Abstract
AIM
To present the current state-of-the art of molecular imaging in the management of patients affected by inflammatory bowel disease (IBD).
METHODS
A systematic review of the literature was performed in order to find important original articles on the role of molecular imaging in the management of patients affected by IBD. The search was updated until February 2016 and limited to articles in English.
RESULTS
Fifty-five original articles were included in this review, highlighting the role of single photon emission tomography and positron emission tomography.
CONCLUSION
To date, molecular imaging represents a useful tool to detect active disease in IBD. However, the available data need to be validated in prospective multicenter studies on larger patient samples.
Keywords: White blood cell scintigraphy, Inflammatory bowel disease, Inflammation, 18F-Fluorodehoxiglucose positron emission tomography/computed tomography, Molecular imaging
Core tip: Inflammatory bowel disease (IBD) is a chronic granulomatous inflammatory condition, in which the integrity of the gut epithelium represents a major pathophysiological step. Although endoscopy and barium radiological examinations are the diagnostic “gold standard” for IBD, both techniques require a specific patient preparation, not always feasible or easily tolerated. Molecular imaging with single photon emission tomography and positron emission tomography seems to be a reliable, non-invasive, accurate and easily reproducible diagnostic tool, able to assess location, extent and activity grade of IBD. We here present the current state-of-the art of molecular imaging in the management of patients affected by IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory condition, in which the integrity of the gut epithelium represents a major pathophysiological step. Both ulcerative colitis (UC) and Crohn’s disease (CD) follow a relapsing and remitting course. As such, tools able to diagnose and manage patients affected by these conditions are highly relevant in clinical practice.
Endoscopy and barium radiological examinations are the diagnostic “gold standard” for IBD[1-3]. Unfortunately, both techniques require a specific patient preparation, which is not always feasible and not easily tolerated. Specifically, endoscopy is an invasive procedure associated with increased risk of bowel perforation, if performed during the acute phase of disease[1,3,4]. Moreover, it cannot study the deepest layers of intestinal wall and discover possible extra-intestinal alterations[5]. Finally, in case of CD complicated by strictures, endoscopic exploration often remains incomplete, due to difficulties in going beyond narrowed bowel segments and in exploring the distal side of the strictures[4].
In this context, the need for a novel non-invasive, accurate and easily reproducible diagnostic tool, able to assess location, extent and activity grade of IBD, is crucial[1,6,7]. Molecular imaging with single photon emission tomography (SPECT) and positron emission tomography (PET) seems to be promising, allowing for tracking molecular changes occurring before clinical manifestations.
We therefore aimed at presenting the current state-of-the art of molecular imaging in the management of patients affected by IBD.
MATERIALS AND METHODS
Search strategy
A comprehensive computer literature search of the PubMed/MEDLINE and Web of Knowledge database was performed in order to find important original articles on the role of molecular imaging in the management of patients affected by IBD. Papers on future perspectives in the field and experimental data were also searched. The adopted algorithm was based on a combination of the terms: (a) IBD and (b1) “positron emission tomography” or “PET” or (b2) “single photon emission tomography” or “SPET” and (c1) diagnosis or (c2) prognosis or (c3) follow-up. The search was updated until February 2016 and limited to articles in English. The references of the retrieved articles were also checked for additional studies as not to exclude possible interesting data.
Article selection
Studies investigating the role of SPECT and PET in patients with IBD were eligible for inclusion. Studies with at least 6 patients were selected. Review articles, articles not in the field of interest, single/double case reports and commentaries were excluded. Meta-analyses were retrieved to provide convincing data for the discussion. Also, abstracts or oral/poster meeting presentations were not included.
RESULTS
Fifty-five original articles were included in this review. Specifically, 10 studies were available on the role of SPECT and 19 on PET. Furthermore, 13 articles were retrieved on the role of molecular imaging in paediatric patients, and 13 articles provided interesting insight on experimental data in the field.
SPECT
Table 1 shows the selected studies on the role of SPECT in adult patients affected by IBD. The majority of the studies were based on the ability of 99mTc-HMPAO-white blood cell (WBC) to diagnose the presence of IBD. Lachter et al[8] showed that 99mTc-HMPAO-WBC scintigraphy can be useful for patients with CD, while it is not sophisticated for the diagnosis of UC. Some years later, Paredes et al[9], reported that patients with suspected recurrent IBD after surgery can be evaluated with 99mTc-HMPAO-WBC scintigraphy as an alternative to ileoendoscopy. The single-injection technique used for the administration of LeukoScan simplifies the labelling techniques required for white cell imaging. Kerry et al[10], investigated 99mTc-leukoscan and 99mTc-white cell scan in suspected IBD in 22 patients. Planar 99mTc-WBC images were acquired at 45 min and 2 h post-injection, while planar 99mTc-leukoscan images were acquired at 1, 2 and 4 h post-injection and with a SPECT after 4 h. From a head-to-head comparison 99mTc-WBC scan showed a better diagnostic accuracy in IBD patients than 99mTc-leukoscan.
Table 1.
Studies on planar scintigraphy and single photon emission tomography included in the present review
| Ref. | Year of pub | Journal | n of pts | Indication | Imaging technique | Gold standard | Conclusion |
| Rowe et al[14] | 1995 | Am J Gastroenterol | 11 | The measurement of the severity colitis | 111In-labelled leukocyte planar scintigraphy | Truelove and Witts criteria | The disappearance of radioactivity from the spleen or whole body during 24 h is likely to be a useful and accurate index of disease severity in inflammatory colitis Scintigraphy is useful for patients with CD, but not for ulcerative colitis. Leukocyte scintigraphy is more useful for the reassessment than initial diagnosis (particularly in case of structuring and fistulising CD). 99mTc-Leukoscan cannot be useful for the evaluation of IBD |
| Lachter et al[8] | 2003 | Hepato-Gastroenterology | 46 | Diagnosis of suspected inflammatory bowel disease | 99mTc-HMPAO planar scintigraphy | Histology | |
| Kerry et al[10] | 2005 | Nuclear Medicine Communication | 22 | Diagnosis of IBD and comparison between 99mTc-HMPAO and 99mTc-Leukoscan | 99mTc-HMPAO planar scintigraphy 99mTc-Leukoscan planar scintigraphy 99mTc-Leukoscan SPECT | Histology Radiology Response to treatment | |
| Biancone et al[11] | 2005 | Am J Gastroenterol | 22 | Comparison between 99mTc-HMPAO planar and SPECT for the assessment of intestinal infiltration in CD | 99mTc-HMPAO planar and SPECT | Histology | SPECT images may better discriminate between intestinal and bone marrow uptake, thus allowing a better visualization of CD lesions in the pelvis (especially for perianal and enterovesical disease) |
| Cheow et al[15] | 2005 | Eur J Nucl Med Mol Imaging | 30 | To quantify disease activity in IBD | 99mTc-granulocytes planar scintigraphy and 111In-granulocytes | NA | A dedicated whole-body counting using 111In can be useful to quantify inflammatory disease, especially IBD |
| Van den Brande et al[16] | 2007 | Gut | 14 | To predict the efficacy of anti-TNF treatment in IBD | 99mTc-annexin V | Histology | The uptake of 99mTc-annexin V correlates with clinical benefit of anti-TNF treatment |
| Mota et al[13] | 2010 | World J Gastroenterol | 20 | To evaluate inflammatory activity in CD patients | 99mTc-HMPAO | NA | Scintigraphy with radiolabeled HMPAO could be useful for the evaluation of intestinal activity in CD |
| Paredes et al[9] | 2010 | Journal of Crohn’s and Colitis | 40 | To assess the accuracy of abdominal ultrasonography, 99mTc-HMPAO in recurrent CD | 99mTc-HMPAO | Histology | 99mTc-HMPAO can be used in case of postsurgical recurrence in CD, in particular for those patients who reject endoscopic examination or for the assessment of neoterminal ileum |
| Hillel et al[12] | 2011 | Nuclear Medicine Communication | 99 | To compare planar and SPECT imaging in IBD | 99mTc-HMPAO | NA | SPECT improves interoperator variability and probably sensitivity for IBD. The size of lesion suggest that planar images underestimates the extent of active disease |
| Aarntzen et al[19] | 2015 | J Nucl Med | 30 | To assess the accuracy of 99mTc-CXCL8 SPECT to detect and to localize disease activity | 99mTc-CXCL8 | Histology | 99mTc-CXCL8 is a novel target for neutrophil recruitment to the intestinal wall, especially in moderate to severe exacerbations of IBD |
CD: Crohn’s disease; IBD: Inflammatory bowel disease; TNF: Tumor necrosis factor; NA: Not available; SPECT: Single photon emission tomography; pts: Patients.
A direct comparison between the diagnostic performance of 99mTc-HMPAO-WBC SPECT and planar images was performed in 2005 and 2011[11,12]. These two studies comprised 22 and 99 patients, respectively and reported comparable results between planar and SPECT images regarding diagnostic accuracy[11,12]. However, SPECT images provided a more detailed visualization of those IBD lesions which were located in critical sites, like terminal ileum (due to the relatively intense overlying background bone marrow activity in the sacro-iliac region), pelvic floor and rectum (because the rectal disease is covered by the intense overlying background activity which is often present in the bladder).
Some authors aimed to quantify the severity of IBD by means of different parameters, like in-house validated scintigraphic indices (SI) at 99mTc-HMPAO-WBC scan[13] or the stool radioactivity (defined as the disappearance of 111In-radiolabelled leukocytes from the spleen[14] or from the whole body retention[14,15], as they migrates toward regions of inflammation). As a matter of fact, such semi-quantitative methods for the assessment of the severity of disease could be important for an objective evaluation of treatment response, although a more extensive demonstration of their usefulness lacks in the literature.
Van den Brande et al[16] investigated whether apoptosis in the intestine underlies the clinical benefit of anti-tumor necrosis factor (TNF) treatment in CD, performing real-time imaging[17,18] of in vivo apoptosis by using 99mTc-annexin V SPECT at baseline and 24 h after Infliximab treatment both in 2 models of murine experimental colitis and in 14 patients with active CD. The authors found a mean increase of 98.7% in colonic uptake of 99mTc-annexin V in 10 of the 14 responding patients compared with 15.2% in non-responding patients (P = 0.03). Thus, the use of 99mTc-annexin V SPECT may be envisioned in order to predict the response to Infliximab, given the correlation between the colonic uptake of 99mTc-annexin V and clinical benefit of anti-TNF treatment. This bears important implications in the choice of the best therapeutic options, also by increasing the cost-effectiveness of Infliximab.
A new emerging radiopharmaceutical agent was investigated to image the presence of Interleukin 8-receptors (IL8-R), which are overexpressed by activated neutrophils into the intestinal wall of patients with IBD. Recently, Aarntzen et al[19] evaluated the accuracy of 99mTc-IL8-R SPECT in a prospective series of patients affected by IBD (15 with CD and 15 affected by UC). Sensitivity and specificity on a per-patient basis for the detection of active disease were 95% and 44% for 99mTc-IL8-R scan and 71% and 70% for endoscopy, respectively. On a per-segment basis, sensitivity and specificity were 82% and 72% for 99mTc-IL8-R scan, and 74% and 85% for endoscopy, respectively. The degree of 99mTc-IL8-R uptake correlated with that of neutrophilic involvement in affected mucosa. As such, the authors showed that 99mTc-IL8-R SPECT provides a novel imaging technique able to target neutrophil recruitment in the intestinal wall, especially in IBD exacerbations of moderate-to-severe degree. These encouraging results suggest to take advantage from 99mTc-IL8-R imaging in future studies, as a biomarker to personalize treatment with immunomodulating drugs.
An overview of diagnostic performance of SPECT in the most relevant studies is reported in Table 2.
Table 2.
Diagnostic accuracies in some selected studies on single photon emission tomography
| Ref. | Year of pub | Tracer | Sensitivity | Specificity |
| Lachter et al[8] | 2003 | 99mTc-HMPAO | 58% | 100% |
| Kerry et al[10] | 2005 | |||
| 99mTc-HMPAO (2 h) | 99mTc-HMPAO | 87% | 86% | |
| 99mTc-Leukoscan (1 h) | 99mTc-Leukoscan | 20% | 86% | |
| 99mTc-Leukoscan (2 h) | 40% | 100% | ||
| 99mTc-Leukoscan (4 h) | 73% | 57% | ||
| 99mTc-Leukoscan (4 h-SPECT) | 87% | 57% | ||
| Paredes et al[9] | 2010 | 99mTc-HMPAO | ||
| Endoscopic recurrence | 88% | 42.9% | ||
| Scintigraphic recurrence | 73.3% | 88.2% | ||
| Aarntzen et al[19] | 2015 | 99mTc-CXCL8 | 95% | 44% |
SPECT: Single photon emission tomography.
PET
PET/CT performance in the diagnosis of IBD: Positron emission tomography with 18F-Fluorodehoxiglucose (18F-FDG-PET) fulfills, at least partially, the required criteria for a non-invasive, accurate and easily reproducible diagnostic tool, able to assess location, extent and activity degree of IBD. Inflamed tissues, especially during the active phase of disease, are often characterized by a high uptake of 18F-FDG on PET images, due to overexpression of glucose transporters on the surface of neutrophils and macrophage. The relatively recent addition of computed tomography (CT) to 18F-FDG-PET scan allows to simultaneously analyze functional and morphologic aspects of disease, thus permitting discriminating between active and quiescent inflammation (Figure 1). Moreover, other complications such as abscesses, fistulas, inflamed lymph nodes can be effectively detected[1-4,6,7,20,21].
Figure 1.
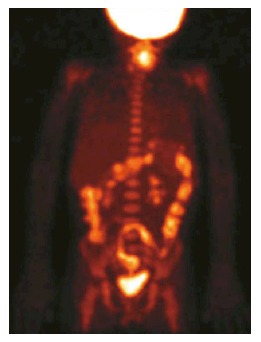
Maximum intensity projection image of an positron emission tomography with 18F-Fluorodehoxiglucose examination shows diffuse and intense radiopharmaceutical uptake in the large bowel. Reprinted with permission of Springer Verlag from Cistaro et al.
In the latest 10 years, a handful of studies explored the role of 18F-FDG-PET, eventually fused with CT, in the evaluation of disease activity in patients with known IBD. In 2007 Meisner et al[6] published the first prospective study on 12 patients with known and at least moderate IBD (7 UC and 5 CD) undergoing 18F-FDG-PET/CT. In this pilot-study, the correlation between active regions on PET scan and those found to be active based on clinical criteria (including colonoscopy and radiologic examinations results) was equal to 95.8% in UC and 81.3% in CD patients. Interestingly, the co-registration with CT was helpful for anatomical identification of different bowel segments in CD, especially in case of small bowel involvement and surgically treated patients, whereas PET alone considered adequate to accurately define anatomic regions in those patients diagnosed with UC.
One of the main limits of 18F-FDG-PET is represented by false positive cases due to physiologic uptake of the radiopharmaceutical in normal bowel, caused by the typically high turnover of intestinal mucosa, the presence of material in the lumen, bacterial flora, lymphoid tissue inside the mucosa, but mostly by the peristaltic activity of muscular layer and the physiologic collapse of bowel loops. However, these two latter factors can be effectively overcome by a proper distention of bowel segments through the administration of great amount of fluids, as in the case of PET/CT enterography[1,3,7,20,21]. To reduce the number of false positive findings in the intestinal tract during a PET scan, Franquet et al[22] proposed to undertake a therapy with Rifaximine 2 d before the PET scan. In their paper, this patient preparation allowed to reduce the unspecific FDG uptake.
For the evaluation of patients with CD, Ahmadi et al[7] retrospectively studied 41 patients with known or suspected active disease of the small bowel, who underwent 18F-FDG-PET/CT enterography. Thirty patients presented a total of 48 pathological bowel segments on CT enterography, of which 38 (79%) also showed an abnormal 18F-FDG uptake. All pathological areas identified by 18F-FDG-PET/CT enterography corresponded to abnormal areas visualized on CT enterography. CT enterography score of activity and semi-quantitative PET parameters showed also a significant correlation (P = 0.03). In a prospective study Groshar et al[20] also explored the ability of 18F-FDG-PET to detect IBD and to assess its degree of activity. The authors compared 18F-FDG-PET to enterography in 28 patients with known or suspected active CD. Eighty-five abnormal segments were found in 22 patients, while the remaining 6 subjects had unremarkable finding both on PET and CT enterography. The authors found a significant difference in abnormal and normal segments in mean wall thickness, mean mural enhancement and maximum standardized uptake value (SUVmax, P < 0.0001). A good correlation was also found between SUVmax and wall thickness or mural enhancement measurements, both in the colon and ileum (P < 0.00001). SUVmax correlated well also with the grade of other CT enterography parameters used to detect active inflammation, such as parietal attenuation, perienteric fat attenuation and perienteric/pericolic hypervascularization (P < 0.001).
A pilot study also analyzed the diagnostic advantage of adding 18F-FDG-PET to CT enterography[21]. The authors compared PET and CT enterography alone in 13 CD patients with clinically suspected active disease, using histology after surgery or biopsy performed during endoscopy as gold standard (in 6 and 7 patients, respectively). In 3 (23%) patients, 18F-FDG-PET allowed to identify signs of active disease otherwise missed by CT enterography alone, including an entero-colic fistula in one patient. Both techniques were able to identify all bowel segments with at least a moderate activity. There was a significant correlation between both techniques and histology in the evaluation of disease activity.
In a prospective study, Das et al[1] compared 18F-FDG-PET/CT and colonoscopy in 15 patients, affected by mild to moderate UC. 18F-FDG-PET/CT detected 66 involved bowel segments, whereas colonoscopy found 67 pathological regions, so that the detection rate of 18F-FDG-PET/CT was 98.5% compared to colonoscopy. A significant correlation between the two techniques was also found in the definition of disease extent (κ = 55.3%, P = 0.02).
18F-FDG-PET was also compared to other imaging techniques, showing in many cases even better diagnostic performance. In 2012 Holtmann et al[2] evaluated 43 patients with active CD, undergoing 18F-FDG-PET, ileo-colonoscopy and hydro-magnetic resonance imaging (MRI). A total of 241 bowel segments in the terminal ileum and colon were examined. Endoscopy detected inflammation in 80 of 241, while 18F-FDG-PET in 72 (sensitivity and a specificity of 90% and 92.6%, respectively). Conversely, hydro-MRI identified 53/80 inflamed segments with a sensitivity of only 66.3% and a specificity of 99.4%. Specifically, hydro-MRI displayed the lowest sensitivity but a 100% specificity for all colon segments, while in the terminal ileum sensitivity and specificity were 100% and 93.3%, respectively (vs 86.4% and 93.3%, respectively, of 18F-FDG-PET). In the proximal ileum, 18F-FDG-PET and hydro-MRI had the same results in all cases, reaching identical sensitivity and specificity.
One of the main issues in CD patients is to distinguish the nature of strictures (predominantly inflammatory or fibrotic, or mixed), as they represent a common complication of CD and show a very high rate of recurrence after surgical resection. As such, it is crucial to discriminate those lesions likely to benefit from surgical treatment (the predominantly fibrotic ones) from those still medically treatable (the inflammatory ones). With this regard, some authors suggested a possible role for 18F-FDG-PET, although data are still limited and controversial[2,4,5]. While Shyn and colleagues reported no significant difference in SUVmax between the strictures deserving or not a surgical resection, although in a small patient sample[21], Holtmann et al[2] showed that both hydro-MRI and 18F-FDG-PET have very high accuracy in detecting and characterizing strictures.
Louis et al[23] prospectively studied 22 patients with CD. Ninety-five ileal segments were comparatively analyzed by endoscopy and 18F-FDG-PET/CT, showing a significant correlation between CD endoscopic index (CDEI) and RSUVmax (the ratios of each positive-segment SUVmax over the SUVmax of the liver). In their paper, 18F-FDG-PET/CT detected and localized the vast majority of gastrointestinal segments with moderate to severe lesions (sensitivity for superficial ulcers, deep ulcers, and strictures was 84.4%), allowing a non-invasive evaluation of the ongoing pathologic process in the gastrointestinal tract. Overall, 18F-FDG-PET/CT detected 35 of 48 endoscopically affected segments (sensitivity for the detection of endoscopic lesions 72.9%). This study demonstrated an important role of PET/CT in detecting active intestinal disease beyond the mucosa. This ability is important because complications of CD such as strictures may develop despite the lack of mucosal lesions visible at endoscopy, and because the presence of extraenteric inflammation is widely recognized as an important prognostic factor in CD.
PET/CT enteroclysis has been suggested in several studies as a new promising approach. This technique essentially consists in a simple extension of CT enteroclysis and provides information both on morphological details and on the metabolic activity of the lesion(s). In a study by Das et al[24], PET/CT enteroclysis, as a single test, detected involvement of a significantly higher number of intestinal segments [total = 50, 23 in the small intestine and 27 in the large intestine, (P < 0.01) compared to conventional barium studies (16 segments of small intestine)] and colonoscopy (17 segments of large intestine). Similarly, a prospective study by Lenze et al[4] compared the performance of 18F-FDG-PET/CT enteroclysis, MR enteroclysis and trans-abdominal ultrasound in the detection of CD strictures and in the differentiation of fibrotic vs inflammatory ones, using invasive endoscopy and histology as reference. In 30 symptomatic patients endoscopy detected 37 strictures, both PET/CT enteroclysis and MR enteroclysis 30/37 (81%) and ultrasound 25/37 (68%). By combining two methods, the sensitivity increased: Specifically, it was reported to be 92% when combining PET/CT and ultrasound and 89% when combining MR and ultrasound or MR and PET/CT. However, the differentiation rates of the strictures nature was low and equal to 57% for MR, 53% for PET/CT and 40% for ultrasound. Considering only inflammatory strictures, MR and PET/CT correctly classified 94% and 83% of them, respectively, vs 66% depicted by ultrasound. By combining two methods (MR and ultrasound or PET/CT with ultrasound), all the strictures deserving surgical resection could be identified.
More recently, a retrospective study of Catalano et al[5] investigated the role of 18F-FDG-PET/MR enterography in discriminating between inflammatory and fibrotic strictures associated with CD, by using “quantitative” methods. Nineteen patients surgically treated within one month from imaging were evaluated and the following parameters were recorded for each resected bowel segment: The “SI” on T2 weighted sequences, the “apparent diffusion coefficient (ADC)”, the SUVmax and the values obtained by multiplying the first two parameters by SUVmax (SI × SUVmax and ADC × SUVmax, respectively). In the 19 patients evaluated, 33 strictured bowel segments were resected. Of these, 7 presented with predominant inflammation, 11 with predominant fibrosis and 15 with a mixed pattern. A significant difference among these three types of stricture was reported in SUVmax (P < 0.03), SI × SUVmax (P = 0.046) and ADC × SUVmax (P = 0.044), but not in SI and ADC. The most accurate biomarker in discriminating fibrotic from inflammatory strictures was ADC × SUVmax, with a cut-off < 3000 (mean accuracy = 0.71), followed by SUVmax < 2.5 (mean accuracy = 0.67). The other quantitative biomarkers tested (SI × SUVmax < 2000, SI < 750, ADC < 1200 × 10-3 mm2/s) showed suboptimal performance (accuracy = 0.63, 0.48 and 0.54, respectively). The selected studies and the most relevant findings are displayed in Tables 3 and 4.
Table 3.
Overview of the selected studies on the role of positron emission tomography in diagnosing inflammatory bowel disease
| Ref. | Year of pub | Journal | n of pts | Indication | Imaging technique | Gold standard | Conclusions |
| Meisner et al[6] | 2007 | Inflamm Bowel Dis | 12 | To identify regions of active inflammation in patients with known and at least moderate UC or CD | 18F-FDG-PET/CT | Clinical evaluation including colonoscopy and radiologic imaging | There is high correlation between 18F-FDG-PET activity and clinical disease activity CT is necessary for anatomical identification of different bowel segments in CD patients with small bowel involvement or surgically treated |
| Das et al[1] | 2010 | Eur J Nucl Med Mol Imaging | 15 | To assess the extent and severity of disease in patients with active, mild to moderate UC | 18F-FDG-PET/CT colonography | Colonoscopy | 18F-FDG-PET/CT colonography is a useful tool for the assessment of extent and activity of UC |
| Ahmadi et al[7] | 2010 | Inflamm Bowel Dis | 41 | To identify disease activity in patients with known or suspected active CD of the small intestine To find out possible risk factors for therapy failure | Localized 18F-FDG-PET/CTe | NA | 18F-FDG-PET scan does not increase CTe in detection of active disease A low 18F-FDG uptake in at least one small bowel segment, resulted to be pathological on CTe, represent a risk factor for medical treatment failure |
| Groshar et al[20] | 2010 | J Nucl Med | 28 | To evaluate disease activity in patients with known or suspected active CD | 18F-FDG-PET/CTe | NA | SUVmax correlates well with CTe findings of active disease. It might be a reliable objective method for quantifying CD’s activity |
| Shyn et al[21] | 2010 | J Nucl Med | 13 | To detect active disease and assess severity of inflammation in patients with clinically suspected active CD | 18F-FDG-PET/CTe | Histology after surgery or after biopsy performed during endoscopy | 18F-FDG-PET added to CTe may improve the detection of active disease |
| Holtmann et al[2] | 2012 | Dig Dis Sci | 43 | To detect bowel segments with active CD | 18F-FDG-PET | Endoscopy for distal ileum and colon, hydro-MRI for proximal ileum | 18F-FDG-PET diagnostic performance in the detection of bowel segments with active disease is high. Compared to 18F-FDG-PET, hydro-MRI shows much lower sensitivity but higher specificity for all colon segments, higher sensitivity and the same specificity for terminal ileum and same performance for proximal ileum. Both methods seem to have high accuracy in strictures detection and characterization of their nature |
| Lenze et al[4] | 2012 | Inflamm Bowel Dis | 30 | To detect CD strictures and differentiate inflammatory from fibrotic ones | 18F-FDG-PET/CT enteroclysis, MR enteroclysis, transabdominal ultrasound | Endoscopy + hystology | All the three studied techniques have good strictures detection rates relating to the gold standard, but none of them can accurately differentiate strictures’ nature. However, a combination of methods allows the detection of all strictures requiring surgery |
| Catalano et al[5] | 2016 | Radiology | 19 | To differentiate fibrotic from inflammatory strictures in CD patients | 18F-FDG-PET/MR enterography | Post-surgical histology | 18F-FDG-PET/MR enterography offers valid biomarkers for stricture evaluation |
SPECT: Single photon emission tomography; CD: Crohn’s disease; UC: Ulcerative colitis; NA: Not available; 18F-FDG-PET: Positron emission tomography with 18F-Fluorodehoxiglucose; CT: Computed tomography; MRI: Magnetic resonance imaging; SUV: Standardized uptake value; CTe: CT esensitivity; pts: Patients.
Table 4.
Diagnostic accuracies in some selected studies on positron emission tomography
| Ref. | Year of pub | Tracer | Sensitivity | Specificity |
| Meisner et al[6] | 2007 | 18F-FDG | ||
| UC | 95.8% | NA | ||
| CD | 81.3% | NA | ||
| Das et al[1] | 2010 | 18F-FDG | 98.5% | NA |
| Ahmadi et al[7] | 2010 | 18F-FDG | NA | NA |
| Groshar et al[20] | 2010 | 18F-FDG | NA | NA |
| Shyn et al[21] | 2010 | 18F-FDG | ||
| Detection of bowel segments with active CD | ||||
| Using a threshold > 1 (at least mild activity) | 63.3% | 100% | ||
| Using a threshold > 2 (at least moderate activity) | 100% | 89.7% | ||
| Holtmann et al[2] | 2012 | 18F-FDG | ||
| Detection of active CD | ||||
| In the terminal ileum + colon (on a per segment-based analysis) | 90% | 92.6% | ||
| In the proximal ileum (on a per patient-based analysis) | 100% | 100% | ||
| Lenze et al[4] | 2012 | 18F-FDG | ||
| Detection of CD strictures | 81% | NA | ||
| Differentiation of the nature of | ||||
| All strictures | 53% | |||
| Only inflammatory ones | 83% | |||
| Only fibromatous ones | 11% | |||
| Only mixed ones | 0% | |||
| Treglia et al[65] (meta-analysis) (on a per segment-based analysis) | 2013 | 18F-FDG | 85% | 87% |
| Zhang et al[3] (meta-analysis) 18F-FDG On per-bowel-segment basis On per-patient basis | 2014 | 18F-FDG, 99mTc-HMPAO, 99mTc-monoclonal antigranulocyteantibody | 0.84 0.59 | 0.86 1 |
| 99mTc-HMPAO | 0.86 | 0.50 | ||
| On per-bowel-segment basis | 0.79 | 0.76 | ||
| On per-patient basis | 0.91 | 0.85 | ||
| 99mTc-monoclonal antigranulocyte antibody on per-bowel-segment basis | 0.45 | 0.94 | ||
| Catalano et al[5] | 2016 | 18F-FDG | (Mean) | (Mean) |
| Detection of fibrotic CD strictures by | ||||
| ADC × SUVmax < 3000 | 0.67 | 0.73 | ||
| SI on T2-weightedimages × SUVmax < 2000 | 0.77 | 0.57 | ||
| SUVmax < 2.5 | 0.79 | 0.61 | ||
| ADC < 1250 × 10-3 mm2/s | 0.84 | 0.26 | ||
| SI on T2-weightedimages < 750 | 0.73 | 0.13 |
SPECT: Single photon emission tomography; CD: Crohn’s disease; UC: Ulcerative colitis; NA: Not available; 18F-FDG: 18F-Fluorodehoxiglucose; SUV: Standardized uptake value; ADC: Apparent diffusion coefficient; SI: Scintigraphic indices.
Therapy response and disease monitoring: Spier et al[25] reported higher FDG uptake in patients with clinically active disease (CD-activity index > 150 or UC-activity index > 6) than in those with inactive disease. This study suggests that FDG PET may be useful in identifying active inflammation in IBD as well as in the long-term monitoring. Glaudemans et al[26] reported a good correlation between clinical symptoms and PET/CT findings in a small cohort of subjects with moderately active IBD (n = 5) undergoing FDG PET/CT before and after successful medical therapy.
Rubin et al[27] evaluated a cohort of ten patients with UC in a strictly defined remission state (at least six month). A total body PET/CT was performed. The bowel was divided in several areas of interest and the uptake of FDG was scored on 3 point scale compared to the liver uptake. PET/CT showed 90% specificity in assessing disease activity in UC quiescent patients, and allowed the choice of the most adequate therapy options. Interestingly, PET could identify residual inflammatory activity in the colon despite negative endoscopic, histologic, and clinical findings.
Lapp et al[28] demonstrated that PET/CT significantly aids in the clinical decision-making in selected patients with known or suspected IBD. In their paper, although used in a heterogeneous group of patients (four with CD, two with pouches, and one without IBD), PET/CT could effectively lead to the choice of an appropriate therapy in each patient and proved superior to currently available modalities (e.g., laboratory, CT, endoscopy). Jacene et al[29] determined single-pixel maximum standardized uptake value corrected for lean body mass (SULmax) on PET/CT for lesions potentially representing active bowel inflammation. Patients with severe chronic inflammation had significantly higher SULmax than those with mild or moderate chronic inflammation. Semiquantitative analyses of 18F-FDG uptake helped in distinguishing predominantly active inflammation from fibrotic strictures and muscle hypertrophy. Specifically, a SULmax cutoff of 8 seemed to represent the most accurate threshold. Interestingly, patients with predominantly active inflammation, underwent surgery closer to the time of PET/CT compared with those with predominantly fibrosis or muscle hypertrophy. This information may be helpful for referring gastroenterologists considering surgery vs medical therapy for patients with CD who present with obstructive symptoms.
In several papers, PET/CT was reported to be superior to other standard radiologic or endoscopic pouch evaluations in the assessment of the response to treatment. Kuwaki et al[30] investigated 12 patients with CD undergoing granulocyte/monocyte apheresis (GMA). The response to treatment was monitored by measuring standard laboratory tests, CD activity index (CDAI) score, International Organization for the Study of Inflammatory Bowel Diseases score and regional and global bowel uptake on FDG-PET (baseline, at the 5th and at the 10th session of GMA). In 6 of 12 patients, a significant correlation was found between clinical and CDAI improvement and FDG uptake reduction vs baseline scan. As such, longitudinal changes in FDG-PET uptake in the involved bowel areas is of potential clinical value for assessing both regional and global bowel disease activity in CD patients during GMA therapy.
Other semiquantitative parameters of FDG PET/CT were investigated by Saboury et al[31] in order to determine the feasibility and potential clinical utility of this technique in the assessment of therapy response in CD. In a cohort of 22 subjects with CD in treatment undergoing 18F-FDG-PET followed by ileocolonscopy and laboratory assay of Fecal Calprotectine and C-reactive protein (CRP), the authors searched for correlation between the global PET quantification measure (GCDAS, global SUVs) with CDAI, fecal calprotectin, CDEIs, and CRP level. A significant correlation was demonstrated between SUVmax, PVC-SUVmean, and PVC-TLG and CDEIS. Similarly, GCDAS was significantly correlated with CDAI and fecal calprotectin. As such, disease activity was effectively monitored through these semiquantitative parameters, allowing for a tailored treatment.
Molecular imaging in paediatrics
An overview of the selected studies on molecular imaging in paediatric patients is shown in Table 5.
Table 5.
Studies on molecular imaging in paediatric patients included in the present review
| Ref. | Year | Pts (n) | Age (range) | Type of study | Clinical setting | Principal results | Technique | Segments evaluated (n) | Criterion for positivity |
| Papós et al[48] | 1996 | 20 | 4-18 | Prospective | IBD | sensitivity, specificity, and accuracy of LS were 93%, 88% and 91%, respectively | 99mTc-HMPAO-WBC planar scintigraphy (30 min and 2 and 3 h) | Scored relative to the normal bone marrow uptake (0, no uptake; 1 < bone marrow uptake; 2 = bone marrow uptake; and 3 > bone marrow uptake) | |
| Charron et al[36] | 1998 | 178 | n.r. | Retrospective | Useful in distinguishing discontinuous from continuous colitis | 99mTc-HMPAO-WBC planar scintigraphy + SPECT (0.5-1 h, 2-4 h) | |||
| Cucchiara et al[35] | 1999 | 48 | 2-17 | Prospective | suspected IBD | significant correlation between results of scintigraphy and endoscopy for the intensity of inflammation | 99mTc-HMPAO-WBC planar scintigraphy (dynamic + 30, 60, 120 and 180 min) | 9 | Abnormal if activity was seen in the gut within the first hour. 0 = no labeling; 1 = less than bone marrow; 2 = greater than bone marrow, less than liver; and 3 = greater than or equal to liver |
| Del Rosario et al[50] | 1999 | 35 | 2-20 | Retrospective | IBD | 83% sensitivity which prompted more aggressive management in 75% of cases | 99mTc-HMPAO-WBC planar scintigraphy (30 min + 2 h) | ||
| Charron et al[33] | 1999 | 184 | n.r. | Retrospective | Sensitivity = 90%, specificity = 97%, overall accuracy = 93% | 99mTc-HMPAO-WBC planar scintigraphy + SPECT (0.5-1 h, 2-4 h ± 6 h ± 24 h) | |||
| Charron et al[37] | 2000 | 262 | n.r. | Retrospective | IBD | Useful as initial screening modality to exclude IBD | 99mTc-HMPAO-WBC planar scintigraphy + SPECT (0.5-1 h, 2-4 h) | ||
| Alberini et al[32] | 2001 | 28 | 2-15 | Retrospective | Sensitivity and specificity were 75% and 92% for 99mTc-HMPAO-WBC | 99mTc-HMPAO-WBC planar scintigraphy (1 + 3 h, p.i.) | |||
| Davison et al[38] | 2001 | 10 | n.r. | Prosepctive | CD | 99mTc-HMPAO leucocyte scintigraphy should not be depended upon as a screening test for Crohn’s disease | 99mTc-HMPAO-WBC planar scintigraphy + (45 min + 3.5 h) | Abdominal isotope uptake equal to or greater than that associated with the bone marrow was considered to indicate significant inflammation | |
| Bruno et al[41] | 2002 | 66 | 4-19 | Prospective | Sensitivity of immunoscitigraphy was 94% for CD and 85% for UC with a relative low specificity | 99mTc-BW250/183 planar scintigraphy (4 + 24 h, p.i.) | |||
| Grahnquist et al[39] | 2003 | 95 | 2-16 | Prospective | Suspected IBD (screening test) | As a screening test for children with suspected IBD the calculated sensitivity was 75%, and the specificity was 82% | 99mTc-HMPAO-WBC planar scintigraphy (45 min + 3.5 h) | 6 | |
| Peacock et al[40] | 2004 | 64 | 2-19 | Retrospective | Suspected IBD | 99mTc-Stannous colloid LS had an 88% sensitivity, 90% specificity | 99mTc-stannous colloid WCS planar + SPECT (1 h, 3 h) | ||
| Chroustova et al[47] | 2009 | 40 | 5-18 | Monitoring IBD (17 = UC, 23 = CD) | 99mTc-HMPAO-WBC provided good information about the current stage of disease in IBD monitoring | 99mTc-HMPAO-WBC planar scintigraphy + SPECT (30-45 min, 2 h, 3 h) | Graded 1-3 according to the uptake intensity. Grade 1 = a barely detectable abnormal uptake, grade 3 = an abnormal uptake at least as intense as that in the bone marrow and grade 2 was between these extremes. The extent of the abnormal uptake was subjectively classified as A (restricted to a single small focus), C (diffuse, such as in pancolitis) or B (between these extremes) | ||
| Caobelli et al[34] | 2011 | 52 | 2-17 | Prospective | Sensitivity of 94%, specificity of 86%, and negative predictive value of 96% to diagnose IBD. During the follow-up, all relapses and remissions were correctly recognized | 99mTc-HMPAO-WBC planar scintigraphy (0.5 h, 3 h, p.i.) | Disease severity was graded by the focal uptake intensity vs iliac bone uptake (Scan Activity Index) and compared with Endoscopy Mayo Score |
SPECT: Single photon emission tomography; CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease; WBC: White blood cell; LS: Leukocyte scintigraphy; pts: Patients.
Diagnosis: Planar scintigraphy and SPECT: As for adult patients, leukocyte-labelled scintigraphy is a useful tool in the diagnosis and therapeutic strategy of IBD, providing information on the presence, the activity and the extent of the disease, particularly in the terminal ileum[32]. Charron et al[33] reported a large series of paediatric IBD patients evaluated by 99mTc-HMPAO-WBC scintigraphy. One hundred and thirty-two patients with IBD were included, along with 52 patients with nonspecific gastrointestinal symptoms and a low probability of IBD and 31 healthy controls. Images were analysed considering bowel activity in 8 segments and scored using a 6-point scale based on liver, spleen and bone marrow uptake. 99mTc-HMPAO-WBC scintigraphy was proven useful in assessing the extent and distribution of inflammation [sensitivity 90%, specificity 97%, positive predictive value (PPV) 97%, negative predictive value (NPV) 93%, overall accuracy 93%]. Caobelli et al[34] found a correspondence between severity of inflammation as assessed by means of Mayo Score and uptake intensity (Scan Activity Index) in 87% of patients and a full correspondence of location and severity of lesions in 15/16 patients. Overall, reported sensitivity and specificity for 99mTc-HMPAO-WBC scintigraphy were 94% and 86%, respectively. On the other hand, other authors found a good correlation between scintigraphy and endoscopy for the intensity of inflammation (r = 0.70) but a poor correlation regarding the number of involved segments (r = 0.30), due to a significantly higher sensitivity of endoscopy as compared with scintigraphy[35]. 99mTc-HMPAO-WBC scintigraphy may distinguish discontinuous from continuous colitis with very good results[36].
The use of 99mTc-HMPAO-WBC scan as initial screening modality to exclude IBD has been suggested, although controversial results have been reported. While a study showed that 99mTc-HMPAO-WBC scintigraphy is more sensitive than upper gastrointestinal small bowel follow-through (SBFT)[37], other authors stated that 99mTc-HMPAO-WBC scintigraphy should not be depended upon as a screening test for CD, since its performance compares unfavourably with each of the conventional investigative techniques[38,39].
Few data are available on other tracers. Peacock et al[40] reported good results using 99mTc-Stannous colloid WBC scintigraphy (88% sensitivity, 90% specificity) although agreement was poor for topographic localization of disease when compared to conventional imaging. However, due to a non-inferiority principle (results at least comparable to those of other WBC tracers) authors concluded that in children, 99mTc-stannous colloid WBC scintigraphy should be preferred in view of lower cost, shorter preparation time, and smaller blood volumes required.
Bruno et al[41] reported a higher sensitivity of immunoscintigraphy in CD than in UC (94% and 85%, respectively), although specificity was suboptimal in identifying clinical remission during follow-up. In fact, scintigraphy resulted positive during clinical remission in 73% of patients with CD and in 66.7% of patients with UC.
Diagnosis: PET: The paediatric literature studying the value of 18F-FDG-PET and 18F-FDG-PET/CT in IBD is limited, heterogeneous, and mostly restricted to retrospective studies.
One of the first article (published in 1999) was a retrospective study performed by Skehan et al[18]. The authors included 25 young patients with suspected IBD who had undergone 18F-FDG-PET scan and colonoscopy with multiple biopsies, SBFT or both, which were used as a reference standard. 18F-FDG-PET uptake in the gut was considered pathological if greater than that of the vertebral spine. 18F-FDG-PET showed a per-patient sensitivity and specificity of 81% and 85%, respectively. On a segment-by-segment basis, PET correctly identified the presence/absence of active disease in 60 of 79 (76%) bowel segments (sensitivity and specificity were 71% and 81%, respectively).
In 2005 Lemberg et al[42] published a prospective study including 65 children with newly diagnosed IBD (37/65 patients), with symptoms suggestive to recurrent disease (18/65 patients) or with recurrent abdominal pain (10/65 patients). 18F-FDG-PET scan results were compared with SBFT, pneumocolon and/or colonoscopy and demonstrated a per-patient sensitivity in UC (n = 17), CD (n = 38) and recurrent abdominal pain of 76%, 82% and 100%, respectively. The specificity of 18F-FDG-PET vs SBFT was 100% both for UC and CD, while it was 50% for CD and 81% for UC if compared to colonoscopy. The authors concluded that, although PET may not be able to replace conventional studies, it may be useful in case of difficult performance or failing of conventional studies. Additionally, Löffler et al[43] studied 23 children with suspected IBD in a retrospective study, comparing 18F-FDG-PET to endoscopy with biopsies and abdominal ultrasound. Inflammation was graded for each bowel segment on a 3-point scale, namely not inflamed, minimally inflamed and moderately-to-severely inflamed. 18F-FDG-PET scans were analysed semi-quantitatively calculating SUV for all bowel segments, normalized to that of the liver. A score ≥ 3 or a SUVmax/SUVliver ratio > 1.2 was considered positive for inflammation. 18F-FDG-PET showed higher sensitivity (98%) but lower specificity compared both to ultrasound (56% vs 92%) and to endoscopy (90% vs 75%). However, 18F-FDG-PET proved to be more reliable when assessing small bowel involvement (sensitivity = 100%; specificity = 86%; accuracy = 90%).
More recently, Däbritz et al[44] compared results of PET/CT to conventional diagnostic procedures on a segment-based analysis. An FDG uptake in the gut greater than that of the liver was considered pathological. Following these criteria, 18F-FDG-PET/CT showed per-patient sensitivity and specificity of 97% and 100%, respectively, and per-segment sensitivity and specificity of 82% and 97%, respectively (PPV 96%, NPV 88%, accuracy 91%). Berthold et al[45] retrospectively analysed 23 young symptomatic patients undergoing endoscopies (gastroscopy and colonoscopy) with biopsies, MRI and 18F-FDG-PET, eventually fused with CT, as part of the diagnostic workup. Positive PET findings were defined as a diffuse and clearly increased FDG uptake in the bowel. In this study, sensitivity and specificity of 18F-FDG-PET was 25% and 100% in the stomach and duodenum, 74% and 88% in the colon and 89% and 75% in the terminal ileum. Especially interesting was the diagnostic performance in assessing lesions involving terminal ileum, for which 18F-FDG-PET appears to be a reliable tool able for the non-invasive assessment of the presence and extent of the disease.
Follow-up: Only a few studies about paediatric patients have been published and are characterized by a smaller number of patients compared to those performed in adults and by the lack of an adequately long follow-up[46]. Chroustova et al[47] reported that 99mTc-HMPAO-WBC scintigraphy may play an important role in the follow-up of patients with IBD if the findings are evaluated in conjunction with clinical symptoms and laboratory examinations, providing good information about the current stage of disease. Similar results were replicated by Papós et al[48], wherein the patient activity index scores (sum of the activity scores as measured in each bowel segment) were compared with laboratory parameters. The scintigraphic activity index showed an excellent correlation with several important activity markers in IBD, such as CRP, α2 globulin, iron, and erythrocyte sedimentation rate. Some impressive cases have been reported, in which 99mTc-HMPAO-WBC scintigraphy could effectively trace the changing in disease activity over time in paediatrics (Figure 2).
Figure 2.
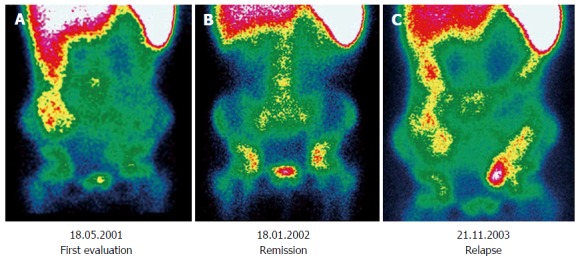
Three sequential 99mTc-HMPAO-white blood cell scintigraphies performed in a patient diagnosed with Crohn’s disease. A: Performed at diagnosis, there is evidence of increased activity involving colon ascendens; B: Performed at follow-up after immunological therapy, a complete remission can be demonstrated; C: There is evidence of a relapse involving colon ascendens and sigma-rectum. Reprinted with permission of Springer Verlag from Caobelli et al[34].
However, despite some advantages of 99mTc-HMPAO-WBC scintigraphy over conventional radiologic techniques in assessing acute inflammation activity, the post-operatory relapse of Crohn disease and the inflammatory component of stenosis[49], some disadvantages of this technique should be recognized. For example, the exclusive use of SPECT without CT combination cannot define anatomic changes in IBD such as stricture formation, fistula formation, or prestenotic dilation. These lesions are best demonstrated by contrast radiography[50]. Moreover, these techniques require a blood sample, followed by reinjection[41].
PET represents a relatively novel technique for monitoring IBD[50]. In contrast to the standard scintigraphy/SPECT, PET offers higher spatial and temporal resolution and is therefore more suitable for quantitative image analysis of physiological and pathologic processes[32]. However, the precise role of PET in the follow-up of paediatric patients diagnosed with IBD still remains to be defined for some clinical indication, such as differential diagnosis of fibrostenotic strictures from inflammatory strictures and as an alternative investigation after failure of conventional imaging[51].
New tracers and preclinical experiments
Radiolabelled autologous leukocytes and 18F-FDG-PET represent two of the most widely adopted tracers to image IBD and other inflammatory diseases and have gained increasing acceptance for routine use. However, during the latest decade many efforts have been made in order to develop new radiopharmaceuticals able to detect inflammation and/or infection, such as labeled monoclonal antibodies, chemotactic peptides, interleukins and chemokines[52] both for SPECT and PET imaging.
SPECT tracers: Neurath et al[53] enrolled 59 patients with CD in a prospective study, in order to assess disease activity by FDG-PET, hydro-MRI, and immunoscintigraphy with anti-nonspecific cross-reacting antigen 95 antigranulocyte antibodies. Twelve patients with irritable bowel syndrome and 20 patients with gut cancer but without gut inflammation served as controls. All three methods showed high specificity (89%) to detect inflamed areas in the terminal ileum and colon, although analyses by hydro-MRI and granulocyte antibody scan had strikingly lower sensitivities (40.9% and 66.7%) than FDG-PET (85.4%). Although hydro-MRI showed a higher sensitivity than immunoscintigraphy, still the diagnostic performance was suboptimal. Authors concluded that FDG-PET may provide improved diagnostic accuracy compared to the other two techniques.
Annovazzi et al[54] compared 99mTc-HMPAO-WBC scintigraphy to 99mTc-IL2 scintigraphy in patients with CD. Both techniques provided high NPV (100% and 91%, respectively), unfortunately at expenses of a weak PPV (44% and 39%, respectively). However, only an unremarkable 99mTc-IL2 scintigraphy was associated with longer disease free survival (log-rank test, P = 0.013). As such, the authors hypothesized a promising role for 99mTc-IL2 scintigraphy is useful in selected patients with CD in clinical remission, who could benefit from preventive therapy to avoid disease relapse.
More recently, Van De Wiele et al[55] investigated three different labelling methods for monocytes (99mTc-HMPAO, 111In-oxine and 99mTc-colloids for SPECT studies or 18F-FDG for PET studies). Best results were yielded by 99mTc-HMPAO. In vitro labelled monocytes specifically accumulated in the intestinal activity foci. In 2015, Aarntzen et al[19] investigated the accuracy of 99mTc-CXCL8 SPECT to detect and localize disease activity in a prospective series of patients with IBD. Radiolabeled CXCL8 (IL-8) targets the CXCL8 receptors mediating chemotaxis of immune cells to the site of inflammation. The overall sensitivity and specificity on a per-patient basis for the detection of active disease was 95% and 44% for 99mTc-CXCL8 scan and 71% and 70% for endoscopy. Interestingly, the degree of 99mTc-CXCL88 accumulation correlated to the degree of neutrophilic influx in affected mucosa.
PET tracers: New more specific PET radiopharmaceuticals for inflammation of the intestinal mucosa have been investigated. A gradual shift from large proteins with a nonspecific uptake mechanism to more specific targeting proteins could be observed in the latest years.
To date, neutrophil-mediated processes, characteristic for both inflammatory and infectious processes, can be targeted in situ by radiolabeled leukocytes, antibodies or its fragments and cytokines. 68Ga is one of the earliest positron-emitting radionuclides applied to clinical medicine, with a short physical half-life (t1/2 = 68 min), allowing favorable dosimetry with consequent lower dose delivered to the patients in case of repeated imaging. This constitutes a major advantage over the SPECT agent 67Ga[56].
WBC labeled with 18F-FDG is a promising method for a non-invasive diagnosis and grading of intestinal inflammation. In both murine models and humans, 18F-FDG-WBC PET imaging demonstrated low tracer uptake in healthy gastrointestinal and urinary tracts, where the often unpredictable glucose metabolism often hinders the specificity of 18F-FDG-PET. In a study, intestinal foci of FDG-labeled WBCs were later confirmed to be inflammatory foci by histopathology. Moreover, the intensity of uptake well correlated with the degree of inflammation based on histopathologic criteria[57].
Preclinical experiments: Interleukins: Interleukins (e.g., IL-1, IL-2, IL-8) have recently been proposed as potential agents for imaging infection and inflammation[52].
In a rabbit model of acute colitis, Gratz et al[58] investigated the diagnostic performance of 99mTc-HYNIC-IL8 scintigraphy compared to 99mTc-HMPAO-WBC scans. While both radiotracers detected colitis within 1 h after injection, 99mTc-HYNIC-IL8 images were more accurate and the absolute uptake in the affected colon continuously increased until 4 h after injection, whereas no further increase was measured for 99mTc-HMPAO-WBC beyond the first hour after the administration. The absolute uptake in the affected colon was significantly higher for 99mTc-HYNIC-IL8 than for 99mTc-HMPAO-WBC. The authors concluded that 99mTc-HYNIC-IL8 scintigraphy, unlike 99mTc-HMPAO-WBC may provide a reliable estimation of the severity of IBD.
Preclinical experiments: (18F)1-(2’-deoxy-2’-arabinofuranosyl) cytosine: The (18F)1-(2’-deoxy-2’-arabinofuranosyl) cytosine (D-FAC) probe was developed as part of a broader effort to identify diverse molecular transport systems representing cellular biologic states[59].
The biodistribution of D-FAC differs from that of FDG for a higher selectivity for immune organs such as the spleen, bone marrow, and thymus. In addition, D-FAC had strikingly higher uptake in the intestine in mouse models[52]. While such increased uptake was thought to reflect the large amount of immune cells resident in the intestine mucosa, several lines of evidence revealed that intestinal D-FAC uptake is predominantly attributable to the intestinal epithelial cells. In fact, the distribution of the signal is strongly focalized in the duodenum, wherein the immune population is not significantly more expressed that in the remaining intestine[60].
Uptake of 18F-D-FAC allows for a noninvasive assessment by PET. Increased uptake of D-FAC radiotracer reflects the activity of the epithelium and lymphocytes, providing a unique early marker of intestinal inflammation[59].
Preclinical experiments: (18F)DPA-714: (18F)DPA-714, a radioligand of a translocator protein (TSPO), is another molecular probe developed for a non-invasive quantification of the inflammatory state of IBD in animal models[61].
The TSPO is located on the outer membrane of the mitochondria and is overexpressed in the activated macrophages and microglia. This molecular event has been widely investigated in the pathologies of the central nervous system, representing a hallmark of brain inflammation. TSPO expression was recently described also in tissue samples of human colon suffering from IBD. Furthermore, its expression has been characterized in a rat model of IBD using autoradiography and immunohistochemistry[62-64].
PET imaging of IBD was conducted using 18F-FDG and 18F-DPA-714 in two rat models[61]. The first model was induced using dextran sodium sulfate (DSS), thus creating global inflammation in the colon, while the second one was induced by a rectal administration of trinitrobenzenesulfonic acid (TNBS), causing a local and acute inflammation.
The degree of inflammation was analyzed using PET imaging on days 7 and 8. In the first rat model, 18F-DPA-714 revealed significant differences between animals treated with DSS and controls (0.50% ± 0.17% ID/cc and 0.35% ± 0.15% ID/cc, respectively). Conversely, no differences could be seen using 18F-FDG. In the second model, the 18F-DPA-714 uptake significantly increased from 0.46% ± 0.23% ID/cc in the controls to 1.30% ± 0.62% ID/cc in the animals treated with TNBS. As such, a correlation between PET with increased TSPO expression at cellular level was fully demonstrated. The results of this study suggest that 18F-DPA-714 is suitable for studying inflammation in IBD models and could be a good molecular probe to define the degree and the localization of inflammation. Moreover, in vivo imaging using this TSPO ligand is potentially a powerful tool to stage and to follow inflammatory disease evolution and therapeutic efficiency at molecular level.
DISCUSSION
IBD represents a diagnostic and therapeutic challenge, not only at initial disease presentation, but also during suspected disease flares. Standard procedures like endoscopy and conventional radiologic studies are frequently required in order to assess the specific IBD subtype and evaluate disease extension and activity[34]. Although endoscopy is an essential procedure for the diagnosis and follow-up of IBD, it is invasive and able to provide only a mucosal and not transmural assessment of the colon and the distal ileal segments. Furthermore, endoscopy is not always applicable during the active phase of IBD, especially in children[48]. As such, there is the need for a noninvasive technique able to replace colonoscopy or conventional radiologic examinations in the follow-up of patients with IBD. This need is of particular importance in a paediatric population, as a highly variable response to therapy has been reported in these patients[46]. As a consequence, a technique able to assess disease activity in paediatric patients plays a major role in yielding an IBD management. Since the simple clinical remission does not well correlate with the taming of the inflammatory process at the tissue level, an effective management of IBD represents a major challenge with the currently available diagnostic modalities.
Molecular imaging presents various advantages over colonoscopy. Firstly, it is minimally invasive and well tolerated by the patients, also by paediatric ones; second, patients can undergo the procedure also during the active state of disease, without any particular limitation or precaution; third, it should be noted that no intestinal preparation is needed; fourth, molecular imaging allows the evaluation of the colon and the terminal ileum. Presence of fibrosis does not impact the results of the examination. The investigation can be repeated over time, considering an adequate time interval. This latter aspect is of relevance especially during follow-up. Finally, molecular imaging has the potential to detect and characterize the anatomic location and the disease activity at diagnosis and on therapy.
All these advantages make molecular imaging particularly suitable in paediatric patients, either when an endoscopy has been refused by the young patient or if there is any specific contraindication[34].
From the data of the literature, 18F-FDG-PET, eventually fused with CT, appears to have the best potential among molecular imaging techniques, since it provides the highest accuracy both in diagnosis and follow-up. In a recent meta-analysis by Treglia et al[65] on 7 selected studies, including 219 patients with IBD, 18F-FDG-PET (and PET/CT) showed pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odd ratio of 85%, 87%, 6.19, 0.19 and 44.35, respectively. In their paper, the area under the ROC curve was 0.933. However, another meta-analysis by Zhang et al[3] including 20 prospective studies published between 1993 and 2013, did not report significant differences in sensitivity, specificity and diagnostic accuracy between 18F-FDG-PET and leukocyte scintigraphy. Following their results, the authors suggested that both techniques can be alternatively used for the diagnosis of IBD, depending on their local availability. Conversely, monoclonal anti-granulocyte antibody scintigraphy showed a significantly lower sensitivity, despite the higher specificity.
During follow-up, molecular imaging proved to effectively monitor therapy response. As a matter of fact, FDG represents the whole inflammatory burden of the gut, and an early post-therapy 18F-FDG-PET or PET/CT (within weeks from the start of the therapy) compared to a pre-therapy scan may allow for the early evaluation of therapy efficacy.
It seems that 18F-FDG-PET or PET/CT may have different indications, depending on the subtype of IBD. Spier et al[25] reported that 18F-FDG-PET has a good sensitivity in inflamed bowel segments in the patients with known IBD (range from 72.9% to 95.8%); while PET alone is at best suitable for patients with UC, PET/CT should be recommended in patients with CD, due to a better anatomical definition. It should also be noted that 18F-FDG-PET allows the visualization of the entire bowel wall in patients with known IBD. This ability is of particular importance in patients diagnosed with CD, since this condition is often characterized by a spread to the deeper layers of the bowel wall, where endoscopy fails to detect inflammatory lesions. Furthermore, PET can assess possible overlaps between irritable bowel syndrome and IBD prior to increasing or changing immunomodulation.
Despite these advantages, the use of FDG PET/CT in a clinical setting is still indeterminate. Indeed, there is no common consensus on the timing and on the number of the post-therapy scan and the predominance of studies involving small cohorts of patients represent a limit to support the use of this imaging technique in clinical practice. Many papers in literature underlined a suboptimal specificity for 18F-FDG-PET. Indeed, false positive cases due to physiologic uptake of the radiopharmaceutical in normal bowel can be often found in clinical practice. However, false positive findings can be effectively reduced with a proper distention of bowel segments through the administration of great amount of fluids[1,3,7,20,21] or by specific patients preparations as reported by Franquet et al[22].
In addition, a crucial question is the lack of a standardized method for quantifying accurately disease activity. Although many papers underlined the strategical role of semiquantitative parameters in PET imaging, still the huge heterogeneity of the published trials prevents to date to establish a robust assessment protocol.
It should also be mentioned that, to date, the widespread use of 18F-FDG-PET/CT (and to a lesser extent of 99mTc-HMPAO-WBC scintigraphy) is hindered by relatively high costs and by relatively long waiting lists given its extensive use in oncology.
Finally, these procedures cause patient exposure to ionizing radiation (e.g., about 10 mSv for 18F-FDG-PET/CT, using the 50 mAs, 120 kV CT-protocol and an administered 18F-FDG activity of 3.7 MBq/kg). Several studies showed that risk of cancer is greater with earlier age of exposure. Therefore it is essential to avoid unneeded ionizing radiation exposure especially in paediatrics[66]. It should be considered, however, that radiation exposure can be kept under acceptable limits by limiting the CT scan only to the abdomen-pelvis and/or using a 3D instead of a 2D scanner and a longer acquisition time with a lower administered 18F-FDG activity, if patient is able to remain still, or by using new reconstruction algorithms. The replacement of CT with MRI would significantly decrease the dose of radiations and improve diagnostic information on structural changes in bowel wall, despite a longer duration of the examination[1,2,4-7,21,67]. Finally, considering an adequate time interval between serial examinations would limit the radioexposure into acceptable limits.
In conclusion, WBC scintigraphy and 18F-FDG-PET (alone or combined with CT or, in a next future, with MRI) represent a useful tool to detect active disease in IBD. However, the available data need to be validated in prospective multicenter studies on larger patient samples. Furthermore, even better diagnostic performance may be envisioned thanks to more specific and accurate radiotracers, which are however to date still far from a clinical validation in the clinical setting.
COMMENTS
Background
Inflammatory bowel disease (IBD) is a chronic inflammatory condition, in which the integrity of the gut epithelium represents a major pathophysiological step. Although endoscopy and barium radiological examinations are the diagnostic “gold standard” for IBD, both techniques require a specific patient preparation, not always feasible or easily tolerated.
Research frontiers
Molecular imaging with single photon emission tomography (SPECT) and positron emission tomography (PET) may be a reliable, non-invasive, accurate and easily reproducible diagnostic tool, able to assess location, extent and activity grade of IBD.
Innovations and breakthroughs
The present Review is to date the most comprehensive, including all imaging modalities (i.e., SPECT, SPECT/CT, PET, PET/CT) and various radiopharmaceuticals. Moreover, interesting data about experimental procedures are provided.
Applications
The present Review aims at becoming a useful guide for the Clinicians who may want to plan an effective diagnostic assessment in patients with IBD. Furthermore, the knowledge of the state-of-the-art of molecular imaging provides a useful tool able to monitor the effectiveness of the therapy.
Terminology
SPECT: Single photon emission tomography. This technique is based on the administration of gamma-emitting radiopharmaceuticals, following the normal metabolic pathways. The uptake is detected by means of gamma-cameras, able to localize possible foci of increased uptakes, consistent with inflammation and/or infection. PET: Positron emission tomography. This technique is based on the administration of positron emitting radiopharmaceuticals, also following the normal metabolic pathways. The uptake is detected by means of PET scanners, whose spatial and temporal resolution is significantly higher than those of SPECT cameras.
Peer-review
This is an extensive review on nuclear medicine investigations in IBD, for diagnosis and follow-up. The authors nicely reported also in tables the large existing literature.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: The authors hereby declare that no one of the coworkers has received fees for serving as a speaker, no one holds a position as advisory board member, none has received research funding, no one owns stocks and/or shares in any Companies, no one owns patents.
Data sharing statement: No additional data are available. A formal consent was not obtained due to the nature of the present paper (Review of already published papers).
Peer-review started: March 26, 2016
First decision: May 17, 2016
Article in press: August 29, 2016
P- Reviewer: Annese V, Capasso R, Fries W, Giron MC S- Editor: Qi Y L- Editor: A E- Editor: Li D
References
- 1.Das CJ, Makharia GK, Kumar R, Kumar R, Tiwari RP, Sharma R, Malhotra A. PET/CT colonography: a novel non-invasive technique for assessment of extent and activity of ulcerative colitis. Eur J Nucl Med Mol Imaging. 2010;37:714–721. doi: 10.1007/s00259-009-1335-2. [DOI] [PubMed] [Google Scholar]
- 2.Holtmann MH, Uenzen M, Helisch A, Dahmen A, Mudter J, Goetz M, Schreckenberger M, Galle PR, Bartenstein P, Neurath MF. 18F-Fluorodeoxyglucose positron-emission tomography (PET) can be used to assess inflammation non-invasively in Crohn’s disease. Dig Dis Sci. 2012;57:2658–2668. doi: 10.1007/s10620-012-2190-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Li LF, Zhu YJ, Qiu H, Xu Q, Yang J, Weng WW, Liu NH. Diagnostic performance of 18F-FDG-PET versus scintigraphy in patients with inflammatory bowel disease: a meta-analysis of prospective literature. Nucl Med Commun. 2014;35:1233–1246. doi: 10.1097/MNM.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 4.Lenze F, Wessling J, Bremer J, Ullerich H, Spieker T, Weckesser M, Gonschorrek S, Kannengiesser K, Rijcken E, Heidemann J, et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis. 2012;18:2252–2260. doi: 10.1002/ibd.22930. [DOI] [PubMed] [Google Scholar]
- 5.Catalano OA, Gee MS, Nicolai E, Selvaggi F, Pellino G, Cuocolo A, Luongo A, Catalano M, Rosen BR, Gervais D, et al. Evaluation of Quantitative PET/MR Enterography Biomarkers for Discrimination of Inflammatory Strictures from Fibrotic Strictures in Crohn Disease. Radiology. 2016;278:792–800. doi: 10.1148/radiol.2015150566. [DOI] [PubMed] [Google Scholar]
- 6.Meisner RS, Spier BJ, Einarsson S, Roberson EN, Perlman SB, Bianco JA, Taylor AJ, Einstein M, Jaskowiak CJ, Massoth KM, et al. Pilot study using PET/CT as a novel, noninvasive assessment of disease activity in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:993–1000. doi: 10.1002/ibd.20134. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi A, Li Q, Muller K, Collins D, Valentine JF, Drane W, Polyak S. Diagnostic value of noninvasive combined fluorine-18 labeled fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography enterography in active Crohn’s disease. Inflamm Bowel Dis. 2010;16:974–981. doi: 10.1002/ibd.21153. [DOI] [PubMed] [Google Scholar]
- 8.Lachter J, Isseroff HN, Yasin K, Keidar Z, Israel O. Radiolabeled leukocyte imaging in inflammatory bowel disease: a prospective blinded evaluation. Hepatogastroenterology. 2003;50:1439–1441. [PubMed] [Google Scholar]
- 9.Paredes JM, Ripollés T, Cortés X, Reyes MD, López A, Martínez MJ, Moreno-Osset E. Non-invasive diagnosis and grading of postsurgical endoscopic recurrence in Crohn’s disease: usefulness of abdominal ultrasonography and (99m)Tc-hexamethylpropylene amineoxime-labelled leucocyte scintigraphy. J Crohns Colitis. 2010;4:537–545. doi: 10.1016/j.crohns.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Kerry JE, Marshall C, Griffiths PA, James MW, Scott BB. Comparison between Tc-HMPAO labelled white cells and Tc LeukoScan in the investigation of inflammatory bowel disease. Nucl Med Commun. 2005;26:245–251. doi: 10.1097/00006231-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Biancone L, Schillaci O, Capoccetti F, Bozzi RM, Fina D, Petruzziello C, Geremia A, Simonetti G, Pallone F. Technetium-99m-HMPAO labeled leukocyte single photon emission computerized tomography (SPECT) for assessing Crohn’s disease extent and intestinal infiltration. Am J Gastroenterol. 2005;100:344–354. doi: 10.1111/j.1572-0241.2005.41123.x. [DOI] [PubMed] [Google Scholar]
- 12.Hillel PG, Lorenz E, Metherall P, Tindale WB. 99mTc white-cell imaging in inflammatory bowel disease: a comparison of planar versus SPECT. Nucl Med Commun. 2011;32:591–596. doi: 10.1097/MNM.0b013e328345b2fd. [DOI] [PubMed] [Google Scholar]
- 13.Mota LG, Coelho LG, Simal CJ, Ferrari ML, Toledo C, Martin-Comin J, Diniz SO, Cardoso VN. Leukocyte-technetium-99m uptake in Crohn’s disease: does it show subclinical disease? World J Gastroenterol. 2010;16:365–371. doi: 10.3748/wjg.v16.i3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe FA, Camilleri M, Forstrom LA, Batts KP, Mullan BP, Thomforde GM, Dunn W, Zinsmeister AR. A pilot study of splenic and whole body retention of autologous radiolabeled leukocytes in the assessment of severity in inflammatory colitis. Am J Gastroenterol. 1995;90:1771–1775. [PubMed] [Google Scholar]
- 15.Cheow HK, Voutnis DD, Evans JW, Szczepura KR, Swift EA, Bird NJ, Ruparelia P, Solanki CK, Ballinger JR, Chilvers ER, et al. Quantification of disease activity in patients undergoing leucocyte scintigraphy for suspected inflammatory bowel disease. Eur J Nucl Med Mol Imaging. 2005;32:329–337. doi: 10.1007/s00259-004-1617-7. [DOI] [PubMed] [Google Scholar]
- 16.Van den Brande JM, Koehler TC, Zelinkova Z, Bennink RJ, te Velde AA, ten Cate FJ, van Deventer SJ, Peppelenbosch MP, Hommes DW. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut. 2007;56:509–517. doi: 10.1136/gut.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicik I, Bauerfeind P, Breitbach T, von Schulthess GK, Fried M. Inflammatory bowel disease activity measured by positron-emission tomography. Lancet. 1997;350:262. doi: 10.1016/S0140-6736(05)62225-8. [DOI] [PubMed] [Google Scholar]
- 18.Skehan SJ, Issenman R, Mernagh J, Nahmias C, Jacobson K. 18F-fluorodeoxyglucose positron tomography in diagnosis of paediatric inflammatory bowel disease. Lancet. 1999;354:836–837. doi: 10.1016/S0140-6736(99)80021-X. [DOI] [PubMed] [Google Scholar]
- 19.Aarntzen EH, Hermsen R, Drenth JP, Boerman OC, Oyen WJ. 99mTc-CXCL8 SPECT to Monitor Disease Activity in Inflammatory Bowel Disease. J Nucl Med. 2016;57:398–403. doi: 10.2967/jnumed.115.165795. [DOI] [PubMed] [Google Scholar]
- 20.Groshar D, Bernstine H, Stern D, Sosna J, Eligalashvili M, Gurbuz EG, Niv Y, Fraser G. PET/CT enterography in Crohn disease: correlation of disease activity on CT enterography with 18F-FDG uptake. J Nucl Med. 2010;51:1009–1014. doi: 10.2967/jnumed.109.073130. [DOI] [PubMed] [Google Scholar]
- 21.Shyn PB, Mortele KJ, Britz-Cunningham SH, Friedman S, Odze RD, Burakoff R, Goldberg JE, Erturk M, Silverman SG. Low-dose 18F-FDG PET/CT enterography: improving on CT enterography assessment of patients with Crohn disease. J Nucl Med. 2010;51:1841–1848. doi: 10.2967/jnumed.110.080796. [DOI] [PubMed] [Google Scholar]
- 22.Franquet E, Palmer MR, Gifford AE, Selen DJ, Chen YC, Sedora-Roman N, Joyce RM, Kolodny GM, Moss AC. Rifaximin suppresses background intestinal 18F-FDG uptake on PET/CT scans. Nucl Med Commun. 2014;35:1026–1031. doi: 10.1097/MNM.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 23.Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med. 2007;48:1053–1059. doi: 10.2967/jnumed.107.040436. [DOI] [PubMed] [Google Scholar]
- 24.Das CJ, Makharia G, Kumar R, Chawla M, Goswami P, Sharma R, Malhotra A. PET-CT enteroclysis: a new technique for evaluation of inflammatory diseases of the intestine. Eur J Nucl Med Mol Imaging. 2007;34:2106–2114. doi: 10.1007/s00259-007-0525-z. [DOI] [PubMed] [Google Scholar]
- 25.Spier BJ, Perlman SB, Jaskowiak CJ, Reichelderfer M. PET/CT in the evaluation of inflammatory bowel disease: studies in patients before and after treatment. Mol Imaging Biol. 2010;12:85–88. doi: 10.1007/s11307-009-0232-1. [DOI] [PubMed] [Google Scholar]
- 26.Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013;2013:623036. doi: 10.1155/2013/623036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DT, Surma BL, Gavzy SJ, Schnell KM, Bunnag AP, Huo D, Appelbaum DE. Positron emission tomography (PET) used to image subclinical inflammation associated with ulcerative colitis (UC) in remission. Inflamm Bowel Dis. 2009;15:750–755. doi: 10.1002/ibd.20819. [DOI] [PubMed] [Google Scholar]
- 28.Lapp RT, Spier BJ, Perlman SB, Jaskowiak CJ, Reichelderfer M. Clinical utility of positron emission tomography/computed tomography in inflammatory bowel disease. Mol Imaging Biol. 2011;13:573–576. doi: 10.1007/s11307-010-0367-0. [DOI] [PubMed] [Google Scholar]
- 29.Jacene HA, Ginsburg P, Kwon J, Nguyen GC, Montgomery EA, Bayless TM, Wahl RL. Prediction of the need for surgical intervention in obstructive Crohn’s disease by 18F-FDG PET/CT. J Nucl Med. 2009;50:1751–1759. doi: 10.2967/jnumed.109.065466. [DOI] [PubMed] [Google Scholar]
- 30.Kuwaki K, Mitsuyama K, Kaida H, Takedatsu H, Yoshioka S, Yamasaki H, Yamauchi R, Fukunaga S, Abe T, Tsuruta O, et al. A longitudinal study of FDG-PET in Crohn disease patients receiving granulocyte/monocyte apheresis therapy. Cytotherapy. 2016;18:291–299. doi: 10.1016/j.jcyt.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Saboury B, Salavati A, Brothers A, Basu S, Kwee TC, Lam MG, Hustinx R, Louis E, Torigian DA, Alavi A. FDG PET/CT in Crohn’s disease: correlation of quantitative FDG PET/CT parameters with clinical and endoscopic surrogate markers of disease activity. Eur J Nucl Med Mol Imaging. 2014;41:605–614. doi: 10.1007/s00259-013-2625-2. [DOI] [PubMed] [Google Scholar]
- 32.Alberini JL, Badran A, Freneaux E, Hadji S, Kalifa G, Devaux JY, Dupont T. Technetium-99m HMPAO-labeled leukocyte imaging compared with endoscopy, ultrasonography, and contrast radiology in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:278–286. doi: 10.1097/00005176-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Charron M, del Rosario FJ, Kocoshis SA. Pediatric inflammatory bowel disease: assessment with scintigraphy with 99mTc white blood cells. Radiology. 1999;212:507–513. doi: 10.1148/radiology.212.2.r99au45507. [DOI] [PubMed] [Google Scholar]
- 34.Caobelli F, Panarotto MB, Andreoli F, Ravelli A, De Agostini A, Giubbini R. Is 99mTc-HMPAO granulocyte scan an alternative to endoscopy in pediatric chronic inflammatory bowel disease (IBD)? Eur J Pediatr. 2011;170:51–57. doi: 10.1007/s00431-010-1269-5. [DOI] [PubMed] [Google Scholar]
- 35.Cucchiara S, Celentano L, de Magistris TM, Montisci A, Iula VD, Fecarotta S. Colonoscopy and technetium-99m white cell scan in children with suspected inflammatory bowel disease. J Pediatr. 1999;135:727–732. doi: 10.1016/s0022-3476(99)70092-2. [DOI] [PubMed] [Google Scholar]
- 36.Charron M, del Rosario JF, Kocoshis S. Use of technetium-tagged white blood cells in patients with Crohn’s disease and ulcerative colitis: is differential diagnosis possible? Pediatr Radiol. 1998;28:871–877. doi: 10.1007/s002470050486. [DOI] [PubMed] [Google Scholar]
- 37.Charron M, Di Lorenzo C, Kocoshis S. Are 99mTc leukocyte scintigraphy and SBFT studies useful in children suspected of having inflammatory bowel disease? Am J Gastroenterol. 2000;95:1208–1212. doi: 10.1111/j.1572-0241.2000.02011.x. [DOI] [PubMed] [Google Scholar]
- 38.Davison SM, Chapman S, Murphy MS. 99mTc-HMPAO leucocyte scintigraphy fails to detect Crohn’s disease in the proximal gastrointestinal tract. Arch Dis Child. 2001;85:43–46. doi: 10.1136/adc.85.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grahnquist L, Chapman SC, Hvidsten S, Murphy MS. Evaluation of 99mTc-HMPAO leukocyte scintigraphy in the investigation of pediatric inflammatory bowel disease. J Pediatr. 2003;143:48–53. doi: 10.1016/S0022-3476(03)00280-4. [DOI] [PubMed] [Google Scholar]
- 40.Peacock K, Porn U, Howman-Giles R, O’Loughlin E, Uren R, Gaskin K, Dorney S, Kamath R. 99mTc-stannous colloid white cell scintigraphy in childhood inflammatory bowel disease. J Nucl Med. 2004;45:261–265. [PubMed] [Google Scholar]
- 41.Bruno I, Martelossi S, Geatti O, Maggiore G, Guastalla P, Povolato M, Ventura A. Antigranulocyte monoclonal antibody immunoscintigraphy in inflammatory bowel disease in children and young adolescents. Acta Paediatr. 2002;91:1050–1055. doi: 10.1080/080352502760311539. [DOI] [PubMed] [Google Scholar]
- 42.Lemberg DA, Issenman RM, Cawdron R, Green T, Mernagh J, Skehan SJ, Nahmias C, Jacobson K. Positron emission tomography in the investigation of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:733–738. doi: 10.1097/01.mib.0000172810.49619.cb. [DOI] [PubMed] [Google Scholar]
- 43.Löffler M, Weckesser M, Franzius C, Schober O, Zimmer KP. High diagnostic value of 18F-FDG-PET in pediatric patients with chronic inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:379–385. doi: 10.1196/annals.1326.014. [DOI] [PubMed] [Google Scholar]
- 44.Däbritz J, Jasper N, Loeffler M, Weckesser M, Foell D. Noninvasive assessment of pediatric inflammatory bowel disease with 18F-fluorodeoxyglucose-positron emission tomography and computed tomography. Eur J Gastroenterol Hepatol. 2011;23:81–89. doi: 10.1097/MEG.0b013e3283410222. [DOI] [PubMed] [Google Scholar]
- 45.Berthold LD, Steiner D, Scholz D, Alzen G, Zimmer KP. Imaging of chronic inflammatory bowel disease with 18F-FDG PET in children and adolescents. Klin Padiatr. 2013;225:212–217. doi: 10.1055/s-0033-1334878. [DOI] [PubMed] [Google Scholar]
- 46.Giaffer MH, Tindale WB, Holdsworth D. Value of technetium-99m HMPAO-labelled leucocyte scintigraphy as an initial screening test in patients suspected of having inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1996;8:1195–1200. doi: 10.1097/00042737-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Chroustova D, Volf V, Kleisner I, Doubravska M. 99mTc-HMPAO-Labelled Leukocytes Scintigraphy in Monitoring Children and Adolescents with IBD. Current Radiopharma. 2009;2:18–23. [Google Scholar]
- 48.Papós M, Várkonyi A, Láng J, Buga K, Tímár E, Polgár M, Bódi I, Csernay L. HM-PAO-labeled leukocyte scintigraphy in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1996;23:547–552. doi: 10.1097/00005176-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Verdú Rico J, Juste Ruiz M, Jover R, Muñoz Acosta J, Muñoz J, Martínez Caballero A, Antón Leal A, Caballero Carpena O. [99mTc-HMPAO-leukocyte-labeled scintigraphy in the detection and follow-up of inflammatory bowel disease] An Pediatr (Barc) 2006;64:457–463. doi: 10.1157/13087873. [DOI] [PubMed] [Google Scholar]
- 50.Del Rosario MA, Fitzgerald JF, Siddiqui AR, Chong SK, Croffie JM, Gupta SK. Clinical applications of technetium Tc 99m hexamethyl propylene amine oxime leukocyte scan in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1999;28:63–70. doi: 10.1097/00005176-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Halpenny DF, Burke JP, Lawlor GO, O’Connell M. Role of PET and combination PET/CT in the evaluation of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:951–958. doi: 10.1002/ibd.20817. [DOI] [PubMed] [Google Scholar]
- 52.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124–145. doi: 10.1053/j.semnuclmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Neurath MF, Vehling D, Schunk K, Holtmann M, Brockmann H, Helisch A, Orth T, Schreckenberger M, Galle PR, Bartenstein P. Noninvasive assessment of Crohn’s disease activity: a comparison of 18F-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am J Gastroenterol. 2002;97:1978–1985. doi: 10.1111/j.1572-0241.2002.05836.x. [DOI] [PubMed] [Google Scholar]
- 54.Annovazzi A, Biancone L, Caviglia R, Chianelli M, Capriotti G, Mather SJ, Caprilli R, Pallone F, Scopinaro F, Signore A. 99mTc-interleukin-2 and (99m)Tc-HMPAO granulocyte scintigraphy in patients with inactive Crohn’s disease. Eur J Nucl Med Mol Imaging. 2003;30:374–382. doi: 10.1007/s00259-002-1069-x. [DOI] [PubMed] [Google Scholar]
- 55.Van De Wiele C, Sathekge M, Maes A. Targeting monocytes and macrophages by means of SPECT and PET. Q J Nucl Med Mol Imaging. 2014;58:269–275. [PubMed] [Google Scholar]
- 56.Silvola JM, Laitinen I, Sipilä HJ, Laine VJ, Leppänen P, Ylä-Herttuala S, Knuuti J, Roivainen A. Uptake of 68gallium in atherosclerotic plaques in LDLR-/-ApoB100/100 mice. EJNMMI Res. 2011;1:14. doi: 10.1186/2191-219X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pio BS, Byrne FR, Aranda R, Boulay G, Spicher K, Song MH, Birnbaumer L, Phelps ME, Czernin J, Silverman DH. Noninvasive quantification of bowel inflammation through positron emission tomography imaging of 2-deoxy-2-[18F]fluoro-D-glucose-labeled white blood cells. Mol Imaging Biol. 2003;5:271–277. doi: 10.1016/s1536-1632(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 58.Gratz S, Rennen HJ, Boerman OC, Oyen WJ, Corstens FH. Rapid imaging of experimental colitis with (99m)Tc-interleukin-8 in rabbits. J Nucl Med. 2001;42:917–923. [PubMed] [Google Scholar]
- 59.Brewer S, Nair-Gill E, Wei B, Chen L, Li X, Riedinger M, Campbell DO, Wiltzius S, Satyamurthy N, Phelps ME, et al. Epithelial uptake of [18F]1-(2’-deoxy-2’-arabinofuranosyl) cytosine indicates intestinal inflammation in mice. Gastroenterology. 2010;138:1266–1275. doi: 10.1053/j.gastro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blumenthal RD, Sharkey RM, Kashi R, Natale AM, Goldenberg DM. Physiological factors influencing radioantibody uptake: a study of four human colonic carcinomas. Int J Cancer. 1992;51:935–941. doi: 10.1002/ijc.2910510617. [DOI] [PubMed] [Google Scholar]
- 61.Bernards N, Pottier G, Thézé B, Dollé F, Boisgard R. In vivo evaluation of inflammatory bowel disease with the aid of μPET and the translocator protein 18 kDa radioligand [18F]DPA-714. Mol Imaging Biol. 2015;17:67–75. doi: 10.1007/s11307-014-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martín A, Boisgard R, Thézé B, Van Camp N, Kuhnast B, Damont A, Kassiou M, Dollé F, Tavitian B. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abourbeh G, Thézé B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dollé F, Tavitian B, Boisgard R. Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [18F]DPA-714. J Neurosci. 2012;32:5728–5736. doi: 10.1523/JNEUROSCI.2900-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ostuni MA, Issop L, Péranzi G, Walker F, Fasseu M, Elbim C, Papadopoulos V, Lacapere JJ. Overexpression of translocator protein in inflammatory bowel disease: potential diagnostic and treatment value. Inflamm Bowel Dis. 2010;16:1476–1487. doi: 10.1002/ibd.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treglia G, Quartuccio N, Sadeghi R, Farchione A, Caldarella C, Bertagna F, Fania P, Cistaro A. Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography in patients with chronic inflammatory bowel disease: a systematic review and a meta-analysis. J Crohns Colitis. 2013;7:345–354. doi: 10.1016/j.crohns.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Sauer CG. Radiation exposure in children with inflammatory bowel disease. Curr Opin Pediatr. 2012;24:621–626. doi: 10.1097/MOP.0b013e32835742a2. [DOI] [PubMed] [Google Scholar]
- 67.Perlman SB, Hall BS, Reichelderfer M. PET/CT imaging of inflammatory bowel disease. Semin Nucl Med. 2013;43:420–426. doi: 10.1053/j.semnuclmed.2013.06.006. [DOI] [PubMed] [Google Scholar]


