Abstract
A highly selective cascade reaction that allows the direct transformation of dienallenes to spirocyclobutenes (spiro[3.4]octenes) as single diastereoisomers has been developed. The reaction involves formation of overall four C–C bonds and proceeds via a palladium-catalyzed oxidative transformation with insertion of olefin, olefin, and carbon monoxide. Under slightly different reaction conditions, an additional CO insertion takes place to give spiro[4.4]nonenes with formation of overall five C–C bonds.
Spirocarbocyclic scaffolds bearing a quaternary carbon center, have received increasing interests from organic chemists.1,2 These structural elements occur in a wide range of natural products, pharmaceutical ingredients, and chiral ligands.3,4 Therefore, chemists have devoted themselves to developing new strategies for addressing the challenges involving spirocarbocycles.1 To date, different methods have been developed to construct this core motif, such as N-heterocyclic carbene-based organocatalysis,5 metal-catalyzed dearomatization reaction,6 and alkene metathesis with Grubbs catalysts.7 However, development of methodologies for the fast and efficient construction of spirocarbocycles are still highly desirable and challenging.
Our research group has been previously involved in the development of Pd-catalyzed oxidative carbocyclization reactions of allenes to carbocyclic skeletons.8−10 An extension of these carbocyclizations to formation of spirocarbocycles would be highly interesting because spirocarbocycles bearing a fully carbon-substituted quaternary carbon center are challenging synthetic targets.11 One class of compounds that we considered were spirocarbocycles bearing a cyclobutene ring.12,13 Recently, we have developed a palladium-catalyzed oxidative carbocyclization–borylation of enallenes A to cyclobutene derivatives (Scheme 1a).13 Initial coordination of the olefin unit to Pd(II) and subsequent allene attack on the metal result in the formation of intermediate Int-A, which undergoes olefin insertion to form cyclobutene intermediate Int-B. The latter intermediate is trapped by the B2pin2 present in the reaction mixture to give B.
Scheme 1. Previous Work and Present Work.
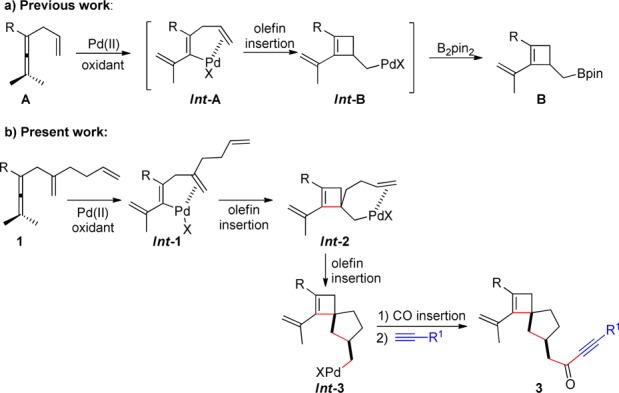
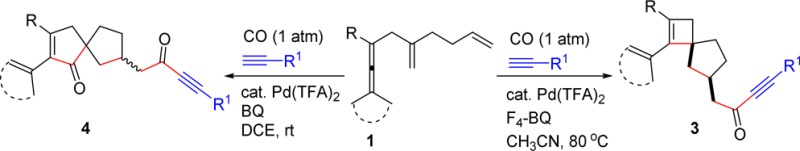
On the basis of these observations, we envisioned that with starting material 1, having an extra olefin chain, the cyclobutene palladium intermediate (Int-2) generated may be able to undergo an insertion reaction to form a spirocarbocyclic intermediate (Int-3) (Scheme 1b). Subsequent carbon monoxide (CO) insertion may provide the spirocyclobutene products 3. Spirocyclobutene derivatives of this type are unique structures and are difficult to prepare with other methods. In this communication, we report on a palladium-catalyzed carbocyclization cascade reaction according to Scheme 1b that provides spirocyclobutene compounds.
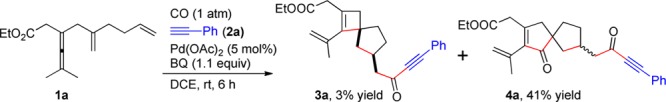
Our study began with the palladium-catalyzed reaction of allene 1a with alkyne 2a (1.5 equiv) using BQ (p-benzoquinone, 1.1 equiv) as oxidant under 1 atm of CO (balloon) at room temperature for 6 h (Scheme 2). Interestingly, the spiro[3.4]octene derivative 3a was formed as envisioned, although the yield was only 3%. Meanwhile, the spiro[4.4]nonene derivative 4a was obtained in 41% yield. To the best our knowledge, there have been no reports to date on efficient synthesis of spirocarbocycles involving a cyclobutene ring via palladium-catalyzed olefin insertion.
Scheme 2. Initial Attempt.
With these inspiring results in hand, we began to optimize the reaction conditions for the formation the spiro[3.4]octene derivative 3a and spiro[4.4]nonene derivative 4a (For details, see Supporting Information, Table S1). Catalyst screening showed that Pd(TFA)2 produced the corresponding 4a in a much higher yield (90%) compared to Pd(OAc)2 or 1,2-bis(phenylsulfinyl)ethane palladium(II), whereas Pd(PPh3)2Cl2 failed to realize such a transformation (Table S1, entries 1–4). Solvent screening revealed that DCE was still the best solvent for the formation of product 4a (Table S1, entries 5–9). Interestingly, the yield of 3a increased to 12% with CH3CN as solvent but conversion was low with starting material 1a being recovered in 45% (Table S1, entry 9). The yield of 3a increased with an increased temperature (Table S1, entries 10–12) and at 80 °C the yield of 3a was 56% (Table S1, entry 12). The favored formation of 3a at higher temperature is probably due to a decrease in the concentration of CO in the solvent, which suppresses CO coordination and hence insertion to form 4a. The yield of 3a was further improved to 65% on dilution (Table S1, entry 13). Finally, the best yield (75%) and selectivity for the formation of 3a was observed when F4-BQ was used as the oxidant (Table S1, entry 15).
Under the optimized reaction conditions for formation of 3, we investigated the scope of terminal alkynes 2 with the substrate dienallene 1a (Table 1). Arylalkynes 2b–2g with both electron-donating and electron-withdrawing groups on the aryl group reacted smoothly and afforded the corresponding spirocyclobutenes 3ab–3ag in good yields (Table 1, entries 2–7). Moreover, selective formation of spirocyclobutenes worked well using heteroaryl acetylenes (Table 1, entries 8 and 9). Aliphatic terminal alkynes also reacted smoothly in the reaction to generate the corresponding products in good yields (Table 1, entries 10 and 11). Gratifyingly, the reaction can be extended to trimethylsilylacetylene to give product 3al in 79% yield (Table 1, entry 12), which after desilylation could be used for further functionalization.
Table 1. Scope of Terminal Alkynesa.
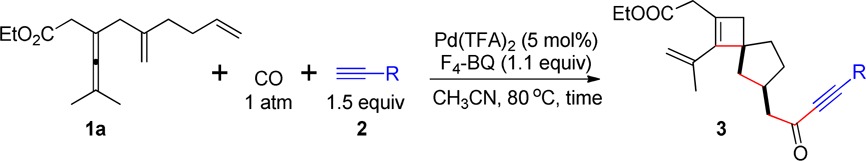
| entry | R | time (h) | yield of 3 (%)b |
|---|---|---|---|
| 1 | Ph | 6 | 75 (3a) |
| 2 | 2-MeOC6H4 | 6 | 72 (3ab) |
| 3 | 3-MeOC6H4 | 6 | 74 (3ac) |
| 4 | 4-MeC6H4 | 6 | 79 (3ad) |
| 5 | 4-FC6H4 | 6 | 64 (3ae) |
| 6 | 4-BrC6H4 | 6 | 66 (3af) |
| 7 | 4-CF3C6H4 | 6 | 77 (3ag) |
| 8 | 2-thiophenyl | 6 | 83 (3ah) |
| 9 | 3-thiophenyl | 6 | 71 (3ai) |
| 10 | Cy | 10 | 66 (3aj) |
| 11 | cinnamyl | 10 | 70 (3ak) |
| 12c | TMS | 15 | 79 (3al) |
The reaction was conducted in MeCN at 80 °C using 1a (0.2 mmol), 2 (1.5 equiv), F4-BQ (1.1 equiv) in the presence of Pd(TFA)2 (5 mol %).
Isolated yield.
TMS-acetylene (3.0 equiv) was used.
We next investigated the scope of the dienallenes for the reaction using phenylacetylene 2a as the terminal alkyne (Scheme 3). In addition to two methyl substituents on the dienallene moiety, cyclopentylidene, cyclohexylidene, and cyclooctylidene dienallenes (1b, 1c, and 1d) also afforded the corresponding products (3b, 3c, and 3d) in good yields. The reaction of the unsymmetric allene 1e, which bears methyl and phenyl groups, afforded 3e in 62% yield. Acetate derivative 1f also worked well under the standard conditions. Furthermore, the reaction tolerated different alkyl groups as R in the oxidative carbocyclization to spirocarbocyclic products 3. For example, R = n-butyl (1g), cyclohexyl (1h), and benzyl (1i),14 afforded the corresponding spirocyclobutene derivatives 3g–i. It is noteworthy that all the spiro[3.4]octene derivatives 3 were obtained as single diastereoisomers with high selectivity.15
Scheme 3. Scope for Formation of 3,
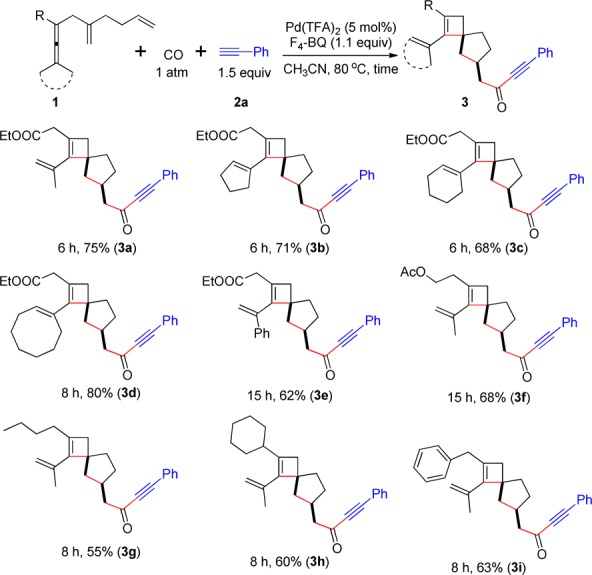
The reaction was conducted in MeCN at 80 °C using 1 (0.2 mmol), 2a (1.5 equiv), F4-BQ (1.1 equiv) in the presence of Pd(TFA)2 (5 mol %).
For stereochemical assignment of products by NOE, see Supporting Information (p. S31).
We next explored the substrate scope under the optimal reaction conditions for the formation of spirocarbocycles 4 (Scheme 4). Notably, the reaction of substrates with two methyl groups, cyclopentylidene, cyclohexylidene, or cyclooctylidene on the dienallene moiety all worked well, affording the corresponding products 4a–4d in good yields with dr values from 91/9 to 95/5. A benzyl group on the allene unit (R) was also tolerated. Arylacetylenes substituted with o-MeO and p-Me groups reacted smoothly and afforded 4ab and 4ad. It is noteworthy that the reaction with heteroaryl acetylenes proceeded well and produced the corresponding spirocarbocycles 4ah and 4ai in good yields.
Scheme 4. Scope for Formation of 4.
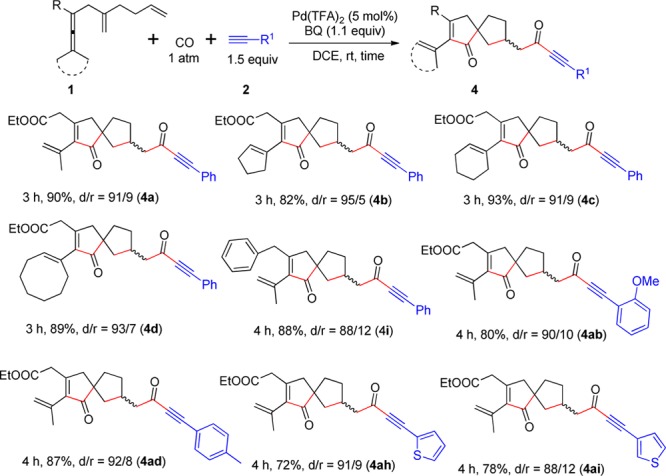
The reaction was conducted in DCE at room temperature using 1 (0.2 mmol), 2 (1.5 equiv), and BQ (1.1 equiv) in the presence of Pd(TFA)2 (5 mol %).
We then explored the effect of the length of the carbon chain.
Substrate 1j with one carbon less, and substrate 1k with one carbon more, compared to the standard substrate 1a, failed to give spirocyclic products 3j and 3k, respectively (eq 1).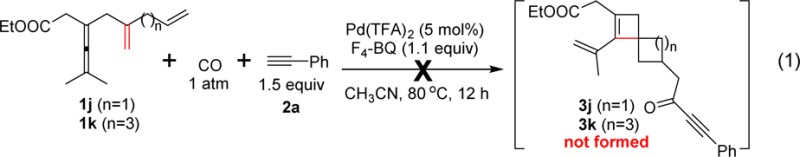 These experiments show that
the second cyclization to give the spirocyclobutene derivatives is
only favored for formation of a five-membered ring.
These experiments show that
the second cyclization to give the spirocyclobutene derivatives is
only favored for formation of a five-membered ring.
To gain further insight into the mechanism for the formation of spirocyclobutenes 3, the deuterium kinetic isotope effects were studied (Scheme 5).16 An intermolecular competition experiment was conducted at 80 °C using a 1:1 mixture of 1a and 1a-d6 (Scheme 5a). The total yield of 3a/3a-d5 was 11%, and the product ratio 3a/3a-d5 (ca. 11.3% conv.) measured was 4.5:1. From these results, the competitive KIE was determined to kH/kD = 4.9. Furthermore, parallel kinetic experiments afforded a KIE (kH/kD from initial rate) value of 2.7 (Scheme 5b,c). These results indicate that the initial allenylic C–H bond cleavage is partially rate-limiting. The large competitive isotope effect in the C–H bond cleavage (kH/kD = 4.9) requires that this step is the first irreversible step.
Scheme 5. Kinetic Isotope Effect Studies.
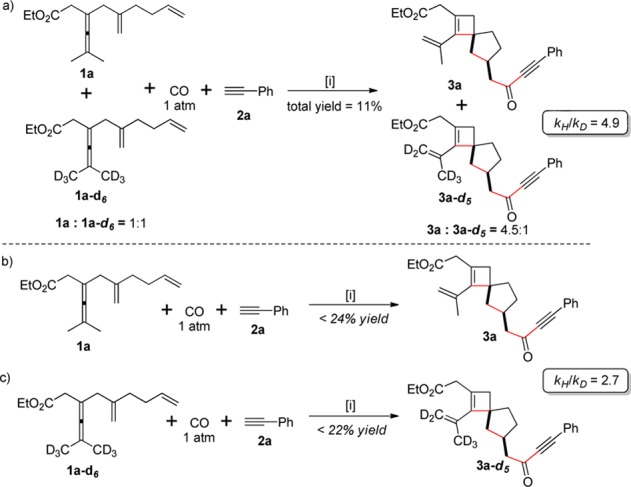
Reaction conditions: allene 1a (or 1a-d6) (0.2 mmol), Pd(TFA)2 (5 mol %), F4-BQ (1.1 equiv), and phenylactylene 2a (1.5 equiv) in CD3CN under CO (1 atm) at 80 °C.
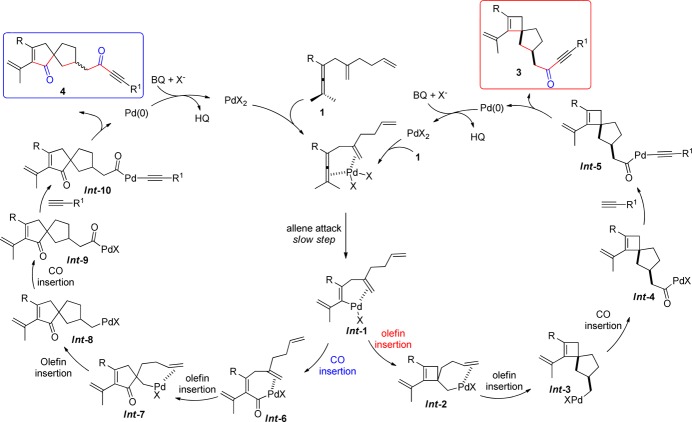
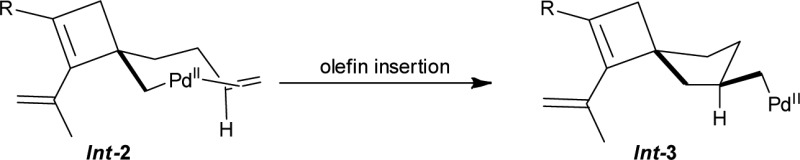
Based on the KIE studies and the reaction outcome, a possible mechanism for the palladium-catalyzed oxidative carbocyclization of dienallene is given in Scheme 6. The reaction of Pd(TFA)2 with 1 could give vinylpalladium intermediate Int-1 through allene attack involving allenic C–H bond cleavage,17,18 which is promoted by the coordination of allene and olefin to Pd(II).19 Intermediate Int-1 could then undergo an olefin insertion to afford cyclobutene intermediate Int-2. Subsequent cascade olefin and CO insertions would produce the intermediate Int-4 via Int-3. Finally, reaction of Int-4 with terminal alkyne 2 would produce Int-5, which on subsequent reductive elimination leads to spiro[3.4]octene derivatives 3.20 On the other hand, Int-1 may undergo a carbonylation to give Int-6, which on olefin–olefin–CO insertion would produce Int-9 via intermediates Int-7 and Int-8. Intermediate Int-9 would then react with terminal alkyne 2 to afford the final spiro[4.4]nonene derivatives 4 via Int-10. The solvent effect by CH3CN to favor Int-2 over Int-6 from Int-1 is most likely due to coordination of CH3CN, which suppresses CO coordination and hence insertion.
Scheme 6. Proposed Mechanisms.
In conclusion, we have developed an efficient palladium-catalyzed oxidative carbocyclization–carbocyclization–carbonylation−alkynylation that selectively gives spirocyclobutene derivatives 3 (spiro[3.4]octenes) as single diastereoisomers with formation of overall four C–C bonds. By changing the reaction conditions, spiro[4.4]nonene derivatives 4 were selectively obtained via cascade CO–olefin–olefin–CO insertion reactions involving formation of overall five C–C bonds. Mechanistic studies showed that the allenylic C–H bond cleavage is partially rate-limiting and also the first irreversible step. The cascade reactions developed here should be useful in synthetic and materials chemistry. Further studies on the scope, synthetic application, and asymmetric variants of these reactions are currently carried out in our laboratory.
Acknowledgments
Financial support from the European Research Council (ERC AdG 247014), The Swedish Research Council (621-2013-4653), the Berzelii Center EXSELENT, and the Knut and Alice Wallenberg Foundation is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b09240.
Experimental procedures and compound characterization data, including the 1H/13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For selected reviews, see:; a Rios R. Chem. Soc. Rev. 2012, 41, 1060. 10.1039/C1CS15156H. [DOI] [PubMed] [Google Scholar]; b Kotha S.; Deb A.; Lahiri K.; Manivannan E. Synthesis 2009, 2009, 165. 10.1055/s-0028-1083300. [DOI] [Google Scholar]; c Ding K.; Han Z.; Wang Z. Chem. - Asian J. 2009, 4, 32. 10.1002/asia.200800192. [DOI] [PubMed] [Google Scholar]; d D'yakonov V. A.; Trapeznikova O. A.; de Meijere A.; Dzhemilev U. M. Chem. Rev. 2014, 114, 5775. 10.1021/cr400291c. [DOI] [PubMed] [Google Scholar]
- For selected reviews involving construction of a quaternary carbon center, see:; a Steven A.; Overman L. E. Angew. Chem., Int. Ed. 2007, 46, 5488. 10.1002/anie.200700612. [DOI] [PubMed] [Google Scholar]; b Shimizu M. Angew. Chem., Int. Ed. 2011, 50, 5998. 10.1002/anie.201101720. [DOI] [PubMed] [Google Scholar]; c Wang B.; Tu Y. Acc. Chem. Res. 2011, 44, 1207. 10.1021/ar200082p. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Zheng Y.; Tice C. M.; Singh S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673. 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]; b Welsch M. E.; Snyder S. A.; Stockwell B. R. Curr. Opin. Chem. Biol. 2010, 14, 347. 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kotha S.; Mandal K. Tetrahedron Lett. 2004, 45, 1391. 10.1016/j.tetlet.2003.12.075. [DOI] [Google Scholar]; d Li F.; Tartakoff S. S.; Castle S. L. J. Am. Chem. Soc. 2009, 131, 6674. 10.1021/ja9024403. [DOI] [PubMed] [Google Scholar]; e Chiang Y.-M.; Kuo Y.-H. Tetrahedron Lett. 2003, 44, 5125. 10.1016/S0040-4039(03)01116-X. [DOI] [Google Scholar]
- a Xie J.; Zhou Q. Acc. Chem. Res. 2008, 41, 581. 10.1021/ar700137z. [DOI] [PubMed] [Google Scholar]; b Zhu S.; Cai Y.; Mao H.; Xie J.; Zhou Q. Nat. Chem. 2010, 2, 546. 10.1038/nchem.651. [DOI] [PubMed] [Google Scholar]; c Coulter M. M.; Kou K. G. M.; Galligan B.; Dong V. M. J. Am. Chem. Soc. 2010, 132, 16330. 10.1021/ja107198e. [DOI] [PubMed] [Google Scholar]; d Zhu S.; Song X.; Li Y.; Cai Y.; Zhou Q. J. Am. Chem. Soc. 2010, 132, 16374. 10.1021/ja1078464. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Fürstner A. Chem. Soc. Rev. 2009, 38, 3208. 10.1039/b816696j. [DOI] [PubMed] [Google Scholar]; b Mahatthananchai J.; Bode J. W. Acc. Chem. Res. 2014, 47, 696. 10.1021/ar400239v. [DOI] [PubMed] [Google Scholar]; c Cohen D. T.; Scheidt K. A. Chem. Sci. 2012, 3, 53. 10.1039/C1SC00621E. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Grossmann A.; Enders D. Angew. Chem., Int. Ed. 2012, 51, 314. 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]; e Fensterbank L.; Malacria M. Acc. Chem. Res. 2014, 47, 953. 10.1021/ar4002334. [DOI] [PubMed] [Google Scholar]; f Hopkinson M. N.; Richter C.; Schedler M.; Glorius F. Nature 2014, 510, 485. 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]; For selected examples, see:; g Dugal-Tessier J.; O'Bryan E. A.; Schroeder T. B. H.; Cohen D. T.; Scheidt K. A. Angew. Chem., Int. Ed. 2012, 51, 4963. 10.1002/anie.201201643. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Li J.; Sahoo B.; Daniliuc C. G.; Glorius F. Angew. Chem., Int. Ed. 2014, 53, 10515. 10.1002/anie.201405178. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Zhuo C.; Zhang W.; You S. Angew. Chem., Int. Ed. 2012, 51, 12662. 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]; b Chen Q.; Ye Z.; Duan Y.; Zhou Y. Chem. Soc. Rev. 2013, 42, 497. 10.1039/C2CS35333D. [DOI] [PubMed] [Google Scholar]; c Ding Q.; Ye Y.; Fan R.. Synthesis 2013, 1. [Google Scholar]; d Zi W.; Zuo Z.; Ma D. Acc. Chem. Res. 2015, 48, 702. 10.1021/ar5004303. [DOI] [PubMed] [Google Scholar]; For selected examples, see:; e Yin Q.; You S. Chem. Sci. 2011, 2, 1344. 10.1039/c1sc00190f. [DOI] [Google Scholar]; f Wu W.; Xu R.; Zhang L.; You S. Chem. Sci. 2016, 7, 3427. 10.1039/C5SC04130A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Kotha S.; Mandal K. Tetrahedron Lett. 2004, 45, 1391. 10.1016/j.tetlet.2003.12.075. [DOI] [Google Scholar]; b Kotha S.; Mandal K.; Tiwari A.; Mobin S. M. Chem. - Eur. J. 2006, 12, 8024. 10.1002/chem.200600540. [DOI] [PubMed] [Google Scholar]; c Brock N. L.; Dickschat J. S. Eur. J. Org. Chem. 2011, 2011, 5167. 10.1002/ejoc.201100688. [DOI] [Google Scholar]; d White D. E.; Stewart I. C.; Grubbs R. H.; Stoltz B. M. J. Am. Chem. Soc. 2008, 130, 810. 10.1021/ja710294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews involving palladium oxidative carbocyclization, see:; a Beccalli E. M.; Broggini G.; Martinelli M.; Sottocornola S. Chem. Rev. 2007, 107, 5318. 10.1021/cr068006f. [DOI] [PubMed] [Google Scholar]; b Dénès F.; Pérez-Luna A.; Chemla F. Chem. Rev. 2010, 110, 2366. 10.1021/cr800420x. [DOI] [PubMed] [Google Scholar]; c Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215. 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]; d Deng Y.; Persson A. K. Å.; Bäckvall J.-E. Chem. - Eur. J. 2012, 18, 11498. 10.1002/chem.201201494. [DOI] [PubMed] [Google Scholar]
- For selected examples involving palladium-catalyzed oxidative carbocyclizations, see:; a Wu T.; Mu X.; Liu G. Angew. Chem., Int. Ed. 2011, 50, 12578. 10.1002/anie.201104575. [DOI] [PubMed] [Google Scholar]; b Mu X.; Wu T.; Wang H.; Guo Y.; Liu G. J. Am. Chem. Soc. 2012, 134, 878. 10.1021/ja210614y. [DOI] [PubMed] [Google Scholar]; c Zhu R.; Buchwald S. L. Angew. Chem., Int. Ed. 2012, 51, 1926. 10.1002/anie.201108129. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jaegli S.; Dufour J.; Wei H.; Piou T.; Duan X.; Vors J.; Neuville L.; Zhu J. Org. Lett. 2010, 12, 4498. 10.1021/ol101778c. [DOI] [PubMed] [Google Scholar]; e Wei Y.; Deb I.; Yoshikai N. J. Am. Chem. Soc. 2012, 134, 9098. 10.1021/ja3030824. [DOI] [PubMed] [Google Scholar]
- For selected examples involving palladium-catalyzed oxidative carbocyclizations of enallenes, see:; a Persson A. K. Å.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2010, 49, 4624. 10.1002/anie.201000726. [DOI] [PubMed] [Google Scholar]; b Persson A. K. Å.; Jiang T.; Johnson M.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2011, 50, 6155. 10.1002/anie.201008032. [DOI] [PubMed] [Google Scholar]; c Jiang T.; Persson A. K. Å.; Bäckvall J.-E. Org. Lett. 2011, 13, 5838. 10.1021/ol202451f. [DOI] [PubMed] [Google Scholar]; d Volla C. M. R.; Mazuela J.; Bäckvall J.-E. Chem. - Eur. J. 2014, 20, 7608. 10.1002/chem.201402688. [DOI] [PubMed] [Google Scholar]; e Volla M. R.; Mazuela J.; Bäckvall J.-E. Org. Lett. 2014, 16, 4174. 10.1021/ol501862z. [DOI] [PubMed] [Google Scholar]; f Jiang T.; Bartholomeyzik T.; Mazuela J.; Willersinn J.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2015, 54, 6024. 10.1002/anie.201501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Ferreira E. M.; Stoltz B. M. J. Am. Chem. Soc. 2003, 125, 9578. 10.1021/ja035054y. [DOI] [PubMed] [Google Scholar]; b Zhang H.; Ferreira E. M.; Stoltz B. M. Angew. Chem., Int. Ed. 2004, 43, 6144. 10.1002/anie.200461294. [DOI] [PubMed] [Google Scholar]; c Ferreira E. M.; Zhang H.; Stoltz B. M. Tetrahedron 2008, 64, 5987. 10.1016/j.tet.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Matsuura B. S.; Condie A. G.; Buff R. C.; Karahalis G. J.; Stephenson C. R. Org. Lett. 2011, 13, 6320. 10.1021/ol202881q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of transition metal-catalyzed formation of cyclobutanes/cyclobutenes, see:; a Kubota K.; Yamamoto E.; Ito H. J. Am. Chem. Soc. 2013, 135, 2635. 10.1021/ja3104582. [DOI] [PubMed] [Google Scholar]; b Innitzer A.; Brecker L.; Mulzer J. Org. Lett. 2007, 9, 4431. 10.1021/ol701697r. [DOI] [PubMed] [Google Scholar]; c Barluenga J.; Riesgo L.; López L. A.; Rubio E.; Tomás M. Angew. Chem., Int. Ed. 2009, 48, 7569. 10.1002/anie.200903902. [DOI] [PubMed] [Google Scholar]; d Luparia M.; Oliveira M. T.; Audisio D.; Frébault F.; Goddard R.; Maulide N. Angew. Chem., Int. Ed. 2011, 50, 12631. 10.1002/anie.201106321. [DOI] [PubMed] [Google Scholar]; e Frébault F.; Luparia M.; Oliveira M. T.; Goddard R.; Maulide N. Angew. Chem., Int. Ed. 2010, 49, 5672. 10.1002/anie.201000911. [DOI] [PubMed] [Google Scholar]; f Audisio D.; Luparia M.; Oliveira M. T.; Klütt D.; Maulide N. Angew. Chem., Int. Ed. 2012, 51, 7314. 10.1002/anie.201202853. [DOI] [PubMed] [Google Scholar]
- The cyclobutene derivative bearing a quaternary carbon centers could not be formed:Qiu Y.; Yang B.; Zhu C.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2016, 55, 6520. 10.1002/anie.201601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allenes 1g, 1h, and 1i were prepared by iron-catalyzed cross coupling of propargyl acetate with the appropriate Grignard reagents:Kessler S. N.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2016, 55, 3734. 10.1002/anie.201511139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The high diastereoselectivity is explained
by re-face coordination in Int-2; see
the Supporting Information (p. S30).
- For details of kinetic isotope effect studies, see the Supporting Information.
- a Deng Y.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2013, 52, 3217. 10.1002/anie.201208718. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhu C.; Yang B.; Bäckvall J.-E. J. Am. Chem. Soc. 2015, 137, 11868. 10.1021/jacs.5b06828. [DOI] [PubMed] [Google Scholar]
- It was demonstrated that triggering the allene for attack on palladium requires coordination of an assisting olefin (or acetylene):Zhu C.; Yang B.; Jiang T.; Bäckvall J.-E. Angew. Chem., Int. Ed. 2015, 54, 9066. 10.1002/anie.201502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples involving alkene directing effects in Pd-catalyzed carbocyclization, see:; a Trost B. M.; Shi Y. J. Am. Chem. Soc. 1993, 115, 9421. 10.1021/ja00074a008. [DOI] [Google Scholar]; b Trost B. M.; Tanoury G. J.; Lautens M.; Chan C.; MacPherson T. J. Am. Chem. Soc. 1994, 116, 4255. 10.1021/ja00089a015. [DOI] [Google Scholar]
- We have tried this cascade reaction with the use of B2pin2 as the quenching reagent; however, no borylated product was obtained. We also tried the reaction without the CO and alkyne, but no product from β-hydride elimination was formed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.