Abstract
Adequate zinc stores in the body are extremely important during periods of accelerated growth. However, zinc deficiency is common in developing countries and low maternal circulating zinc concentrations have previously been associated with pregnancy complications. We reviewed current literature assessing circulating zinc and dietary zinc intake during pregnancy and the associations with preeclampsia (PE); spontaneous preterm birth (sPTB); low birthweight (LBW); and gestational diabetes (GDM). Searches of MEDLINE; CINAHL and Scopus databases identified 639 articles and 64 studies were reviewed. In 10 out of 16 studies a difference was reported with respect to circulating zinc between women who gave birth to a LBW infant (≤2500 g) and those who gave birth to an infant of adequate weight (>2500 g), particularly in populations where inadequate zinc intake is prevalent. In 16 of our 33 studies an association was found between hypertensive disorders of pregnancy and circulating zinc; particularly in women with severe PE (blood pressure ≥160/110 mmHg). No association between maternal zinc status and sPTB or GDM was seen; however; direct comparisons between the studies was difficult. Furthermore; only a small number of studies were based on women from populations where there is a high risk of zinc deficiency. Therefore; the link between maternal zinc status and pregnancy success in these populations cannot be established. Future studies should focus on those vulnerable to zinc deficiency and include dietary zinc intake as a measure of zinc status.
Keywords: zinc, pregnancy, pregnancy complications, dietary zinc intake, circulating zinc
1. Introduction
Adequate maternal nutrition, particularly before and during pregnancy, is imperative to the health of both the mother and child [1,2]. Poor nutrition in pregnancy may lead to inappropriate nutrient partitioning between the mother and fetus, which can be deleterious to the health of both [3]. Each year, 3.5 million deaths in women and children are attributed to undernutrition [4]. Zinc deficiency is predicted to be responsible for 1% of all deaths globally and 4.4% of deaths in children aged 6 months to 5 years [5]. The World Health Organization (WHO) prioritized minimizing zinc deficiency in developing nations as part of the Millennium Development Goal 1: to eradicate extreme poverty and hunger [6]. Therefore, understanding the effects of zinc deficiency on pregnancy and fetal growth is very important.
Zinc is an essential component of over 1000 proteins including antioxidant enzymes, metalloenzymes, zinc-binding factors and zinc transporters. These are required for a variety of biological processes including carbohydrate and protein metabolism, DNA and RNA synthesis, cellular replication and differentiation, and hormone regulation [7,8,9,10]. The importance of zinc to the growth of the fetus is demonstrated by the active transport of zinc across the placenta into the fetal circulation resulting in higher cord blood concentrations compared to those in the maternal circulation [11,12,13,14]. Rodent models of severe maternal zinc deficiency show increased rates of fetal loss and congenital malformations in the surviving fetuses [15] as well as reduced fetal growth [16,17,18], lower implantation rates and impaired placental growth [19], all highlighting the teratogenic effects of zinc deficiency in pregnancy.
Diet is the main factor that determines zinc status [20]. In the United States and Australia, an additional 2–4 mg zinc per day is recommended for pregnant women compared to non-pregnant women [21,22]. It is widely acknowledged that many pregnant women do not meet this recommendation [23,24,25], particularly in developing countries where diets are often plant-based. Grains and legumes contain a significant amount of phytic acid and phytate binding of zinc limits its absorption in the small intestine, contributing to zinc deficiency [22]. Estimates based on bioavailability of zinc, physiological requirements and predicted zinc absorption suggest the prevalence of zinc deficiency to range from 4% (European countries including the United Kingdom, Sweden, Germany and France) to 73% in Bangladesh, India and Nepal [26]. A more recent evaluation, based on similar estimates, also predicted inadequate zinc intakes in over 25% in populations in Southeast Asia and Africa [27].
A recent Cochrane review assessed the effects of zinc supplementation versus no supplementation (with or without placebo) on the success of pregnancy in 21 randomized controlled trials (RCTs) [28]. It was concluded that zinc supplementation reduced the risk of spontaneous preterm birth (sPTB) by 14% (RR: 0.86, 95% CI: 0.76–0.97; 16 RCTs) but there was no effect on other outcomes such as stillbirth/neonatal death, birthweight and pregnancy-induced hypertension [28]. However, this review did not include the effects of zinc supplementation on reducing the risk of gestational diabetes (GDM) and analysis of maternal circulating zinc concentrations provides evidence that low maternal zinc may be associated with GDM, as well as preeclampsia (PE), gestational hypertension (GH), sPTB and infant birthweight [24,29]. The association between serum zinc and PE has been reviewed recently [30] but there has been no extensive review that has assessed maternal zinc concentrations with respect to a range of pregnancy complications. Here, we review the current literature based on observational studies assessing the association between maternal zinc status and a number of pregnancy complications in order to determine whether maternal circulating zinc or dietary zinc intake are important factors associated with pregnancy outcome.
2. Materials and Methods
2.1. Eligibility Criteria
Studies included human prospective cohorts, case-control, longitudinal and cross-sectional studies assessing maternal circulating zinc concentrations and pregnancy complications including PE, eclampsia, GH, GDM, small for gestational age (SGA), intrauterine growth restriction (IUGR; ˂10th percentile), low-birthweight (LBW; ≤2500 g) and sPTB. Only studies that measured maternal circulating zinc during pregnancy or at delivery and/or dietary zinc intake at these times were included. Studies that assessed zinc concentrations in placenta, amniotic fluid, in offspring (post-natally), cord blood only and breast milk were excluded. There were no restrictions imposed on age of women included in the studies or on any other population characteristic such as race or body mass index (BMI). Given the heterogeneity of the observational strategies, a meta-analysis was not possible.
2.2. Information Sources and Search
The search strategy and procedure was guided by the PRISMA statement [31]. Potential studies were located through electronic databases (Ovid Medline (1946–present), CINAHL (1937–present) and Scopus (1995–present)), as well as manual searches of references in review articles and relevant articles known by the authors. Limits included full text articles written in English and published in academic journals. The last search was performed on 25 August 2016. Search terms and MeSH headings in the title, abstract, and index terms, were initially identified in Medline and subsequent key words were used for the remaining databases (Appendix A). Briefly, the search included the following: zinc; dietary zinc; zinc intake; plasma zinc; serum zinc; preeclampsia; eclampsia; gestational hypertension; gestational diabetes mellitus; fetal macrosomia; small for gestational age; intrauterine growth restriction; low birthweight; preterm birth.
2.3. Data Collection
An independent search of the literature was performed in April 2015 and again in August 2016. Titles and abstracts were examined independently by two of the authors who documented reasons for excluding full text articles. Any differences between the two reviewers were clarified; a third reviewer resolved any disagreements. If an article appeared in duplicate from two or three of the databases, only the search containing the most relevant and useful information was included. For each eligible study, the following data was extracted: author, year and country of publication; inclusion/exclusion criteria; sample size; zinc measure including sample type, collection time during pregnancy and method of analysis and pregnancy outcome. Most studies did not report on exclusion/inclusion criteria; these were therefore not included in the results table. Values determining zinc status were all converted to μg/L for easier comparisons between studies (Appendix B).
3. Results
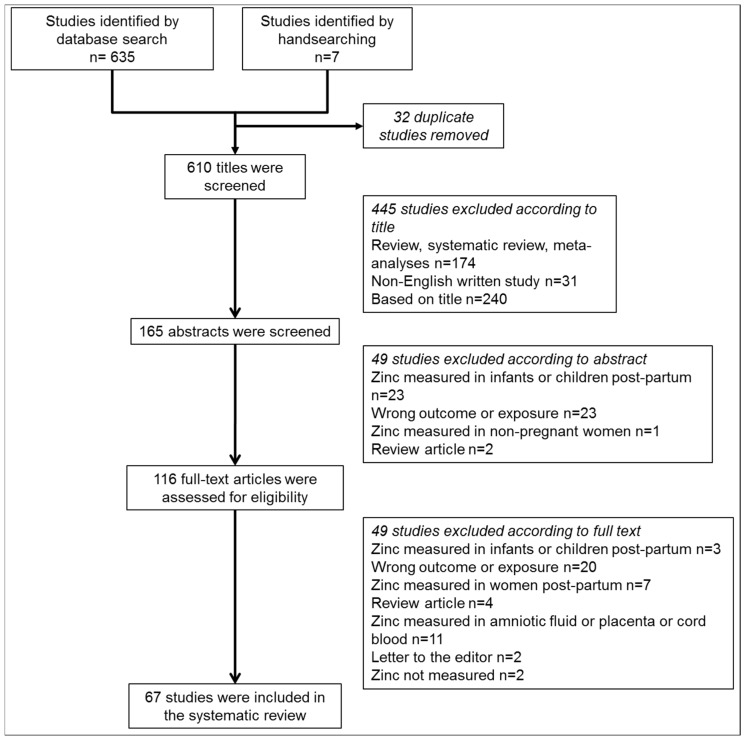
Figure 1 outlines the literature search and selection of studies. We identified 635 citations after searching Medline (OVID), CINAHL and Scopus databases. A further seven were added by the authors. After screening the title and abstract, 116 full text papers were read. Of these, 67 studies met the inclusion criteria, including 29 on SGA/LBW (Table 1), 34 on hypertensive disorders of pregnancy (Table 2), 11 on sPTB (Table 3) and 9 on GDM (Table 4). Eleven studies assessed multiple pregnancy outcomes and are included in the relevant pregnancy outcome tables. Table 5 summarizes all included studies and whether there was a positive, negative or no association between zinc status and the pregnancy complication. The included studies were tabulated based on those that measured dietary zinc intake, then those that measured serum/plasma zinc. Globally, the average percentage of people affected by inadequate zinc intake is estimated to be 17.3% [27]. As dietary consumption of zinc is most influential on zinc status, studies that measured circulating zinc were further categorized based on whether they sampled from countries where inadequate zinc intake has been predicted to affect <17% or ≥17% of the population. We did not limit the studies to a specific period during gestation when zinc was measured and this information was not provided in eight studies [32,33,34,35,36,37,38,39]. However, zinc concentrations decline across gestation due to a combination of factors including hemodilution and increased fetal demand [40,41] and this made direct comparison of the studies difficult.
Figure 1.
Flow diagram of the search strategy used in this review including the relevant number of papers at each point.
Table 1.
Included studies assessing maternal zinc status and birthweight.
| Author, Country | Sample Size | Zinc Measure | Outcome of the Study |
|---|---|---|---|
| (1) Sample Type | |||
| (2) Time at Which Gestation Diet Was Assessed or Sample Collected | |||
| (3) Method of Analysis | |||
| [42] Simmer, United Kingdom a | 28 SGA 29 uncomplicated |
Dietary zinc intake Third trimester of pregnancy 7 day dietary recall |
↓ mean (SEM) dietary intake in the SGA mothers compared to the women with uncomplicated pregnancies. SGA: 11.3 (0.5) vs. uncomplicated: 13.0 (0.6) mg/day, p < 0.05 |
| [43] Negandhi, India b | 144 LBW 240 uncomplicated |
Dietary zinc intake 26–30 weeks 24 h dietary recall |
↓ mean dietary zinc intake in women with a LBW infant compared to those with an uncomplicated pregnancy. LBW: 5.39 mg/day vs. uncomplicated 6.77 mg/day, p < 0.001 |
| [44] Scholl, United States c | 115 with zinc intake ≤6 mg/day 699 with zinc intake ˃6 mg/day |
Dietary zinc intake 28 and 36 weeks 24 h dietary recall |
2-fold ↓ risk of delivering a LBW infant with dietary zinc intake ˃6 mg/day. OR: 2.01, 95% CI: 1.11–3.66 |
| [45] Neggers, United States d | 180 LBW 1218 uncomplicated |
Dietary zinc intake 18 and 30 weeks 24 h dietary recall using the nutrient data base developed by the University of Minnesota |
NS association between low dietary zinc intake (less than median) and risk of LBW. OR: 1.4, 95% CI: 0.9–2.1 |
| Inadequate dietary zinc intake estimated to affect <17% of the studied population | |||
| [46] Wang, China b | 247 with serum zinc <560 µg/L 2940 with serum zinc ≥560 µg/L |
Fasting serum zinc Across gestation Flame AAS |
↑ incidence of LBW in the mothers with serum zinc <560 µg/L compared to those with serum zinc ≥560 µg/L. Adjusted RR: 3.41, 95% CI: 1.97, 5.91 |
| [47] Voss Jepsen, Denmark a | 10 SGA 30 uncomplicated |
Heparin plasma zinc Collected at 35–41 weeks AAS |
↑ mean (SD) plasma zinc between SGA mothers and those with uncomplicated pregnancies. SGA: 732 (85) vs. uncomplicated: 654 (78) μg/L, p = 0.03 |
| [48] Borella, Italy a | 16 SGA 35 uncomplicated |
Heparin plasma zinc Collected in the third trimester Flame AAS |
↑ mean (SD) plasma zinc in SGA women compared to women with uncomplicated pregnancies. SGA: 685.6 (119.6) vs. uncomplicated: 627.5 (150) µg/L, p < 0.001 |
| [49] Neggers, USA e | 39 LBW 437 uncomplicated |
Serum zinc Collected across gestation Flame AAS |
8-fold ↑ prevalence of LBW with serum zinc in the lowest quartile (457.5–797.4 µg/L) compared to the highest (1039.2–1660.1 µg/L). OR: 8.2, 95% CI:2.4–27.5 |
| [50] Bro, Denmark a | 47 SGA and 34 preterm 220 uncomplicated |
Serum zinc Collected at delivery Flame AAS |
NS mean (SD) serum zinc levels between SGA and women with uncomplicated pregnancies. SGA: 764.7 (119.6) vs. uncomplicated: 679.7 (98) µg/L |
| [38] Hyvonen-Dabek, Finland f | 4 SGA 10 uncomplicated |
Serum zinc Collection time not specified Particle induced X-ray emission |
NS mean (SD) serum zinc in SGA women compared to those with uncomplicated pregnancies. SGA: 1270 (320) vs. uncomplicated: 1150 (220) µg/L |
| [51] Mistry, UK a* | 19 SGA 107 uncomplicated |
Heparin plasma zinc Collected at 28–32 weeks Inductively coupled plasma mass spectrometry |
NS in mean (95% CI) plasma zinc between SGA women and those with uncomplicated pregnancies. SGA: 708.1 (510.4–905.8) vs. uncomplicated: 634.4 (580.5–688.2) μg/L |
| [52] Tamura, USA g | 80 SGA 80 uncomplicated |
Serum zinc Collected at 18 weeks and 30 weeks Flame AAS |
NS in mean (SD) plasma zinc between SGA and women with uncomplicated pregnancies at 18 weeks. SGA: 627 (118) vs. uncomplicated: 667 (98) µg/L NS in mean (SD) plasma zinc between SGA and women with uncomplicated pregnancies at 30 weeks. SGA: 562 (92) vs. uncomplicated: 575 (92) µg/L |
| [53] Tamura, USA a | 139 SGA 2038 uncomplicated |
Non-fasting heparin plasma zinc Collected at first prenatal visit (6 to 34 weeks) Flame AAS |
NS in the prevalence (n (%)) of SGA measured between the lowest quartile and upper 3 quartiles of zinc. Highest: 103 (4.4) vs. lowest: 36 (4.8) |
| [54] Ghosh, China a | 22 SGA 38 uncomplicated |
Serum zinc Collected within 24 h of delivery AAS |
NS in mean (SD) serum zinc levels between SGA and women with uncomplicated pregnancies. SGA: 508.1 (185.9) vs. uncomplicated: 542.3 (162.8) μg/L |
| [55] Cherry, USA b | 29 LBW 230 uncomplicated |
Heparin plasma zinc Collected across gestation AAS |
NS mean (SEM) plasma zinc in mothers with a LBW infant compared to mothers with uncomplicated pregnancies. LBW: 604.9 (22.4) vs. uncomplicated: 577.2 (7.7) μg/L |
| [56] Bogden, USA h | 22 LBW 50 uncomplicated |
EDTA plasma zinc Collected at delivery Flame AAS |
NS mean (SEM) plasma zinc in women with a LBW infant compared to women with uncomplicated pregnancies. LBW: 640 (20) vs. uncomplicated: 620 (20) µg/L |
| Inadequate dietary zinc intake estimated to affect ≥17% of the studied population | |||
| [57] Atinmo, Nigeria h | 20 LBW 30 uncomplicated |
Heparin plasma zinc Collected at delivery AAS |
↓ mean (SD) serum zinc in women with a LBW infant compared to those with uncomplicated pregnancies. LBW: 663.1 (144.6) vs. uncomplicated: 731.5 (235.6) µg/L, p < 0.05 |
| [58] Abass, Sudan b | 50 LBW 50 uncomplicated |
Serum zinc AAS Atomic absorption spectrometry |
↓ median (IQR) serum zinc in women with a LBW infant compared to those with uncomplicated pregnancies. LBW: 629 (363–968) vs. uncomplicated 962 (846–1257) µg/L, p < 0.001 |
| [59] Rwebembera, Tanzania c | 81 LBW 84 uncomplicated |
EDTA plasma zinc Collected at delivery Flame AAS |
3-fold ↓ risk of delivering a LBW infant with serum zinc ≥ 392.2 µg/L OR: 3.07, 95% CI: 1.07–8.97 |
| [60] Bahl, India c | 19 LBW 56 uncomplicated |
Serum zinc Collected at delivery Flame AAS |
↓ mean (SD) serum zinc in women with a LBW infant compared to those with uncomplicated pregnancies. LBW: 553 (43) vs. 692 (95) µg/L, p < 0.001 |
| [61] Singh, India e | 47 LBW 45 uncomplicated |
Serum zinc Collected at delivery AAS |
↓ mean (SD) serum zinc in women with a LBW infant compared to those with uncomplicated pregnancies. LBW: 623 (330) vs. uncomplicated: 895 (514) μg/L, p < 0.001 |
| [62] Prema, India e | 23 LBW 208 uncomplicated |
Serum zinc Collected at delivery between 9–11.30 a.m. Flame AAS |
↑ mean (SD) serum zinc in mothers with a LBW infant compared to mothers with an uncomplicated pregnancy. LBW: 660 (162) vs. uncomplicated: 620 (146) µg/L, p < 0.01 |
| [63] Badakhsh, Iran b | 30 LBW 110 uncomplicated |
Serum zinc Collected at delivery AAS |
↑ mean (SD) serum zinc in mothers with a LBW infant compared to mothers with an uncomplicated pregnancy. LBW: 686.2 (204.8) vs. uncomplicated: 514.3 (138.8) µg/L, p < 0.001 |
| [64] Goel, India a | 20 LBW 25 uncomplicated |
Heparin plasma zinc Collected at delivery AAS |
NS mean (SD) plasma zinc in women with a LBW infant compared to those with an uncomplicated pregnancy. LBW: 726 (61) vs. uncomplicated: 763 (56) μg/L |
| [65] Srivastava, India b | 26 LBW 25 uncomplicated |
Heparin plasma zinc Collected at delivery Flame AAS |
NS mean (SD) plasma zinc between mothers with a LBW infant and mothers with uncomplicated pregnancies. LBW: 6470 (4860) vs. uncomplicated: 5670 (2490) µg/L |
| [66] Jeswani, India a | 10 SGA 25 uncomplicated |
Serum zinc Collected at 28–40 weeks AAS |
NS mean (SD) serum zinc in SGA women compared to those with uncomplicated pregnancies. SGA: 938 (76.2) vs. uncomplicated: 962.8 (194.8) µg/L |
| [67] George, India a | 65 SGA 51 uncomplicated |
Heparin plasma zinc Collected before labor between 8–10 a.m. AAS |
NS in mean (SD) plasma zinc between SGA and women with uncomplicated pregnancies. SGA: 675 (90) vs. uncomplicated: 706.7 (139) µg/L |
| [68] Akman, Turkey f | 22 SGA 34 uncomplicated |
Serum zinc Collected at delivery AAS |
NS mean (SD) serum zinc between SGA women and women with uncomplicated pregnancies. SGA: 1218 (543) vs. uncomplicated 1038 (343) µg/L |
| [69] Ozdemir, Turkey b | 16 LBW 59 uncomplicated |
Serum zinc Collected at 38–42 weeks Flame AAS |
NS mean (SD) serum zinc between mothers with a LBW infant and mothers with uncomplicated pregnancies. Data represented on graphs |
a SGA defined as ˂10th percentile; b LBW defined as ˂2500 g; c LBW defined as ≤2000 g; d LBW defined as ˂2750 g; e LBW defined as ˂2000; f SGA not defined; a* SGA defined as ˂10th percentile based on customised centiles; g SGA defined as ˂15th percentile; h LBW defined as ≤2500 g. Bold print signifies results that were significantly different. Abbreviations: AAS: atomic absorption spectrometry; CI: confidence interval; IQR: interquartile range; LBW: low birth weight; NS: non-significant; OR: odds ratio; SD: standard deviation; SEM: standard error of the mean; SGA: small for gestational age.
Table 2.
Included studies assessing maternal zinc status and hypertensive disorders of pregnancy.
| Author, Country | SAMPLE SIZE | Zinc Measure | Outcome of the Study |
|---|---|---|---|
| (1) Sample Type | |||
| (2) Time at Which Gestation Diet Was Assessed or Sample Collected | |||
| (3) Method of Analysis | |||
| [70] Tande, United States a,b | 13 hypertensive (11 PE + 2 GH) 44 uncomplicated |
Dietary and supplement intake First 3 months of pregnancy Harvard food frequency questionnaire |
NS in mean (SEM) dietary zinc intake between those with and without gestational hypertension. Hypertensive: 16.9 (1.56) vs. uncomplicated: 15.4 (1.03) mg/day |
| Inadequate dietary zinc intake estimated to affect <17% of the studied population | |||
| [71] Lazebnik, United States a,b | 17 PE and 14 hypertensive 31 uncomplicated |
Plasma zinc Collected within 1 h of delivery AAS |
↓ mean (SD) serum zinc in women with PE when compared to women with uncomplicated pregnancies. PE: 420 (100) vs. uncomplicated: 520 (130) µg/L, p < 0.05 NS mean (SD) plasma zinc in hypertensive women compared to those whose pregnancies remained uncomplicated. Hypertensive: 530 (110) vs. uncomplicated: 520 (110) µg/L |
| [55] Cherry, United States a | 48 toxemic/ hypertensive 207 uncomplicated |
Heparin plasma zinc Collected across gestation AAS |
↓ mean (SEM) plasma zinc in women with toxemia/ hypertension compared to women with uncomplicated pregnancies. Toxemic: 541.5 (16.8) vs. uncomplicated: 590.7 (8) μg/L, p < 0.009 |
| [72] Kim, Korea a | 29 PE 30 uncomplicated |
Serum zinc Collected at delivery Instrumental neutron activation analysis |
↓ mean (SEM) serum zinc in mothers with PE compared to women with uncomplicated pregnancies. PE: 700 (200) vs. uncomplicated: 1900 (500) μg/L, p < 0.0001 |
| [73] Kiilholma, Finland c,d | 10 mild PE and 10 severe PE 20 uncomplicated |
Serum zinc Collected at delivery Particle induced X-ray emission |
↓ mean (SD) serum zinc in women with mild and severe PE compared to women with uncomplicated pregnancies. Mild PE: 510 (70) and severe PE: 370 (10) vs. uncomplicated: 630 (90) μg/L, p < 0.001 for both, respectively ↓ mean (SD) serum zinc in women with severe PE compared to those with mild PE. Severe PE: 370 (10) vs. mild PE: 510 (70) μg/L, p < 0.005 |
| [74] Araujo Brito, Brazil e | 20 mild PE and 24 severe PE 50 uncomplicated |
Fasting sodium citrate plasma zinc Collected before delivery Flame AAS |
↓ mean (SD) plasma zinc in mothers with severe PE compared to mothers with uncomplicated pregnancies. Severe PE: 388 (82) vs. uncomplicated: (483 (83) µg/L, p < 0.05 NS mean (SD) plasma zinc in women with mild PE compared to women with uncomplicated pregnancies. Mild PE: 500 (94) vs. uncomplicated: (483 (83) µg/L |
| [75] Magri, Malta b | 33 GH 110 uncomplicated |
Serum zinc Collected in third trimester Electro-thermal AAS |
NS in mean (SD) serum zinc between women with GH and women with uncomplicated pregnancies. PE: 606 (80) vs. uncomplicated: 636 (100) μg/L |
| [76] Fenzl, Croatia a,b | 30 PE and 30 GH 37 uncomplicated |
Fasting serum zinc Collected at the time of diagnosis Flame AAS |
NS in mean (SD) serum zinc between both women with PE or GH women and women with uncomplicated pregnancies. PE: 603 (93) and GH: 599 (83) vs. uncomplicated: 578 (93) μg/L |
| [77] Katz, Israel d | 43 severe PE 80 uncomplicated |
Plasma zinc Collected immediately after delivery Inductively coupled plasma mass spectrometry |
NS mean (SD) plasma zinc in mothers with severe PE vs. mothers with uncomplicated pregnancies. Severe PE: 685 (875) vs. uncomplicated: 534 (139) µg/L |
| [38] Hyvonen-Dabek, Finland f | 10 hypertensive 10 uncomplicated |
Serum zinc Collection time not specified Particle induced X-ray emission |
NS mean (SD) serum zinc in women with PE compared to women with an uncomplicated pregnancy. PE: 1070 (320) and hypertensive: 1090 (170) vs. uncomplicated: 1150 (220) |
| [48] Borella, Italy a | 24 hypertensive 35 uncomplicated |
Heparin plasma zinc Collected in the third trimester Flame AAS |
NS mean (SD) plasma zinc in the hypertensive women compared to those who remained uncomplicated. Hypertensive: 685.6 (149) vs. uncomplicated: 627.5 (150) µg/L |
| [78] Mistry, United Kingdom a | 244 PE 472 uncomplicated |
Non-fasting heparin plasma zinc Collected at 15 weeks gestation Inductively coupled plasma mass spectrometry |
NS median (interquartile range) plasma zinc in women with PE women compared to those with uncomplicated pregnancies. PE: 579.6 (521.1–638.6) vs. uncomplicated: 575.7 (515.6–641.7) µg/L |
| [53] Tamura, United States a | 271 hypertensive 2038 uncomplicated |
Non-fasting heparin plasma zinc Collected at first prenatal visit (6 to 34 weeks) Flame AAS |
NS in the prevalence (n (%)) of hypertension measured between the lowest quartile and upper 3 quartiles of zinc. Highest: 205 (7.9) vs. Lowest: 66 (7.7) |
| [79] Lao TT, China a | 28 PE 28 uncomplicated |
Heparin plasma zinc Collected after diagnosis, before delivery Flame AAS |
NS mean (SD) plasma zinc in women with PE compared to women with uncomplicated pregnancies. PE: 641 (163) vs. uncomplicated: 647 (111) µg/L |
| Inadequate dietary zinc intake estimated to affect ≥17% of the studied population | |||
| [80] Sarwar, Bangladesh a | 50 PE 58 uncomplicated |
Fasting serum zinc Collected ˃20 weeks gestation Flame AAS |
↓ mean (SEM) serum zinc in mothers with PE compared to mothers with uncomplicated pregnancies. PE: 770 (50) vs. uncomplicated: 980 (30) µg/L, p < 0.001 |
| [34] Kumru, Turkey a | 30 PE 30 uncomplicated |
Serum zinc Collection time not specified AAS |
↓ mean serum zinc in women with PE when compared to women with uncomplicated pregnancies. Data represented on graphs, p < 0.001 |
| [81] IIhan, Turkey a | 21 PE 20 uncomplicated |
Serum zinc Collected at 31–38 weeks Flame AAS |
↓ mean (SD) serum zinc in women with PE when compared to those with an uncomplicated pregnancy. PE: 829.4 (289.3) vs. uncomplicated: 1251.9 (242.3) μg/L, p < 0.001 |
| [82] Bakacak, Turkey a | 38 PE 40 uncomplicated |
Fasting serum zinc 32–38 weeks Flame AAS |
↓ median (max-min) serum zinc in women with PE when compared to those with an uncomplicated pregnancy. PE: 812.4 (1106.5–624) vs. uncomplicated: 1084.5 (1385.5–881.2) μg/L, p < 0.001 |
| [36] Farzin, Iran a | 60 PE 60 uncomplicated |
Fasting heparin plasma zinc Collection time not specified Flame AAS |
↓ mean (SEM) serum zinc in mothers with PE compared to mothers with uncomplicated pregnancies. PE: 764.9 (176.2) vs. uncomplicated: 1006.1 (201.2) µg/L, p < 0.001 |
| [83] Al-Jameil, Saudi Arabia a | 40 PE 40 uncomplicated |
Serum zinc Collected in the third trimester Inductively coupled plasma optical emission spectrometry |
↓ mean (SD) serum zinc in mothers with PE compared to mothers with uncomplicated pregnancies. PE: 670 (590) vs. uncomplicated: 1300 (830) µg/L, p < 0.05 |
| [33] Akinloye, Nigeria a | 49 PE 40 uncomplicated |
Serum zinc Collection time not specified Flame AAS |
↓ mean (SD) serum zinc between women with PE and women with uncomplicated pregnancies. PE: 562 (92) vs. uncomplicated: 614 (52) μg/L, p < 0.05 |
| [39] Jain, India e | 25 mild PE and 25 severe PE 50 uncomplicated |
Serum zinc Collection time not specified AAS |
↓ mean (SD) serum zinc between women with mild PE and those with uncomplicated pregnancies. Mild PE: 831 (111) vs. uncomplicated: 1022 (157) μg/L, p < 0.05 ↓ mean (SD) serum zinc between women with severe PE and women with uncomplicated pregnancies. Severe PE: 787 (92) vs. uncomplicated: 1022 (157) μg/L, p < 0.05 |
| [37] Gupta, India b,e | 47 mild PE and. 18 severe PE and 10 eclamptic 74 uncomplicated |
Non-fasting heparin plasma zinc Collection time not specified AAS |
↓ mean (SD) serum zinc in mothers with severe PE and eclampsia compared to mothers with uncomplicated pregnancies. Severe PE: 607 (107) and eclampsia: 607 (171) vs. uncomplicated: 695 (119) μg/L, p < 0.01 NS in mean (SD) serum zinc between women with mild PE and women with uncomplicated pregnancies. Mild PE: 684 (134) vs. uncomplicated: 695 (119) μg/L |
| [84] Bassiouni, Egypt g,d | 52 PE (28 mild and 24 severe) 20 uncomplicated |
Heparin plasma zinc Collected at delivery AAS |
NS in mean (SD) plasma zinc in women with mild PE compared to women with uncomplicated pregnancies. Mild PE: 604.2 (162.7) vs. uncomplicated: 646 (173.7) μg/L ↓ mean (SD) plasma zinc in women with severe PE compared to the women with uncomplicated pregnancies. Severe PE: 410.8 (116.5) vs. uncomplicated: 646.0 (173.7 μg/L, p < 0.001 |
| [85] Harma, Turkey a | 24 PE 44 uncomplicated |
Heparin plasma zinc Collected just during the latent phase of labor AAS |
↑ mean (SD) plasma zinc levels in women with PE when compared to women with uncomplicated pregnancies. PE: 15.53 (4.92) vs. uncomplicated: 11.93 (3.11) μg/g protein, p = 0.003 |
| [86] Rafeeinia, Iran h | 35 PE and 15 severe PE 50 uncomplicated |
Fasting serum zinc Collected in the third trimester AAS |
NS mean (SD) serum zinc in mothers with PE or severe PE and uncomplicated pregnancies. Mild PE: 690 (40) and severe PE: 780 (80) vs. uncomplicated: 720 (40) µg/L |
| [87] Vafaei, Iran e | 20 mild PE and 20 severe PE 40 uncomplicated |
Serum zinc Collected at 28–40 weeks Auto-analyser |
NS mean (SD) serum zinc in either the mild or severe PE women compared to women with uncomplicated pregnancies. Data represented on graphs |
| [88] Ahsan, Bangladesh a,i | 44 PE and 33 eclampsia 27 uncomplicated |
Serum zinc Collected at 28–42 weeks Flame AAS |
NS mean (SD) serum zinc in PE or eclamptic women compared to women with uncomplicated pregnancies. PE: 1045.8 (131) and eclampsia: 915 (131) vs. uncomplicated: 980.4 (131) µg/L |
| [89] Rathore, India a | 14 PE 47 uncomplicated |
Serum zinc Collected at delivery Flame AAS |
NS mean (SD) serum zinc between women with PE and those with uncomplicated pregnancies. PE: 492 (178) vs. uncomplicated: 575 (216) μg/L |
| [90] Kolusari, Turkey a | 47 PE 48 uncomplicated |
Serum zinc Collected between 29 and 38 weeks AAS |
NS mean (SD) serum zinc between women with PE women and those with uncomplicated pregnancies. PE: 10.6 (4.4) vs. uncomplicated: 12.7 (4.1) µg/L |
| [91] Atamer, Turkey a | 32 PE 28 uncomplicated |
Fasting serum zinc Collected at 28–29 weeks Flame AAS |
NS in mean (SD) serum zinc between women with PE and women with uncomplicated pregnancies. PE: 792 (180) vs. uncomplicated: 1086 (199) μg/L |
| [92] Adam, Turkey a | 20 PE 20 uncomplicated |
Plasma zinc Collected before the onset of labor Flame AAS |
NS mean (SD) plasma zinc in women with PE compared to women with an uncomplicated pregnancy. PE: 313 (47) vs. uncomplicated: 341 (44) µg/L |
| [93] Vigeh, Iran a | 31 PE 365 uncomplicated |
Heparin plasma zinc Collected at delivery Inductively coupled plasma mass spectrometry |
NS mean (SD) plasma zinc between women with PE women and women with uncomplicated pregnancies. PE: 5200 (1444) vs. uncomplicated: 5561 (1057) µg/L |
| [32] Adeniyi, Nigeria a | 55 pregnant women | Plasma zinc Collection time not specified AAS |
NS mean (SD) plasma zinc in women with PE compared to women with uncomplicated pregnancies. PE: 940 (270) vs. uncomplicated: 970 (230) μg/L |
a PE defined as high blood pressure (≤140/90 mmHg) after 20 weeks gestation and proteinuria (≥300 mg/24 h); b GH defined as high blood pressure (≤140/90 mmHg) after 20 weeks gestation without proteinuria; c PE not defined; d Severe PE not defined; e Mild PE defined as blood pressure ≥140/90 but less than 160/110 mmHg and severe PE defined as ≥160/110 mmHg; f PE defined as blood pressure ˃130/85 and proteinuria ≥1 by dipstick, severe PE defined as blood pressure ˃160/110; g PE defined by the classification proposed by the Paris meeting of the Gestosis Organisation, 1970; h PE defined as blood pressure ˃ 130/85 and proteinuria ≥1 by dipstick, severe PE defined as blood pressure ˃160/110; i eclampsia defined as women diagnosed with PE whom also suffer seizures that cannot be attributed to other causes. Bold print signifies results that were significantly different. Abbreviations: AAS: atomic absorption spectrometry; GH: gestational hypertension; PE: preeclampsia; SD: standard deviation; SEM: standard error of the mean.
Table 3.
Included studies assessing maternal zinc status and sPTB.
| Author, Country | Sample Size | Zinc Measure | Outcome of the Study |
|---|---|---|---|
| (1) Sample Type | |||
| (2) Time at Which Gestation Diet Was Assessed or Sample Collected | |||
| (3) Method of Analysis | |||
| [44] Scholl, United States a | 115 with zinc intake ≤6 mg/day 699 with zinc intake ˃6 mg/day |
Dietary zinc intake 28 and 36 weeks 24 h dietary recall |
2-fold ↓ risk of delivering a preterm infant with dietary zinc intake ˃6 mg/day. OR (LMP): 1.85, 95% CI: 1.09–3.12, OR (OE): 2.13, 95% CI: 1.20–3.79 2.75 to 3.44-fold ↓ risk of delivering a very preterm infant with dietary zinc intake ˃9 mg/day. OR (LMP): 2.75, 95% Cl: 1.31–5.77, OR (OE): 3.44, 95% Cl: 1.39–8.55 |
| [94] Carmichael, United States a,b | 413 preterm and 58 early preterm 5267 term |
Dietary zinc intake Harvard food frequency questionnaires |
2-fold ↓ for preterm birth <32 weeks with zinc intake ˃ 8.0 mg/day compared to 8.0–14.2 mg/day. OR: 2.3, 95% CI: 1.2–4.5 |
| [45] Neggers, United States a | 238 preterm 1160 term |
Dietary zinc intake 18 and 30 weeks 24 h dietary recall using the nutrient database developed by the University of Minnesota |
NS association between low dietary zinc intake (less than median) and risk of PTB. OR: 1.1, 95% CI: 0.7–1.7 |
| [95] Hsu, Taiwan c | 28 preterm 423 term |
Dietary zinc intake Each trimester 24 h dietary recall |
NS in dietary zinc intake between each of the trimesters and in those who delivered preterm versus term. Preterm: 9.6–10.8 mg/day vs. term: 8.90–10.9 mg/day |
| Inadequate dietary zinc intake estimated to affect <17% of the studied population | |||
| [96] Wang, China a | 169 preterm 2912 uncomplicated |
Fasting serum zinc First and second trimester Flame AAS |
↑ risk of preterm birth with serum zinc <767 μg/L and serum zinc between 767 and 996 μg/L. aOR: 2.41, 95% CI: 1.57, 3.70; aOR: 1.97, 95% CI: 1.27, 3.05, p < 0.001 for both, respectively |
| [50] Bro, Denmark c | 34 preterm 220 uncomplicated |
Serum zinc Collected at delivery Flame AAS |
NS mean (SD) serum zinc levels in women who delivered preterm compared to term women. Preterm: 666.7 (104.6) vs. term: 679.7 (98) μg/L |
| [54] Tamura, United States c | 505 preterm and 136 early preterm 2038 uncomplicated |
Non-fasting heparin plasma zinc Collected at first prenatal visit (6 to 34 weeks) Flame AAS |
NS in the prevalence or n (%) of PTB measured between the lowest quartile and upper three quartiles of zinc. Highest: 373 (14.5) vs. lowest: 132 (15.3) NS in the prevalence (n (%)) of early PTB measured between the lowest quartile and upper three quartiles of zinc. Highest: 107 (4.2) vs. lowest: 29 (3.4) |
| Inadequate dietary zinc intake estimated to affect ≥17% of the studied population | |||
| [66] Jeswani, India c | 25 preterm 25 term |
Serum zinc Collected at 28–40 weeks AAS |
↑ mean (SD) serum zinc in women who delivered preterm women compared to term. Preterm: 1154.4 (154.1) vs. uncomplicated: 962.8 (194.8) µg/L, p ˂ 0.01 |
| [64] Goel, India d | 20 preterm 25 term |
Heparin plasma zinc Collected at delivery AAS |
↑ mean (SD) plasma zinc in mothers who delivered preterm compared to term mothers. Preterm: 842 (43) vs. term: 744 (51) μg/L, p ˂ 0.001 |
| [60] Bahl, India a | 10 preterm 97 term |
Serum zinc Collected at delivery Flame AAS |
NS mean (SD) in women who delivered Preterm that were an appropriate weight for date compared to uncomplicated. Preterm: 627 (212) vs. uncomplicated: 670 (96) µg/L |
| [65] Srivastava, India c | 26 preterm 23 term |
Heparin plasma zinc Collected at delivery Flame AAS |
NS mean (SD) plasma zinc between preterm and term mothers. Preterm: 6350 (2640) vs. term: 6310 (5090) μg/L |
a PTB defined as ˂37 weeks gestation; b Early PTB defined as ˂32 weeks gestation; c PTB defined as ≤37 weeks gestation; d PTB not defined. Bold print signifies results that were significantly different. Abbreviations: AAS: atomic absorption spectrometry; aOR: adjusted odds ratio; CI: confidence interval; LMP: last menstrual period; OE: obstetric estimate; PTB: preterm birth; SD: standard deviation.
Table 4.
Included studies assessing maternal zinc status and GDM.
| Author, Country | Sample Size | Zinc Measure | Outcome of the Study |
|---|---|---|---|
| (1) Sample Type | |||
| (2) Time at Which Gestation Diet Was Assessed or Sample Collected | |||
| (3) Method of Analysis | |||
| [97] Bo, Italy a,b | 126 GDM and 84 aOGTT 294 uncomplicated |
Dietary zinc intake 24–28 weeks Food frequency questionnaire |
↓ mean (SD) daily zinc intake between GDM and aOGTT women and women with uncomplicated pregnancies. GDM: 8.5 (2.4) and aOGTT: 8.7 (2.5) vs. uncomplicated: 9.4 (2.8) mg/day, p = 0.007 |
| [98] Behboudi-Gandevani S, Iran a | 72 with GDM 961 uncomplicated |
Dietary zinc intake 14–20 weeks Semi-quantitative food frequency questionnaire |
NS in mean (SD) daily zinc intake between GDM and those with uncomplicated pregnancies. GDM: 6.91 (3.42) vs. uncomplicated: 10.1 (7.45) mg/day |
| Inadequate dietary zinc intake estimated to affect <17% of the studied population | |||
| [48] Borella, Italy a | 18 GDM 35 uncomplicated |
Heparin plasma zinc Collected in the third trimester Flame AAS |
↑ mean (SD) plasma zinc in GDM women compared to women with uncomplicated pregnancies. GDM: 766.6 (117.6) vs. uncomplicated: 627.5 (150) µg/L, p ˂0.001 |
| [35] Wang, China a,c | 46 GDM and 98 IGT 90 uncomplicated |
Plasma zinc Collection time not specified Inductively coupled plasma atomic emission spectroscopy |
NS in mean (SD) plasma zinc between women with IGT and women with uncomplicated pregnancies. IGT: 1080 (270) vs. uncomplicated: 1130 (330) μg/L NS mean (SD) plasma zinc between women with GDM and those with uncomplicated pregnancies. GDM:1020 (190) vs. uncomplicated: 1130 (330) μg/L |
| [38] Hyvonen-Dabek, Finland d | 5 GDM 10 uncomplicated |
Serum zinc Collection time not specified Particle induced X-ray emission |
NS mean (SD) serum zinc in women with GDM compared to women with uncomplicated pregnancies. GDM: 1070 (190) vs. uncomplicated: 1150 (220) µg/L |
| [99] Wibell, Sweden d | 20 GDM 13 uncomplicated |
Serum zinc Collected across gestation AAS |
NS mean (SD) serum zinc between women with GDM and those with uncomplicated pregnancies. GDM: 700 (100) vs. uncomplicated: 700 (80) μg/L |
| Inadequate dietary zinc intake estimated to affect ≥17% of the studied population | |||
| [98] Behboudi-Gandevani, Iran a | 72 with GDM 961 uncomplicated |
Serum zinc Collected 14–20 weeks Flame AAS |
NS mean serum zinc between GDM and women with uncomplicated pregnancies. GDM: 844 (440) vs. uncomplicated: 835 (444) μg/L |
| [100] Al-Saleh, Kuwait a | 30 GDM 30 uncomplicated |
Serum zinc Collected at delivery Furnace AAS |
NS mean (SEM) serum zinc in women with GDM compared to women with uncomplicated pregnancies. GDM: 610.3 (60.1) vs. uncomplicated: 656.2 (241.4) µg/L |
a GDM defined as high blood glucose levels in pregnant women who have not previously been diagnosed with diabetes which over a 3 h oral glucose tolerance test provided at least two values over the criteria of Carpenter and Coustan; b aOGTT defined as high blood glucose levels in pregnant women who have not previously been diagnosed with diabetes which over a 3 h oral glucose tolerance test provided one abnormal value over the criteria of Carpenter and Coustan; c IGT defined as women with blood glucose consistently higher than 7.8 mmol/L; d GDM diagnosed with an intravenous glucose tolerance test at 30 weeks gestation. Bold print signifies results that were significantly different. Abbreviations AAS: atomic absorption spectrometry; aOGTT: abnormal oral glucose tolerance test; BMI: body mass index; GDM: gestational diabetes mellitus; IGT: impaired glucose tolerance; OGTT: oral glucose tolerance test; SD: standard deviation.
Table 5.
Summary of all the studies reviewed and whether zinc status was positively, negatively or not associated with the studied pregnancy complication.
| Dietary Zinc Intake | ||||
| Total No. Reference | LBW/SGA | Hypertensive Disorders of Pregnancy | sPTB | GDM |
| 9 | 4 | 1 | 4 | 2 |
| 3 reported a negative association [42,43,44] | Reported no association [70] | 2 reported a negative association [44,93] | 1 reported a negative association [96] | |
| 1 reported no association [45] | 2 reported no association [45,94] | 1 reported no association [97] | ||
| Serum/Plasma Zinc | ||||
| Total No. Reference | LBW/SGA | Hypertensive Disorders of Pregnancy | sPTB | GDM |
| 58 | 26 | 33 | 7 | 6 |
| No. where inadequate zinc intake affects <17% of the population | ||||
| 12 | 13 | 3 | 4 | |
| 2 reported a negative association [46,49] | 5 reported a negative association [55,71,72,73,74] | 1 reported a positive association [95] | 1 reported a positive association [48] | |
| 2 reported a positive association [47,48] | 8 reported no association [38,48,53,75,76,77,78,79] | 2 reported no association [50,53] | 3 reported no association [35,38,98] | |
| 8 reported no association [38,50,51,52,53,54,55,56] | ||||
| No. where inadequate zinc intake affects ≥17% of the population | ||||
| 14 | 20 | 4 | 2 | |
| 5 reported a negative association [57,58,59,60,61] | 10 reported a negative association [33,34,36,37,39,80,81,82,83,100] | 2 reported a positive association [64,66] | 2 reported no association [97,99] | |
| 2 reported a positive association [63,101] | 1 reported a positive association [84] | 2 reported no association [60,65] | ||
| 6 reported no association [64,65,66,67,68,69] | 9 reported no association [32,85,86,87,88,89,90,91,92] | |||
Abbreviations: GDM: gestational diabetes mellitus; LBW: low birth weight; SGA: small for gestational age; sPTB: spontaneous preterm birth.
3.1. Infant Birthweight
There were four studies that assessed dietary zinc intake and birthweight with three based on women from countries where the estimated prevalence of low dietary zinc intake is <17% (Table 1) [42,43,44,45]. Lower zinc intake was reported in women from the United Kingdom (UK) who gave birth to an SGA infant compared to those who gave birth to an appropriate-for-gestational-age (AGA) infant (SGA: mean (SEM) 11.3 (0.5) vs. AGA 13.0 (0.6) mg/day, p ˂ 0.05) [45]. This was similar to another study of Indian women that reported lower zinc intakes in women who delivered an infant weighing <2500 g compared to those who delivered an infant that was ≥2500 g [42]. Logistic regression analysis in one study from the United States reported daily zinc intake ˂6 mg/day to be associated with a 2-fold increase in the risk of delivering a LBW infant (aOR: 2.01, 95% CI: 1.11–3.66) [44] although dietary zinc intakes <median were not found to be associated with LBW in another study of American women (OR: 1.4, 95% CI: 0.9–2.1) [43]. While both studies used a 24 h recall questionnaire to determine zinc intakes, there were differences in ethnicity of the women studied as Neggers et al., [43] predominantly studied African-American women as opposed to Scholl et al. who studied Caucasian women [44].
Twelve studies were identified that measured maternal circulating zinc in countries where inadequate zinc intake is predicted to be <17%, and looked at the association with birthweight (Table 1) [38,46,47,48,49,50,51,52,53,54,55,56]. Only one study, based on 3817 women in China, reported a 3.4-fold increase in the risk of delivering a LBW infant with serum zinc <560 µg/L (adjusted RR: 3.41, 95% CI: 1.97, 5.91) [56]. This is in contrary to two studies that reported significantly higher zinc concentrations in women with an SGA infant in the third trimester [47,55]. However, these findings were based on a relatively small number of women: 40–51 pregnant women including 10–16 women with SGA. Conversely, another study, which followed 476 women of whom 39 gave birth to an SGA infant, found the incidence of LBW to be 8 times higher in women with serum zinc in the lowest quartile (457.5–797.4 µg/L) compared to the highest (1039.2–1660.1 µg/L) (8.2, 95% CI: 2.4–27.5) [52]. The remaining eight studies found no differences in maternal zinc concentrations between women with a SGA infant and those with an uncomplicated pregnancy. However, one study found a positive correlation between maternal zinc status and birthweight (r = 0.632, p ˂ 0.001) [50].
The association between maternal circulating zinc and birthweight was assessed in 14 studies based on women where inadequate dietary zinc intake was predicted to affect ≥17% of the population [57,58,59,60,61,62,63,64,65,66,67,68,69], of which 7 reported a significant association (Table 1) [57,59,60,61,66,67,68]. All three of the studies based on women from Africa reported serum/plasma zinc on average 72–333 µg/L lower in women who gave birth to a LBW infant compared to those who gave birth to an appropriate weight infant [57,59,67]. In another study, the risk of delivering a LBW infant was also reported to be 3-fold greater in women with serum zinc levels ≤392.2 µg/L compared to those with levels above this figure (3.07, 95% CI: 1.07–8.97) [67]. Conversely, two other studies reported serum zinc to be 40–172 µg/L higher in women who gave birth to a LBW infant compared to those who gave birth to an appropriate weight infant [60,66]. A further four studies, also based on women from India, reported no association between circulating zinc levels and birthweight [62,63,64,69] and this was also reported in two studies of Turkish women [58,65]. However, univariate analysis and small sample size in these studies may not provide an accurate assessment of the effects of maternal circulating zinc and birthweight.
3.2. Hypertensive Disorders of Pregnancy
Only one study assessed dietary zinc intake and the association with hypertensive disorders (Table 2) and found no significant differences in dietary zinc intake between 13 women who developed a hypertensive disorder in pregnancy and 44 whose pregnancies remained uncomplicated [70].
Thirteen studies analyzed serum/plasma zinc in women who developed a hypertensive disorder of pregnancy in women residing in countries where inadequate zinc intake is estimated to be low (<17%) (Table 2). Three studies reported mean serum/plasma zinc to be on average 120–1200 µg/L lower in women who developed PE compared to women whose pregnancies remained uncomplicated [49,71,72] and included one study that reported a reduction in risk of PE with serum levels above 1360 µg/L after adjusting for maternal age, height and weight before pregnancy (aOR: 0.005, 95% CI: 0.001–0.07) [71]. A further two studies reported circulating zinc to be lower in women who developed severe PE (blood pressure BP ≥ 160/110) compared to women whose pregnancies remained uncomplicated [73,74]. The remaining eight studies, whose sample sizes ranged from 10–271 women with PE/GH and 10–2038 women with an uncomplicated pregnancy, reported no difference in maternal zinc status between women with a hypertensive disorder of pregnancy and those without [38,47,54,75,76,77,78,79].
There were twenty studies that analyzed circulating zinc in women with a hypertensive disorder of pregnancy in populations where inadequate zinc intake is estimated to be ≥17% (Table 2) [32,33,34,36,37,39,80,81,82,83,84,85,86,87,88,89,90,91,92,93]. Ten studies reported mean serum/plasma zinc to be significantly lower in women who developed PE and/or GH [33,34,36,37,39,80,81,82,83,93] however, one reported plasma zinc to be higher in women with PE compared to those whose pregnancies remained uncomplicated when measured during the latent phase of labor; with (PE mean (SD): 15.53 (4.92) vs. uncomplicated: 11.93 (3.11) μg/g protein, p = 0.003) [89]. These studies also included three which found circulating zinc to be 80–260 μg/L lower in women who developed severe PE when compared to women whose pregnancies remained uncomplicated [37,39,83]. A further nine studies reported no difference in circulating zinc between women with PE/GH and those whose pregnancies remained uncomplicated.
3.3. Spontaneous Preterm Birth
The literature search identified four studies which measured dietary zinc intakes during pregnancy and sPTB with varying conclusions (Table 3) [43,44,94,95]. Two of these studies, which analyzed 5738 and 818 women respectively, determined that low zinc intake (≤6 mg/day which is ≤54% of the recommended 11 mg/day [21]) was associated with a more than 2-fold increase in the risk of delivering preterm (aOR: 2.3, 95% CI: 1.2–4.5 and aOR: 1.85 95% CI: 1.09–3.12, respectively), after adjusting for factors such as ethnicity, pre-pregnancy BMI, smoking, alcohol and multivitamin consumption [44,94]. If delivery date was calculated by last menstrual period (LMP), zinc intake below 9 mg/day was associated with a 2.75-fold increased risk in delivering <32 weeks gestation (aOR: 2.75, 95% CI: 1.31–5.77) [44]. However, another study reported no association between low dietary zinc intake (less than the median) and the risk of sPTB (OR: 1.1, 95% CI: 0.7–1.7) [43] but mean zinc intake of the women in this study was 14 mg/day, higher than the recommended 11 mg/day, indicating that low zinc intake was not prevalent within this studied population.
When separated based on estimates of inadequate zinc intake, there were three studies which assessed whether there was an association between circulating zinc and sPTB in low-risk populations (Table 3). While two showed no significant difference between serum/plasma zinc levels during gestation in women who gave birth preterm and those who gave birth at term [48,54], one study which recruited 3081 women in China found a 2.4-fold increase risk of PTB with serum levels <767 µg/L (aOR: 2.41, 95% CI: 1.57, 3.70) [96].
The association between maternal circulating zinc and sPTB was determined in four studies on populations with inadequate zinc intake ≥17%, all of which sampled women in India (Table 4) [61,63,64,69]. Two of the studies reported serum/plasma zinc to be higher in women who delivered preterm compared to those who delivered at term (average 98–1991 µg/L increase) [63,64]. However, no difference in circulating zinc measured at delivery was reported in the remaining two studies [61,69].
3.4. Gestational Diabetes Mellitus
Two studies looked at the association between dietary zinc intake and GDM (Table 4) [97,98]. One collected data at 24–28 weeks gestation, and found an 11% reduction in the risk of gestational hyperglycaemia with every 1 mg/day increase in dietary zinc intake (aOR: 0.89, 95% CI: 0.82–0.96) [97]. The second, which sampled women at 14–20 weeks’ gestation, found no association between maternal dietary zinc intakes below 50% of the recommended daily allowance and GDM (OR: 1.4, 95% CI: 0.6–2.9) [98]. Differences between the studies included when dietary zinc was measured (early versus late second trimester) as well as ethnicity (Italian versus Iranian in which, genetic and cultural differences are likely).
Of the five studies which assessed the association between circulating zinc and GDM in countries where inadequate zinc intake is estimated to be <17%, two, both studying Italian women, reported a significant difference in serum/plasma zinc in women who developed GDM compared to women whose pregnancies remained uncomplicated (Table 4) [47,97]. However, while one study reported that serum zinc was negatively associated with the risk of hyperglycemia in pregnancy (aOR: 0.94, 95% CI: 0.91–0.96) [97], the other found that there was in increase in serum zinc in women with GDM compared to women whose pregnancy remained uncomplicated (GDM mean (SD): 766.6 (117.6) vs. uncomplicated: 627.5 (150) µg/L, p ˂ 0.001) [47]. Both studies sampled women at similar times during pregnancy and used atomic absorption spectrometry to quantitate zinc. The remaining three studies found no difference in circulating zinc [35,38,99] however, given the small sample size of women with GDM in these studies (n = 5–46), it is likely they were underpowered and not suitable for the chosen statistical tests.
There were two studies that sampled women from countries where inadequate zinc intake was estimated to be ≥17% and assessed the association between maternal circulating zinc and GDM (Table 4) [98,100]. Neither study reported a difference in serum zinc in early pregnancy or at delivery in women with GDM compared to those whose pregnancies remained uncomplicated.
4. Discussion
This systematic review assessed whether maternal circulating zinc levels and/or dietary zinc intake were associated with a number of pregnancy complications. Overall, the evidence regarding the association between maternal zinc status and PE/GH, LBW/SGA, sPTB and GDM is weak and heterogeneity between the studies made comparisons difficult. However, systematic analysis of the available literature indicated some trends between maternal zinc status and infant birthweight as well as the development of severe PE (BP ≥160/110 mmHg).
There is consistent evidence in animal models that maternal dietary zinc deficiency during pregnancy reduces fetal growth [16,17,18,19]. From the studies that measured maternal zinc intake during pregnancy reviewed here, a possible relationship between low zinc intake (≤54% of the recommended 11 mg/day) and decreased infant birthweight may exist in human populations. Both food frequency questionnaires and 24 h recalls are limited by the preparedness of the participants to accurately record their diets, the food composition tables used and their ability to capture variations within diets [102]. This may explain the conflicting results between studies which assessed dietary zinc intake and the association with infant birthweight, sPTB and GDM. However, three of the four studies that measured dietary zinc intake in pregnancy and recorded infant birthweight reported a significant reduction in maternal zinc status in those who delivered a LBW/SGA infant [42,44,45]. The relationship between infant birthweight and maternal serum/plasma zinc is less clear. Plasma measures of zinc are considered preferable over serum as erythrocytes can be a source of zinc contamination within serum samples [22]. However, plasma zinc only accounts for approximately 0.1% of total body zinc [103], is heavily influenced by confounding factors like stress, infection and hormones [101,104,105,106,107] and does not directly correlate with dietary zinc intake [108]. This limits how useful measuring circulating zinc is as a biomarker for health and disease. When studies on LBW/SGA that measured maternal circulating zinc were separated based on populations where inadequate zinc intake is predicted to be ≥17%, 7 of the 13 studies reported a difference in serum/plasma zinc between women who delivered LBW/SGA infant and those whose infants were of an appropriate weight. Given the lack of suitable alternatives, particularly in studies of pregnant women, determining zinc status by measuring serum/plasma zinc can still be informative about the importance of zinc to pregnancy, especially if measured in conjunction with dietary zinc intakes.
Other maternal factors such as age, BMI, smoking status and alcohol consumption in pregnancy not only influence pregnancy outcome but also circulating zinc [109,110]. BMI is a significant factor in influencing the risk for developing PE and GH [111,112]. However, only 11 of the 32 studies on PE/GH [33,36,54,71,75,79,80,82,84,88,92] reported on BMI, making it difficult to comment on whether differences in BMI may be influencing the outcomes of the studies included in this review. Despite this, there may be a relationship between maternal circulating zinc levels and the severity of PE. Mean maternal zinc concentrations in women with severe PE (ranging from 388 to 410 μg/L) [73,74,83] were well below 562.1 μg/L, which is the defined zinc deficiency cut-off [26,113]. In women with mild PE and those with uncomplicated pregnancies, mean maternal zinc concentrations ranged between 684–831 µg/L [37,39,83] and 630–1022 µg/L [37,39,74,83] respectively. A current leading hypothesis relating to the development of PE is increased placental oxidative stress [114]. Zinc itself has antioxidant capabilities and is an integral structural component of superoxide dismutase, a first line defense antioxidant [115] which has reduced activity in cell lines, animal models and human studies of zinc deficiency [116,117,118,119,120]. Hence, it is possible in pregnancies complicated by PE, that low maternal zinc concentration (˂562.1 µg/L) may reduce the potential to combat rises in free radical production and increase the severity of the complication.
Zinc levels in maternal circulation decrease across gestation; this is thought to be due to a combination of increased maternal blood volume and fetal demands [40,121,122,123], and therefore comparisons between studies which measured zinc in maternal serum or plasma early in pregnancy versus late should be interpreted with caution. Overall, regardless of pregnancy outcome, the majority (31 out of 59 studies which measured maternal circulating zinc) collected samples during labor or at delivery. Physiologically, parturition results in huge changes to maternal hormonal profile with rises in estrogen, oxytocin and prostaglandin required to initiate labor [124]. Furthermore, there is an increase in the production of inflammatory cytokines and a withdrawal of anti-inflammatory cytokines within the gestational tissues [125]. Infection and inflammation decrease plasma zinc [104] and use of the contraceptive pill, which raises estrogen and progesterone levels, also decreases circulating zinc [101,105]. Given that pregnancy itself is likely to confound zinc status, this has implications for interpreting studies that have measured serum/plasma zinc at delivery. In addition, how zinc may be associated with a pregnancy outcome needs to be measured before the pregnancy complication has manifested. Only five studies of 6795 pregnant women in total measured either circulating zinc or dietary zinc intake prior to 20 weeks gestation [44,53,54,79,98]. All found no significant difference in maternal zinc status during this time period between women who developed a pregnancy complication and those who did not, indicating that zinc status in early pregnancy may not be associated with adverse pregnancy outcomes.
Due to the additional demands associated with pregnancy and fetal growth, pregnant women are more vulnerable to multiple nutrient deficiencies [126] and this is potentially another cofounding factor when assessing the association between maternal zinc status and pregnancy outcome. This is because nutrients can interact with each other in both a positive (e.g., vitamin A and zinc [127]) and negative manner (e.g., calcium or iron and zinc [128,129]). A number of studies reviewed here measured serum/plasma concentrations of other nutrients as well as zinc, including copper [35,97], iron [75,98], selenium [51,88], magnesium [95,99] and lead [78]. While circulating zinc levels were not different for the pregnancy outcomes studied in these articles, those of other micronutrients were. Serum copper concentrations were found to be higher in women with GDM or those who delivered an SGA infant when compared to women with an uncomplicated pregnancy in two studies [35,97]. Furthermore, serum iron was higher in women with PE and GDM compared to women whose pregnancies were uncomplicated [75,98]. Two other studies found selenium to be lower in the serum of women with PE or those who delivered an SGA infant compared to women with an uncomplicated pregnancy [51,88]. Therefore, it is important to consider other nutritional factors that may influence pregnancy outcome as well as micronutrient ratios in order to fully understand the importance of micronutrient status on pregnancy success.
Finally, the lack of studies identified in this review analyzing truly zinc deficient women, nor those in populations at high risk of zinc deficiency, is a major limitation in determining the effects of zinc on pregnancy outcome. Only 8 of the 64 studies reported mean circulating zinc below 562.1 μg/L [49,50,60,72,74,83,87,91] and there were very few studies based on women in countries where inadequate zinc intake is predicted to be prevalent like South-East Asia and parts of Africa [26,27]. The majority of studies were based on populations in the United States and Europe where zinc deficiency is estimated to only affect 3.9%–12.7% of the population [26]. Therefore, there is the potential that the results from this review may be skewed given the lack of evidence based on women living in areas predicted to be at high risk of zinc deficiency.
5. Conclusions
The current review has explored the connection between maternal zinc status and pregnancy complications including hypertensive disorders of pregnancy, infant birthweight, spontaneous preterm birth (sPTB) and gestational diabetes mellitus (GDM). While it appears that there may be a relationship between maternal dietary zinc intake and infant birthweight and the development of severe PE, there is little evidence to suggest an association between zinc and sPTB or GDM. However, heterogeneity in the studies identified in this review reflects real uncertainty in the evidence linking zinc deficiency and pregnancy complications and therefore this warrants further study, particularly in developing countries whose populations are at increased risk of zinc deficiency. If we are to continue to reduce preventable deaths of newborns and children under the age of five [6], understanding the importance of micronutrients like zinc in child development, particularly in utero, will greatly increase the likelihood of success. Future studies need to focus on women more vulnerable to zinc deficiency in pregnancy in order to fully determine the effects of zinc status on pregnancy outcome.
Acknowledgments
This project was funded in part by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (GNT1020754) awarded to CTR. CTR is supported by a NHMRC Senior Research Fellowship GNT1020749. RLW is supported by an Australian Postgraduate Award.
Appendix A
Outline of the search terms and MeSH headings identified in the Medline search and used for the remaining database searches.
Search strategy: MEDLINE (OVID).
| Searches | Results | |
|---|---|---|
| 1 | exp Zinc/ or zinc.mp. | 108,695 |
| 2 | plasma zinc.mp. | 1365 |
| 3 | zinc intake.mp. | 679 |
| 4 | dietary zinc.mp. | 1158 |
| 5 | serum zinc.mp. | 2083 |
| 6 | 1 or 2 or 3 or 4 or 5 | 108,695 |
| 7 | preterm birth.mp. or exp Premature Birth/ | 14,642 |
| 8 | premature birth.mp. or Premature Birth/ | 11,131 |
| 9 | small for gestational age.mp. | 9255 |
| 10 | exp Infant, Small for Gestational Age/ | 5977 |
| 11 | gestational hypertension.mp. or exp Hypertension, Pregnancy-Induced/ | 32,078 |
| 12 | pre?eclampsia.mp. or exp Pre-Eclampsia/ | 30,863 |
| 13 | exp Pre-Eclampsia/ or exp Eclampsia/ or eclampsia.mp. | 32,471 |
| 14 | exp HELLP Syndrome/ or HELPP syndrome.mp. | 1613 |
| 15 | gestational diabetes.mp. or exp Diabetes, Gestational/ | 11,382 |
| 16 | fetal macrosomia.mp. or exp Fetal Macrosomia/ | 2369 |
| 17 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 69,148 |
| 18 | infant, low birth weight.mp. or Infant, Low Birth Weight/ | 16,571 |
| 19 | infant, very low birth weight.mp. or exp Infant, Very Low Birth Weight/ | 8491 |
| 20 | 17 or 18 or 19 | 89,771 |
| 21 | 6 and 20 | 380 |
| 22 | limit 21 to (english language and full text and humans) | 165 |
Appendix B
List of conversion factors used to convert all measures of zinc to μg/L.
| Units | Conversion |
|---|---|
| μg/100 mL or μg/dL | Multiply 10 |
| μmol/L or μM | Divide 0.153 |
| mg/L | Multiply 1000 |
| μg/mL | Multiply 1000 |
Author Contributions
All authors contributed to conceiving and designing the systematic review. R.L.W. performed the literature search and along with J.A.G. read and compiled the results. R.L.W. wrote the paper and J.A.G., T.B.M. and C.T.R. edited the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M., Ezzati M., Grantham-McGregor S., Katz J., Martorell R., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 2.Grieger J.A., Clifton V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients. 2015;7:153–178. doi: 10.3390/nu7010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman P.D., Hanson M.A. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr. Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 4.Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M., Mathers C., Rivera J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 5.Fischer Walker C.L., Ezzati M., Black R.E. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur. J. Clin. Nutr. 2009;63:591–597. doi: 10.1038/ejcn.2008.9. [DOI] [PubMed] [Google Scholar]
- 6.Organisation WH (2015) Fact Sheet No 290—Millennium Development Goals (MDGs) [(accessed on 13 January 2016)]. Available online: http://www.who.int/mediacentre/factsheets/fs290/en/#.
- 7.Cousins R., Zinc I., Bowman B.A., Russell R.M. Present Knowledge in Nutrition. Volume 9. DC ILSI Press; Washington, DC, USA: 2006. pp. 445–457. [Google Scholar]
- 8.MacDonald R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000;130:1500s–1508s. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 9.Maret W. Molecular aspects of human cellular zinc homeostasis: Redox control of zinc potentials and zinc signals. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- 10.Prasad A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008;43:370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Butler Walker J., Houseman J., Seddon L., McMullen E., Tofflemire K., Mills C., Corriveau A., Weber J.-P., LeBlanc A., Walker M. Maternal and umbilical cord blood levels of mercury, lead, cadmium, and essential trace elements in Arctic Canada. Environ. Res. 2006;100:295–318. doi: 10.1016/j.envres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Perveen S., Altaf W., Vohra N., Bautista M.L., Harper R.G., Wapnir R.A. Effect of gestational age on cord blood plasma copper, zinc, magnesium and albumin. Early Hum. Dev. 2002;69:15–23. doi: 10.1016/S0378-3782(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 13.Tsuzuki S., Morimoto N., Hosokawa S., Matsushita T. Associations of maternal and neonatal serum trace element concentrations with neonatal birth weight. PLoS ONE. 2013;8:641. doi: 10.1371/journal.pone.0075627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson C.A., King J.C. Reduced serum zinc concentration during pregnancy. Obstet. Gynecol. 1983;62:313–318. doi: 10.1097/00006250-198309000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hurley L.S., Swenerton H. Congenital malformations resulting from zinc deficiency in rats. Proc. Soc. Exp. Biol. Med. 1966;123:692–696. doi: 10.3181/00379727-123-31578. [DOI] [PubMed] [Google Scholar]
- 16.Dempsey C., McCormick N.H., Croxford T.P., Seo Y.A., Grider A., Kelleher S.L. Marginal maternal zinc deficiency in lactating mice reduces secretory capacity and alters milk composition. J. Nutr. 2012;142:655–660. doi: 10.3945/jn.111.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.T., Baek S.H., Lee S.H., Park E.K., Kim E.C., Kwun I.S., Shin H.I. Zinc-deficient diet decreases fetal long bone growth through decreased bone matrix formation in mice. J. Med. Food. 2009;12:118–123. doi: 10.1089/jmf.2007.0647. [DOI] [PubMed] [Google Scholar]
- 18.McCormick N.H., King J., Krebs N., Soybel D.I., Kelleher S.L. Redistribution of tissue zinc pools during lactation and dyshomeostasis during marginal zinc deficiency in mice. J. Trace Elem. Med. Biol. 2014;29:170–175. doi: 10.1016/j.jtemb.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian X., Anthony K., Neuberger T., Diaz F.J. Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol. Reprod. 2014;90:83. doi: 10.1095/biolreprod.113.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown K.H., Wuehler S.E., Peerson J.M. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr. Bull. 2001;22:113–125. doi: 10.1177/156482650102200201. [DOI] [Google Scholar]
- 21.National Health and Medical Research Council . Nutrient Reference Values for Australia and New Zealand. NHMRC; Canberra, Australia: 2005. [Google Scholar]
- 22.Food and Nutrition Board: Institute of Medicine . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. The National Academies Press; Washington, DC, USA: 2001. [PubMed] [Google Scholar]
- 23.Caulfield L.E., Zavaleta N., Shankar A.H., Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am. J. Clin. Nutr. 1998;68:499s–508s. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- 24.King J.C. Determinants of maternal zinc status during pregnancy. Am. J. Clin. Nutr. 2000;71:1334s–1343s. doi: 10.1093/ajcn/71.5.1334s. [DOI] [PubMed] [Google Scholar]
- 25.Parr R. Assessment of dietary intakes. Trace Elem. Hum. Nutr. Health. 1996;1996:265–288. [Google Scholar]
- 26.Caulfield L.E., Black R.E. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors Geneva. World Health Organization; Geneva, Swizterland: 2004. Zinc deficiency; pp. 257–279. [Google Scholar]
- 27.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE. 2012;7:641. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ota E., Mori R., Middleton P., Tobe-Gai R., Mahomed K., Miyazaki C., Bhutta Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD000230.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah D., Sachdev H.P. Effect of gestational zinc deficiency on pregnancy outcomes: Summary of observation studies and zinc supplementation trials. Br. J. Nutr. 2001;85:S101–S108. doi: 10.1079/BJN2000301. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y., Shen X., Zhang D. The relationship between serum zinc level and preeclampsia: A Meta-Analysis. Nutrients. 2015;7:7806–7820. doi: 10.3390/nu7095366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Adeniyi F.A. The implications of hypozincemia in pregnancy. Acta Obstet. Gynecol. Scand. 1987;66:579–582. doi: 10.3109/00016348709022059. [DOI] [PubMed] [Google Scholar]
- 33.Akinloye O., Oyewale O.J., Oguntibeju O.O. Evaluation of trace elements in pregnant women with pre-eclampsia. Afr. J. Biotechnol. 2010;9:5196–5202. [Google Scholar]
- 34.Kumru S., Aydin S., Simsek M., Sahin K., Yaman M., Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in preeclamptic and healthy pregnant women. Biol. Trace Elem. Res. 2003;94:105–112. doi: 10.1385/BTER:94:2:105. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Tan M., Huang Z., Sheng L., Ge Y., Zhang H., Jiang M., Zhang G. Elemental contents in serum of pregnant women with gestational diabetes mellitus. Biol. Trace Elem. Res. 2002;88:113–118. doi: 10.1385/BTER:88:2:113. [DOI] [PubMed] [Google Scholar]
- 36.Farzin L., Sajadi F. Comparison of serum trace element levels in patients with or without pre-eclampsia. J. Res. Med. Sci. 2012;17:938–941. [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S., Jain N.P., Avasthi K., Wander G.S. Plasma and erythrocyte zinc in pre-eclampsia and its correlation with foetal outcome. J. Assoc. Phys. India. 2014;62:306–310. [PubMed] [Google Scholar]
- 38.Hyvonen-Dabek M., Nikkinen-Vilkki P., Dabek J.T. Selenium and other elements in human maternal and umbilical serum, as determined simultaneously by proton-induced X-ray emission. Clin. Chem. 1984;30:529–533. [PubMed] [Google Scholar]
- 39.Jain S., Sharma P., Kulshreshtha S., Mohan G., Singh S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol. Trace Elem. Res. 2010;133:162–170. doi: 10.1007/s12011-009-8423-9. [DOI] [PubMed] [Google Scholar]
- 40.Donangelo C.M., King J.C. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients. 2012;4:782–798. doi: 10.3390/nu4070782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura T., Goldenberg R.L. Zinc nutriture and pregnancy outcome. Nutr. Res. 1996;16:139–181. doi: 10.1016/0271-5317(95)02068-3. [DOI] [Google Scholar]
- 42.Simmer K., Iles C.A., Slavin B., Keeling P.W., Thompson R.P. Maternal nutrition and intrauterine growth retardation. Hum. Nutr. Clin. Nutr. 1987;41:193–197. [PubMed] [Google Scholar]
- 43.Negandhi P.H., Negandhi H.N., Zodpey S.P., Ughade S.N., Biranjan J.R. Risk factors for low birth weight in an Indian urban setting: A nested case control study. Asia Pac. J. Public Health. 2014;26:461–469. doi: 10.1177/1010539511431486. [DOI] [PubMed] [Google Scholar]
- 44.Scholl T.O., Hediger M.L., Schall J.I., Fischer R.L., Khoo C.S. Low zinc intake during pregnancy: Its association with preterm and very preterm delivery. Am. J. Epidemiol. 1993;137:1115–1124. doi: 10.1093/oxfordjournals.aje.a116615. [DOI] [PubMed] [Google Scholar]
- 45.Neggers Y.H., Goldenberg R.L., Tamura T., Cliver S.P., Hoffman H.J. The relationship between maternal dietary intake and infant birthweight. Acta Obstet. Gynecol. Scand. Suppl. 1997;165:71–75. [PubMed] [Google Scholar]
- 46.Wang H., Hu Y.-F., Hao J.-H., Chen Y.-H., Su P.-Y., Wang Y., Yu Z., Fu L., Xu Y.-Y., Zhang C., et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Sci. Rep. 2015;5:11262. doi: 10.1038/srep11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voss Jepsen L., Clemmensen K. Zinc in Danish women during late normal pregnancy and pregnancies with intra-uterine growth retardation. Acta Obstet. Gynecol. Scand. 1987;66:401–405. doi: 10.3109/00016348709022042. [DOI] [PubMed] [Google Scholar]
- 48.Borella P., Szilagyi A., Than G., Csaba I., Giardino A., Facchinetti F. Maternal plasma concentrations of magnesium, calcium, zinc and copper in normal and pathological pregnancies. Sci. Total Environ. 1990;99:67–76. doi: 10.1016/0048-9697(90)90212-D. [DOI] [PubMed] [Google Scholar]
- 49.Neggers Y.H., Cutter G.R., Acton R.T., Alvarez J.O., Bonner J.L., Goldenberg R.L., Go R.C., Roseman J.M. A positive association between maternal serum zinc concentration and birth weight. Am. J. Clin. Nutr. 1990;51:678–684. doi: 10.1093/ajcn/51.4.678. [DOI] [PubMed] [Google Scholar]
- 50.Bro S., Berendtsen H., Norgaard J., Host A., Jorgensen P.J. Serum zinc and copper concentrations in maternal and umbilical cord blood. Relation to course and outcome of pregnancy. Scand. J. Clin. Lab. Investig. 1988;48:805–811. doi: 10.3109/00365518809088764. [DOI] [PubMed] [Google Scholar]
- 51.Mistry H.D., Kurlak L.O., Young S.D., Briley A.L., Pipkin F.B., Baker P.N., Poston L. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Matern. Child. Nutr. 2014;10:327–334. doi: 10.1111/j.1740-8709.2012.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura T., Goldenberg R.L., Johnston K.E., Cliver S.P., Hoffman H.J. Serum concentrations of zinc, folate, vitamins A and E, and proteins, and their relationships to pregnancy outcome. Acta Obstet. Gynecol. Scand. Suppl. 1997;165:63–70. [PubMed] [Google Scholar]
- 53.Tamura T., Goldenberg R.L., Johnston K.E., DuBard M. Maternal plasma zinc concentrations and pregnancy outcome. Am. J. Clin. Nutr. 2000;71:109–113. doi: 10.1093/ajcn/71.1.109. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh A., Fong L.Y., Wan C.W., Liang S.T., Woo J.S., Wong V. Zinc deficiency is not a cause for abortion, congenital abnormality and small-for-gestational age infant in Chinese women. Br. J. Obstet. Gynaecol. 1985;92:886–891. doi: 10.1111/j.1471-0528.1985.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 55.Cherry F.F., Bennett E.A., Bazzano G.S., Johnson L.K., Fosmire G.J., Batson H.K. Plasma zinc in hypertension/toxemia and other reproductive variables in adolescent pregnancy. Am. J. Clin. Nutr. 1981;34:2367–2375. doi: 10.1093/ajcn/34.11.2367. [DOI] [PubMed] [Google Scholar]
- 56.Bogden J.D., Thind I.S., Kemp F.W., Caterini H. Plasma concentrations of calcium, chromium, copper, iron, magnesium, and zinc in maternal and cord blood and their relationship to low birth weight. J. Lab. Clin. Med. 1978;92:455–462. [PubMed] [Google Scholar]
- 57.Atinmo T., Mbofung C., Osinusi B.O. Relationship of zinc and copper concentrations in maternal and cord blood and birth weight. Int. J. Gynaecol. Obstet. 1980;18:452–454. doi: 10.1002/j.1879-3479.1980.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 58.Abass R.M., Hamdan H.Z., Elhassan E.M., Hamdan S.Z., Ali N.I., Adam I. Zinc and copper levels in low birth weight deliveries in Medani Hospital, Sudan. BMC Res. Notes. 2014;7:641. doi: 10.1186/1756-0500-7-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rwebembera A.A.-B., Munubhi E.K.D., Manji K.P., Mpembeni R., Philip J. Relationship between infant birth weight </=2000 g and maternal zinc levels at Muhimbili National Hospital, Dar Es Salaam, Tanzania. J. Trop. Pediatr. 2006;52:118–125. doi: 10.1093/tropej/fmi077. [DOI] [PubMed] [Google Scholar]
- 60.Bahl L., Chaudhuri L.S., Pathak R.M. Study of serum zinc in neonates and their mothers in Shimla hills (Himachal Pradesh) Indian J. Pediatr. 1994;61:571–575. doi: 10.1007/BF02751721. [DOI] [PubMed] [Google Scholar]
- 61.Singh P.P., Khushlani K., Veerwal P.C., Gupta R.C. Relationship between birth weight and zinc status of newly born infants and their mothers. Indian J. Physiol. Pharmacol. 1989;33:134–135. [PubMed] [Google Scholar]
- 62.Prema K. Predictive value of serum copper and zinc in normal and abnormal pregnancy. Indian J. Med. Res. 1980;71:554–560. [PubMed] [Google Scholar]
- 63.Badakhsh M.H., Khamseh M.E., Seifoddin M., Kashanian M., Malek M., Shafiee G., Baradaran H.R. Impact of maternal zinc status on fetal growth in an Iranian pregnant population. Gynecol. Endocrinol. 2011;27:1074–1076. doi: 10.3109/09513590.2011.569792. [DOI] [PubMed] [Google Scholar]
- 64.Goel R., Misra P.K. Study of plasma zinc in neonates and their mothers. Indian Pediatr. 1982;19:611–614. [PubMed] [Google Scholar]
- 65.Srivastava S., Mehrotra P.K., Srivastava S.P., Siddiqui M.K.J. Some essential elements in maternal and cord blood in relation to birth weight and gestational age of the baby. Biol. Trace Elem. Res. 2002;86:97–105. doi: 10.1385/BTER:86:2:097. [DOI] [PubMed] [Google Scholar]
- 66.Jeswani R.M., Vani S.N. A study of serum zinc levels in cord blood of neonates and their mothers. Indian J. Pediatr. 1991;58:683–686. doi: 10.1007/BF02820191. [DOI] [PubMed] [Google Scholar]
- 67.George S.S., Swaminathan S., Kanagasabapathy A.S., Seshadri L. Maternal zinc indices and small babies. Natl. Med. J. India. 1998;11:120–121. [PubMed] [Google Scholar]
- 68.Akman I., Arioglu P., Koroglu O.A., Sakalli M., Ozek E., Topuzoglu A., Eren S., Bereket A. Maternal zinc and cord blood zinc, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 levels in small-for-gestational-age newborns. Clin. Exp. Obstet. Gynecol. 2006;33:238–240. [PubMed] [Google Scholar]
- 69.Ozdemir U., Gulturk S., Aker A., Guvenal T., Imir G., Erselcan T. Correlation between birth weight, leptin, zinc and copper levels in maternal and cord blood. J. Physiol. Biochem. 2007;63:121–128. doi: 10.1007/BF03168223. [DOI] [PubMed] [Google Scholar]
- 70.Tande D.L., Ralph J.L., Johnson L.K., Scheett A.J., Hoverson B.S., Anderson C.M. First trimester dietary intake, biochemical measures, and subsequent gestational hypertension among nulliparous women. J. Midwifery Womens Health. 2013;58:423–430. doi: 10.1111/jmwh.12007. [DOI] [PubMed] [Google Scholar]
- 71.Lazebnik N., Kuhnert B.R., Kuhnert P.M. Zinc, cadmium, and hypertension in parturient women. Am. J. Obstet. Gynecol. 1989;161:437–440. doi: 10.1016/0002-9378(89)90538-3. [DOI] [PubMed] [Google Scholar]
- 72.Kim J., Kim Y.J., Lee R., Moon J.H., Jo I. Serum levels of zinc, calcium, and iron are associated with the risk of preeclampsia in pregnant women. Nutr. Res. 2012;32:764–769. doi: 10.1016/j.nutres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Kiilholma P., Paul R., Pakarinen P., Gronroos M. Copper and zinc in pre-eclampsia. Acta Obstet. Gynecol. Scand. 1984;63:629–631. doi: 10.3109/00016348409155551. [DOI] [PubMed] [Google Scholar]
- 74.Araujo Brito J., do Nascimento Marreiro D., Moita Neto J.M., Michelle Costa e Silva D., Goncalves de Sousa Almondes K., Valadares Neto J.D.D., do Nascimento Nogueira N. Enzyme activity of superoxide dismutase and zincemia in women with preeclampsia. Nutr. Hosp. 2013;28:486–490. doi: 10.3305/nh.2013.28.2.6179. [DOI] [PubMed] [Google Scholar]
- 75.Magri J., Sammut M., Savona-Ventura C. Lead and other metals in gestational hypertension. Int. J. Gynaecol. Obstet. 2003;83:29–36. doi: 10.1016/S0020-7292(03)00212-1. [DOI] [PubMed] [Google Scholar]
- 76.Fenzl V., Flegar-Mestric Z., Perkov S., Andrisic L., Tatzber F., Zarkovic N., Duic Z. Trace elements and oxidative stress in hypertensive disorders of pregnancy. Arch. Gynecol. Obstet. 2013;287:19–24. doi: 10.1007/s00404-012-2502-4. [DOI] [PubMed] [Google Scholar]
- 77.Katz O., Paz-Tal O., Lazer T., Aricha-Tamir B., Mazor M., Wiznitzer A., Sheiner E. Severe pre-eclampsia is associated with abnormal trace elements concentrations in maternal and fetal blood. J. Matern. Fetal Neonatal Med. 2012;25:1127–1130. doi: 10.3109/14767058.2011.624221. [DOI] [PubMed] [Google Scholar]
- 78.Mistry H.D., Gill C.A., Kurlak L.O., Seed P.T., Hesketh J.E., Meplan C., Schomburg L., Chappell L.C., Morgan L., Poston L. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks gestation in nulliparous women who subsequently develop preeclampsia. Free Radic. Biol. Med. 2015;78:147–155. doi: 10.1016/j.freeradbiomed.2014.10.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lao T.T., Chin R.K., Swaminathan R., Mak Y.T. Plasma and erythrocyte zinc concentrations in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989;30:117–122. doi: 10.1016/0028-2243(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 80.Sarwar M.S., Ahmed S., Ullah M.S., Kabir H., Rahman G.K.M.M., Hasnat A., Islam M.S. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol. Trace Elem. Res. 2013;154:14–20. doi: 10.1007/s12011-013-9721-9. [DOI] [PubMed] [Google Scholar]
- 81.Ilhan N., Ilhan N., Simsek M. The changes of trace elements, malondialdehyde levels and superoxide dismutase activities in pregnancy with or without preeclampsia. Clin. Biochem. 2002;35:393–397. doi: 10.1016/S0009-9120(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 82.Bakacak M., Kilinc M., Serin S., Ercan O., Kostu B., Avci F., Kiran H., Kiran G. Changes in copper, zinc, and malondialdehyde levels and superoxide dismutase activities in pre-eclamptic pregnancies. Med. Sci. Monit. 2015;21:2414–2420. doi: 10.12659/MSM.895002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Jameil N., Tabassum H., Al-Mayouf H., Aljohar H.I., Alenzi N.D., Hijazy S.M., Khan F.A. Analysis of serum trace elements-copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: A prospective case controlled study in Riyadh, Saudi Arabia. Int. J. Clin. Exp. Pathol. 2014;7:1900–1910. [PMC free article] [PubMed] [Google Scholar]
- 84.Bassiouni B.A., Foda A.I., Rafei A.A. Maternal and fetal plasma zinc in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1979;9:75–80. doi: 10.1016/0028-2243(79)90002-9. [DOI] [PubMed] [Google Scholar]
- 85.Harma M., Harma M., Kocyigit A. Correlation between maternal plasma homocysteine and zinc levels in preeclamptic women. Biol. Trace Elem. Res. 2005;104:97–105. doi: 10.1385/BTER:104:2:097. [DOI] [PubMed] [Google Scholar]
- 86.Rafeeinia A., Tabandeh A., Khajeniazi S., Marjani A.J. Serum copper, zinc and lipid peroxidation in pregnant women with preeclampsia in gorgan. Open Biochem. J. 2014;8:83–88. doi: 10.2174/1874091X01408010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vafaei H., Dalili M., Hashemi S.A. Serum concentration of calcium, magnesium and zinc in normotensive versus preeclampsia pregnant women: A descriptive study in women of Kerman province of Iran. Iran. J. Reprod. Med. 2015;13:23–26. [PMC free article] [PubMed] [Google Scholar]
- 88.Ahsan T., Banu S., Nahar Q., Ahsan M., Khan M.N., Islam S.N. Serum trace elements levels in preeclampsia and eclampsia: Correlation with the pregnancy disorder. Biol. Trace Elem. Res. 2013;152:327–332. doi: 10.1007/s12011-013-9637-4. [DOI] [PubMed] [Google Scholar]
- 89.Rathore S., Gupta A., Batra H.S., Rathore R. Comparative study of trace elements and serum ceruloplasmin level in normal and pre-eclamptic pregnancies with their cord blood. Biomed. Res. 2011;22:207–210. [Google Scholar]
- 90.Kolusari A., Kurdoglu M., Yildizhan R., Adali E., Edirne T., Cebi A., Demir H., Yoruk I.H. Catalase activity, serum trace element and heavy metal concentrations, and vitamin A, D and E levels in pre-eclampsia. J. Int. Med. Res. 2008;36:1335–1341. doi: 10.1177/147323000803600622. [DOI] [PubMed] [Google Scholar]
- 91.Atamer Y., Kocyigit Y., Yokus B., Atamer A., Erden A.C. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005;119:60–66. doi: 10.1016/j.ejogrb.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 92.Adam B., Malatyalioglu E., Alvur M., Talu C. Magnesium, zinc and iron levels in pre-eclampsia. J. Matern. Fetal Med. 2001;10:246–250. doi: 10.1080/jmf.10.4.246.250-14. [DOI] [PubMed] [Google Scholar]
- 93.Vigeh M., Yokoyama K., Ramezanzadeh F., Dahaghin M., Sakai T., Morita Y., Kitamura F., Sato H., Kobayashi Y. Lead and other trace metals in preeclampsia: A case-control study in Tehran, Iran. Environ. Res. 2006;100:268–275. doi: 10.1016/j.envres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Carmichael S.L., Yang W., Shaw G.M., National Birth Defects Prevention Sdudy Maternal dietary nutrient intake and risk of preterm delivery. Am. J. Perinatol. 2013;30:579–588. doi: 10.1055/s-0032-1329686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu W.-Y., Wu C.-H., Hsieh C.T.-C., Lo H.-C., Lin J.-S., Kao M.-D. Low body weight gain, low white blood cell count and high serum ferritin as markers of poor nutrition and increased risk for preterm delivery. Asia Pac. J. Clin. Nutr. 2013;22:90–99. doi: 10.6133/apjcn.2013.22.1.05. [DOI] [PubMed] [Google Scholar]
- 96.Wang H., Hu Y.-F., Hao J.-H., Chen Y.-H., Wang Y., Zhu P., Zhang C., Xu Y.-Y., Tao F.-B., Xu D.-X. Maternal serum zinc concentration during pregnancy is inversely associated with risk of preterm birth in a Chinese population. J. Nutr. 2016;146:509–515. doi: 10.3945/jn.115.220632. [DOI] [PubMed] [Google Scholar]
- 97.Bo S., Lezo A., Menato G., Gallo M.-L., Bardelli C., Signorile A., Berutti C., Massobrio M., Pagano G.F. Gestational hyperglycemia, zinc, selenium, and antioxidant vitamins. Nutrition. 2005;21:186–191. doi: 10.1016/j.nut.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 98.Behboudi-Gandevani S., Safary K., Moghaddam-Banaem L., Lamyian M., Goshtasebi A., Goshtasbi A., Alian-Moghaddam N. The relationship between maternal serum iron and zinc levels and their nutritional intakes in early pregnancy with gestational diabetes. Biol. Trace Elem. Res. 2013;154:7–13. doi: 10.1007/s12011-013-9703-y. [DOI] [PubMed] [Google Scholar]
- 99.Wibell L., Gebre-Medhin M., Lindmark G. Magnesium and zinc in diabetic pregnancy. Acta Paediatr. Scand. Suppl. 1985;320:100–106. doi: 10.1111/j.1651-2227.1985.tb10146.x. [DOI] [PubMed] [Google Scholar]
- 100.Al-Saleh E., Nandakumaran M., Al-Shammari M., Al-Harouny A. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in patients with gestational diabetes. J. Matern. Fetal Neonatal Med. 2004;16:15–21. doi: 10.1080/14767050412331283139. [DOI] [PubMed] [Google Scholar]
- 101.Prema K., Ramalakshmi B.A., Babu S. Serum copper and zinc in hormonal contraceptive users. Fertil. Steril. 1980;33:267–271. doi: 10.1016/S0015-0282(16)44591-7. [DOI] [PubMed] [Google Scholar]
- 102.Kristal A.R., Peters U., Potter J.D. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol. Biomark. Prev. 2005;14:2826–2828. doi: 10.1158/1055-9965.EPI-12-ED1. [DOI] [PubMed] [Google Scholar]
- 103.Jackson M. Zinc in Human Biology. Springer; London, UK: 1989. Physiology of zinc: General aspects; pp. 1–14. [Google Scholar]
- 104.Goode H.F., Kelleher J., Walker B.E. The effects of acute infection on indices of zinc status. Clin. Nutr. 1991;10:55–59. doi: 10.1016/0261-5614(91)90082-N. [DOI] [PubMed] [Google Scholar]
- 105.Halsted J.A., Hackley B.M., Smith J.C., Jr. Plasma-zinc and copper in pregnancy and after oral contraceptives. Lancet. 1968;2:278–279. doi: 10.1016/S0140-6736(68)92375-1. [DOI] [PubMed] [Google Scholar]
- 106.Prasad A.S. Clinical, endocrinological and biochemical effects of zinc deficiency. Clin. Endocrinol. Metab. 1985;14:567–589. doi: 10.1016/S0300-595X(85)80007-4. [DOI] [PubMed] [Google Scholar]
- 107.Singh A., Smoak B.L., Patterson K.Y., LeMay L.G., Veillon C., Deuster P.A. Biochemical indices of selected trace minerals in men: Effect of stress. Am. J. Clin. Nutr. 1991;53:126–131. doi: 10.1093/ajcn/53.1.126. [DOI] [PubMed] [Google Scholar]
- 108.Moran V.H., Skinner A.L., Medina M.W., Patel S., Dykes F., Souverein O.W., Dullemeijer C., Lowe N.M. The relationship between zinc intake and serum/plasma zinc concentration in pregnant and lactating women: A systematic review with dose-response meta-analyses. J. Trace Elem. Med. Biol. 2012;26:74–79. doi: 10.1016/j.jtemb.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Benes B., Spevackova V., Smid J., Batariova A., Cejchanova M., Zitkova L. Effects of age, BMI, smoking and contraception on levels of Cu, Se and Zn in the blood of the population in the Czech Republic. Cent. Eur. J. Public Health. 2005;13:202–207. [PubMed] [Google Scholar]
- 110.Ghayour-Mobarhan M., Taylor A., New S.A., Lamb D.J., Ferns G.A. Determinants of serum copper, zinc and selenium in healthy subjects. Ann. Clin. Biochem. 2005;42:364–375. doi: 10.1258/0004563054889990. [DOI] [PubMed] [Google Scholar]
- 111.North R.A., McCowan L.M., Dekker G.A., Poston L., Chan E.H., Stewart A.W., Black M.A., Taylor R.S., Walker J.J., Baker P.N., et al. Clinical risk prediction for pre-eclampsia in nulliparous women: Development of model in international prospective cohort. BMJ. 2011;342:d1875. doi: 10.1136/bmj.d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kenny L.C., Black M.A., Poston L., Taylor R., Myers J.E., Baker P.N., McCowan L.M., Simpson N.A., Dekker G.A., Roberts C.T., et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: The Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014;64:644–652. doi: 10.1161/HYPERTENSIONAHA.114.03578. [DOI] [PubMed] [Google Scholar]
- 113.Brown K.H., Rivera J.A., Bhutta Z., Gibson R.S., King J.C., Lonnerdal B., Ruel M.T., Sandtrom B., Wasantwisut E., Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004;25:S99–S203. [PubMed] [Google Scholar]
- 114.Burton G.J., Jauniaux E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Marklund S. Distribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluids. Acta Physiol. Scand. Suppl. 1980;492:19–23. [PubMed] [Google Scholar]
- 116.Bruno R.S., Song Y., Leonard S.W., Mustacich D.J., Taylor A.W., Traber M.G., Ho E. Dietary zinc restriction in rats alters antioxidant status and increases plasma F2 isoprostanes. J. Nutr. Biochem. 2007;18:509–518. doi: 10.1016/j.jnutbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 117.Oteiza P.I., Clegg M.S., Zago M.P., Keen C.L. Zinc deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Radic. Biol. Med. 2000;28:1091–1099. doi: 10.1016/S0891-5849(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 118.Prasad A.S., Beck F.W., Bao B., Fitzgerald J.T., Snell D.C., Steinberg J.D., Cardozo L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 119.Sankavaram K., Chong L., Bruno R.S., Freake H.C. Zinc status alters growth and oxidative stress responses in rat hepatoma cells. Nutr. Cancer. 2014;66:104–116. doi: 10.1080/01635581.2014.851713. [DOI] [PubMed] [Google Scholar]
- 120.Song Y., Leonard S.W., Traber M.G., Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J. Nutr. 2009;139:1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Habib Z., Abdulla M. Plasma levels of zinc, copper, magnesium and calcium during early weeks of gestation. Acta Pharmacol. Toxicol. 1986;59(Suppl. 7):602–605. doi: 10.1111/j.1600-0773.1986.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 122.Donangelo C.M., Zapata C.L., Woodhouse L.R., Shames D.M., Mukherjea R., King J.C. Zinc absorption and kinetics during pregnancy and lactation in Brazilian women. Am. J. Clin. Nutr. 2005;82:118–124. doi: 10.1093/ajcn.82.1.118. [DOI] [PubMed] [Google Scholar]
- 123.Fung E.B., Ritchie L.D., Woodhouse L.R., Roehl R., King J.C. Zinc absorption in women during pregnancy and lactation: A longitudinal study. Am. J. Clin. Nutr. 1997;66:80–88. doi: 10.1093/ajcn/66.1.80. [DOI] [PubMed] [Google Scholar]
- 124.Sherwood L. Human Physiology: From Cells to Systems. Cengage Learning; Boston, MA, USA: 2015. [Google Scholar]
- 125.Bowen J.M., Chamley L., Keelan J.A., Mitchell M.D. Cytokines of the placenta and extra-placental membranes: Roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 126.Allen L.H. Multiple micronutrients in pregnancy and lactation: An overview. Am. J. Clin. Nutr. 2005;81:1206S–1212S. doi: 10.1093/ajcn/81.5.1206. [DOI] [PubMed] [Google Scholar]
- 127.Christian P., West K.P., Jr. Interactions between zinc and vitamin A: An update. Am. J. Clin. Nutr. 1998;68:435S–441S. doi: 10.1093/ajcn/68.2.435S. [DOI] [PubMed] [Google Scholar]
- 128.Lonnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 129.Solomons N.W. Competitive interaction of iron and zinc in the diet: Consequences for human nutrition. J. Nutr. 1986;116:927–935. doi: 10.1093/jn/116.6.927. [DOI] [PubMed] [Google Scholar]