. CaMKII also suppresses inflammation resolution through 5-LOX-mediated leukotriene B4 (LTB4) generation. CaMKII, calcium/calmodulin-dependent protein kinase II; STAT1, signal transducer and activator of transcription 1; JNK, c-Jun N-terminal kinase; FAS, TNF receptor superfamily member 6; NOX, NADPH oxidase; ROS, reactive oxygen species; MK2, mitogen-activated protein kinase-activated protein kinase 2; p-5-LOX, phosphorylated-5-lipoxygenase; LTB4, leukotriene B4.
As discussed above, atherosclerosis is the most important complication of type 2 diabetes. In this regard, insulin resistance has been suggested to promote atherogenesis through activating proatherogenic ER stress pathways in macrophages. In one scenario, downregulation of insulin receptor signalling in macrophages caused by hyperinsulinaemia promotes ER stress and apoptosis through elevation of cytosolic calcium via SERCA inhibition [73, 74]. Additionally, elevated levels of free fatty acids associated with insulin resistance trigger ER stress-induced apoptosis of macrophages through multiple mechanisms [62, 75]. Finally, obesity and insulin resistance impair the ability of macrophages to carry out efferocytosis and lead to secondary necrosis as demonstrated in studies using ob/ob; Ldlr−/− mice [76]. Collectively, these results demonstrate the link between enhanced atherosclerosis development and insulin resistance states.
Conclusions
The incidence of obesity and of its associated diseases, notably diabetes and atherosclerosis, have increased dramatically over the past decade and constitute a global threat to human health. Studies in the last several years, using experimental animal models and human specimens, have identified disturbed calcium signalling and ER stress as upstream pathogenic pathways that contribute to multiple pathological processes in different cell types in metabolic diseases. Markers of ER stress were upregulated in both insulin-sensitive tissues in models of type 2 diabetes and in atherosclerotic plaques. Moreover, strategies aimed at relieving ER stress have shown beneficial effects in experimental mouse models. Therefore, therapeutic targeting of these pathways may provide a new avenue of treatment. Nonetheless, these upstream pathways have important functions in many biological processes, and thus, further work is required to understand their physiological roles in order to prevent side effects and effectively improve treatment of metabolic diseases.
Footnotes
Content List – Read more articles from the symposium: 12th Key Symposium - Insulin Resistance in Common Diseases.
Conflict of interest statement
No conflict of interest was declared.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–50. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 3.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 4.Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–73. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 5.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–31. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–8. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 9.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–55. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Mongillo M, Chin KT, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–92. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–7. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 12.van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J Biol Chem. 2003;278:15558–64. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 13.Ye J, Rawson RB, Komuro R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–64. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 14.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–28. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adeshara KA, Diwan AG, Tupe RS. Diabetes and Complications: Cellular Signaling Pathways, Current Understanding and Targeted Therapies. Curr Drug Targets. 2016;17:1309–28. doi: 10.2174/1389450117666151209124007. [DOI] [PubMed] [Google Scholar]
- 16.Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999-2010. N Engl J Med. 2013;369:287–8. doi: 10.1056/NEJMc1306652. [DOI] [PubMed] [Google Scholar]
- 17.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajvani UB, Accili D. The new biology of diabetes. Diabetologia. 2015;58:2459–68. doi: 10.1007/s00125-015-3722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47:1281–6. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- 20.Brozinick JT, Jr, Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 2003;52:935–41. doi: 10.2337/diabetes.52.4.935. [DOI] [PubMed] [Google Scholar]
- 21.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 22.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–6. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am. 2008;37:603–21. viii. doi: 10.1016/j.ecl.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Calay ES, Fan J, et al. METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 2015;349:500–6. doi: 10.1126/science.aaa0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–7. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engin F, Nguyen T, Yermalovich A, Hotamisligil GS. Aberrant islet unfolded protein response in type 2 diabetes. Sci Rep. 2014;4:4054. doi: 10.1038/srep04054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 28.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–32. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Investig. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozawa K, Miyazaki M, Matsuhisa M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–63. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 31.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kars M, Yang L, Gregor MF. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu S, Yalcin A, Lee GY, et al. Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Sci Transl Med. 2015;7:292ra98. doi: 10.1126/scitranslmed.aaa9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci USA. 2011;108:8885–90. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Investig. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Li G, Goode J, et al. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485:128–32. doi: 10.1038/nature10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2 + -ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci USA. 2010;107:19320–5. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozcan L, Wong CC, Li G, et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 2012;15:739–51. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–6. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 42.Tao L, Xie Q, Ding YH, et al. CAMKII and calcineurin regulate the lifespan of Caenorhabditis elegans through the FOXO transcription factor DAF-16. Elife. 2013;2:e00518. doi: 10.7554/eLife.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozcan L, Cristina de Souza J, Harari AA, Backs J, Olson EN, Tabas I. Activation of calcium/calmodulin-dependent protein kinase II in obesity mediates suppression of hepatic insulin signaling. Cell Metab. 2013;18:803–15. doi: 10.1016/j.cmet.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozcan L, Xu X, Deng SX, et al. Treatment of obese insulin-resistant mice with an allosteric MAPKAPK2/3 inhibitor lowers blood glucose and improves insulin sensitivity. Diabetes. 2015;64:3396–405. doi: 10.2337/db14-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2011;108:16381–5. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang X, Shen H, Chen J, et al. Activating transcription factor 6 protects insulin receptor from ER stress-stimulated desensitization via p42/44 ERK pathway. Acta Pharmacol Sin. 2011;32:1138–47. doi: 10.1038/aps.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meex SJ, van Greevenbroek MM, Ayoubi TA, et al. Activating transcription factor 6 polymorphisms and haplotypes are associated with impaired glucose homeostasis and type 2 diabetes in Dutch Caucasians. J Clin Endocrinol Metab. 2007;92:2720–5. doi: 10.1210/jc.2006-2280. [DOI] [PubMed] [Google Scholar]
- 48.Thameem F, Farook VS, Bogardus C, Prochazka M. Association of amino acid variants in the activating transcription factor 6 gene (ATF6) on 1q21-q23 with type 2 diabetes in Pima Indians. Diabetes. 2006;55:839–42. doi: 10.2337/diabetes.55.03.06.db05-1002. [DOI] [PubMed] [Google Scholar]
- 49.Ozcan L, Ghorpade DS, Zheng Z, et al. Hepatocyte DACH1 is increased in obesity via nuclear exclusion of HDAC4 and promotes hepatic insulin resistance. Cell Rep. 2016;15:2214–25. doi: 10.1016/j.celrep.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu-Farha M, Tiss A, Abubaker J, et al. Proteomics analysis of human obesity reveals the epigenetic factor HDAC4 as a potential target for obesity. PLoS One. 2013;8:e75342. doi: 10.1371/journal.pone.0075342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–64. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azim S, Baker WL, White WB. Evaluating cardiovascular safety of novel therapeutic agents for the treatment of type 2 diabetes mellitus. Curr Cardiol Rep. 2014;16:541. doi: 10.1007/s11886-014-0541-0. [DOI] [PubMed] [Google Scholar]
- 53.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–79. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 56.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–95. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–85. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou AX, Tabas I. The UPR in atherosclerosis. Semin Immunopathol. 2013;35:321–32. doi: 10.1007/s00281-013-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorp E, Iwawaki T, Miura M, Tabas I. A reporter for tracking the UPR in vivo reveals patterns of temporal and cellular stress during atherosclerotic progression. J Lipid Res. 2011;52:1033–8. doi: 10.1194/jlr.D012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–21. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 61.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–81. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukano H, Gotoh T, Endo M, et al. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2010;30:1925–32. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 64.Chung J, Kim KH, Lee SC, An SH, Kwon K. Ursodeoxycholic acid (UDCA) exerts anti-atherogenic effects by inhibiting endoplasmic reticulum (ER) stress induced by disturbed flow. Mol Cells. 2015;38:851–8. doi: 10.14348/molcells.2015.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong Y, Zhang M, Liang B, et al. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myoishi M, Hao H, Minamino T, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–33. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Ge M, Ciani L, et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–9. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 68.Timmins JM, Ozcan L, Seimon TA, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim WS, Timmins JM, Seimon TA, et al. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–51. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–25. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fredman G, Ozcan L, Spolitu S, et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc Natl Acad Sci USA. 2014;111:14530–5. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han S, Liang CP, DeVries-Seimon T, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–66. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Liang CP, Han S, Li G, Tabas I, Tall AR. Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment. Diabetes. 2012;61:2609–20. doi: 10.2337/db11-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–82. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S, Sun Y, Liang CP, et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–82. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]