Abstract
One goal of the HIV care continuum is achieving viral suppression (VS), yet disparities in suppression exist among subpopulations of HIV-infected persons. We sought to identify disparities in both the ability to achieve and sustain VS among an urban cohort of HIV-infected persons in care. Data from HIV-infected persons enrolled at the 13 DC Cohort study clinical sites between January 2011 and June 2014 were analyzed. Univariate and multivariate logistic regression were conducted to identify factors associated with achieving VS (viral load <200 copies/ml) at least once, and Kaplan–Meier (KM) curves and Cox proportional hazards models were used to identify factors associated with sustaining VS and time to virologic failure (VL≥200 copies/ml after achievement of VS). Among the 4,311 participants 95.4% were either virally suppressed at study enrollment or able to achieve VS during the follow-up period. In multivariate analyses, achieving VS was significantly associated with age (aOR: 1.04; 95%CI: 1.03–1.06 per 5-year increase) and having a higher CD4 (aOR: 1.05, 95% CI 1.04–1.06 per 100 cells/mm3). Patients infected through perinatal transmission were less likely to achieve VS compared to MSM patients (aOR: 0.63, 95% CI 0.51–0.79). Once achieved, most participants (74.4%) sustained VS during follow-up. Blacks and perinatally-infected persons were less likely to have sustained VS in KM survival analysis (log rank chi-square p≤0.001 for both) compared to other races and risk groups. Earlier time to failure was observed among females, Blacks, publically insured, perinatally infected, those with longer-standing HIV infection, and those with diagnoses of mental health issues or depression. Among this HIV-infected cohort, most people achieved and maintained VS; however, disparities exist with regard to patient age, race, HIV transmission risk, and co-morbid conditions. Identifying populations with disparate outcomes allows for appropriate targeting of resources to improve outcomes along the care continuum.
Keywords: viral suppression, care continuum, HIV, disparities, cohort
Introduction
The ultimate goal of the HIV care continuum is the ability to achieve and sustain viral suppression (VS) as this can result in improved individual and population-level outcomes with reductions in comorbidities, mortality, and reduced risks of HIV transmission to others. (Cohen et al., 2011) Thus, achieving and sustaining VS is essential to meet the care continuum goals and successfully implement HIV prevention approaches such as treatment as prevention.
Data from the United States highlights gaps along the continuum with as few as 30% of persons being able to achieve VS.(United States Centers for Disease Control and Prevention, 2014a) Disparate outcomes have been observed along the continuum and it is well documented that blacks, women, men who have sex with men (MSM), and youth are disproportionately impacted by HIV and have lower rates of suppression.( Hall et al., 2012; Mugavero et al., 2009; Zanoni & Mayer, 2014; T. P. Giordano, Hartman, Gifford, Backus, & Morgan, 2009; T. P. Giordano et al., 2005; Olatosi, Probst, Stoskopf, Martin, & Duffus, 2009; Torian & Wiewel, 2011) Accordingly, one of the National HIV AIDS Strategy (NHAS) goals is to reduce HIV-related health disparities.(United States Office of National AIDS Policy, 2010) To reach this goal, identifying groups at higher risk of failing to achieve and maintain suppression is necessary to inform the development of targeted interventions.
In Washington DC, where the HIV prevalence is 2.5%, blacks, women, and MSM represent 75%, 27%, and 44% of persons living with HIV, respectively (District of Columbia HIV/AIDS, Hepatitis, STD, TB Administration, 2014) Upon measurement of DC’s care continuum, an estimated 57% of persons have achieved VS and only 46% remain suppressed over time.(District of Columbia HIV/AIDS, Hepatitis, STD, TB & Administration, 2014) However, more detailed analysis of subpopulations has not been performed to confirm and monitor the relative success of subpopulations in achieving the NHAS goals.
In response to the high burden of HIV disease in Washington, DC in 2011, the DC Cohort study was launched to provide a platform from which to develop strategies and programs to reduce the burden of HIV/AIDS on the nation’s capital. The DC Cohort is a longitudinal observational cohort study of HIV-infected persons receiving care at 13 clinical sites in DC and is able to provide timely data to monitor the quality of HIV care being provided. (Castel, 2012; Greenberg et al., 2015) Briefly, after obtaining informed consent, data are routinely collected from electronic medical records (EMR) on patients’ socio-demographics, general medical and HIV transmission risk factors, and HIV/AIDS diagnosis dates; these data are supplemented with manual abstraction of historical data. Additionally, information on clinical encounters, diagnoses including hepatitis, treatments, and laboratory tests including CD4 cell counts, viral load (VL), and resistance, are collected on all participants. As an observational cohort, follow-up visits, and therefore data collection, correspond with the frequency of clinical visits. Monthly, data are imported into a centralized database via an internet application called Discovere®, and processed into analytic files via SAS 9.4 (Cary, NC).
The objectives of this analysis were to identify disparities in the achievement of VS and sustained VS over time among DC Cohort participants so that population-based interventions to improve care could be based on specific, real-time data relevant to the HIV care environment in Washington, DC.
Methods
Data source
For this analysis, DC Cohort participants enrolled in the study between January 1, 2011 and June 15, 2014 were included. VL information collected through September 15, 2014 was also included. Among the 6,162 persons enrolled during this time period, the analysis was restricted to persons who were antiretroviral treatment experienced at enrollment and had at least two reported VL values at least 60 days apart over the course of the study (n= 4,311). Persons whose ARV status at enrollment was not known were excluded from the analysis. All data reported, including behavioral risk factors and clinical diagnoses, were abstracted from the EMR. Data on persons who refuse to be in the Cohort are routinely collected and, thus far, persons refusing to participate are significantly more likely to be female, white, and have public insurance.
Definitions
VS was defined as having at least one VL test result less than 200 copies/ml; a commonly used cutoff for measuring suppression in other population-based HIV VS analyses. (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2014; Benator et al., 2014; United States Centers for Disease Control and Prevention, 2014a; United States Centers for Disease Control and Prevention, 2014b; United States Department of Health and Human Services, 2015; Yehia et al., 2015) Among those who achieved VS, sustained VS was defined as having all subsequent VL values less than 200 copies/ml after achieving suppression. Virologic failure (VF) was defined as having a VL that was greater than or equal to 200 copies/ml after achieving suppression.
Statistical Analysis
Univariate analyses, using chi-square and Wilcoxon rank sum tests, were conducted describing the characteristics of DC Cohort participants and assessing differences between those who achieved suppression and those who did not.
For each six-month time interval between January 1, 2011, and June 15, 2014, we calculated trends in the percentage of persons on ARVs and the percentage with a suppressed VL, median VL among those not suppressed, and the percentage of VLs greater than 100,000 copies/ml among those who were not suppressed. Univariate and multivariate Cox proportional hazards analyses were performed to identify factors at enrollment that were associated with achieving VS. Similarly, we assessed factors associated with time to earlier VF among patients who were suppressed at enrollment or during observation. For this analysis, observation time began at the date of enrollment or VS, and all covariates were measured at that time. All variables were included in the multivariate models regardless of significance.
To assess the time to VF, Kaplan-Meier survival curves were generated among those participants who were suppressed at study enrollment or thereafter. Mean time in months from the time of suppression to the first date of “failure”, defined as a VL test result of 200 copies/ml or greater, or last VL test date, was calculated stratifying by race and HIV transmission mode.
Given the inherent variability in the 13 clinics, for all analyses, site type (hospital vs. community based clinic) was adjusted for as a random effect. P-values less than or equal to 0.05 were considered significant and all analyses were performed in SAS 9.4 (Cary, NC).
Results
Among the 4,311 participants enrolled between January 1, 2011, and June 15, 2014, 4,111 (95.4 %) were either virally suppressed at study enrollment or able to achieve VS during the follow-up period (Table 1). Most participants were male (75.5%), non-Hispanic black (73.9%), MSM (39.6%), and publically insured (65.7%), and the median age at enrollment was 47.5 years (data not shown). At the time of study enrollment, among those achieving suppression, the median time since HIV diagnosis was 11.2 years; participants had a median CD4 count of 534 cells/μl. Compared to participants who achieved VS, a significantly higher proportion of persons not suppressed were younger, female, black, unstably housed, publically insured, perinatally infected, and had lower CD4 counts at enrollment (p<0.05 for all). There were no statistically significant differences between the two groups with respect to length of HIV diagnosis, history of alcohol or substance abuse, and other co-morbid conditions such as hepatitis B or C, mental health diagnoses, or HIV care facility.
Table 1.
Characteristics of ARV-experienced DC Cohort Participants by Viral Suppression Status (N=4,311).1
| Characteristics2 | Participants Ever Achieving VS (n=4,111) | Participants Not Achieving VS (n= 200) | P-value3 |
|---|---|---|---|
| Age (yrs) (median, IQR) | 47.9 (38.8, 55.0) | 39.6 (21.8, 47.8) | <0.001 |
| Sex at birth, n (%) | |||
| Male | 3131 (76.2) | 122 (61.0) | <0.001 |
| Female | 980 (23.8) | 78 (39.0) | |
| Race/Ethnicity, n (%) | |||
| Non-Hispanic black | 3,07 (73.1) | 180 (90.0) | <0.001 |
| Non-Hispanic white | 698 (17.0) | 11 (5.5) | |
| Hispanic | 179 (4.4) | 4 (2.0) | |
| Other4 | 73 (1.8) | 5 (2.5) | |
| Unknown | 154 (3.7) | 0 (0.0) | |
| Housing status, n (%) | |||
| Permanent/stable | 3480 (84.7) | 154 (77.0) | 0.022 |
| Temporary/unstable | 345 (8.4) | 27 (13.5) | |
| Homeless | 54 (1.3) | 5 (2.5) | |
| Other/Unknown | 232 (5.6) | 14 (7.0) | |
| Insurance status, n (%) | |||
| Public | 2682 (65.2) | 151 (75.5) | <0.001 |
| Private | 1218 (29.6) | 33 (16.5) | |
| Other | 99 (2.4) | 9 (4.5) | |
| Unknown | 112 (2.7) | 7 (3.5) | |
| Mode of transmission, n (%) | |||
| MSM | 1654 (40.2) | 53 (26.5) | <0.001 |
| High risk heterosexual | 1142 (27.8) | 61 (30.5) | |
| IDU | 310 (7.5) | 10 (5.0) | |
| Perinatal | 132 (3.2) | 42 (21.0) | |
| MSM/IDU | 55 (1.3) | 2 (1.0) | |
| Other | 77 (1.9) | 3 (1.5) | |
| Unknown | 741 (18.0) | 29 (14.5) | |
| Years HIV positive (median, IQR) | 11.2 (5.5, 17.4) | 11.3 (5.8, 17.8) | 0.88 |
| CD4 at enrollment (cells/μl) (median, IQR) | 534 (356, 737) | 297 (106, 470) | <0.001 |
| Alcohol abuse, n (%) | |||
| No | 2,576 (62.7) | 121 (60.5) | 0.79 |
| Yes | 442 (10.8) | 24 (12.0) | |
| Missing | 1,093 (26.6) | 55 (27.5) | |
| History of substance abuse, n (%) | |||
| No | 2,219 (54.0) | 102 (51.0) | 0.70 |
| Yes | 695 (16.9) | 37 (18.5) | |
| Missing | 1,197 (29.1) | 61 (30.5) | |
| Hepatitis C status, n (%) | |||
| Negative | 3,562 (86.6) | 181 (90.5) | 0.12 |
| Positive | 549 (13.4) | 19 (9.5) | |
| Hepatitis B status, n (%) | |||
| Negative | 3,982 (96.9) | 196 (98.0) | 0.36 |
| Positive | 129 (3.1) | 4 (2.0) | |
| Mental Health/Depression, n (%) | |||
| No | 2,781 (67.6) | 145 (72.5) | 0.15 |
| Yes | 1,330 (32.4) | 55 (27) | |
| HIV care facility | |||
| Hospital | 2,245 (54.6) | 115 (57.5) | 0.42 |
| Community-based organization | 1,866 (45.4) | 85 (42.5) | |
This includes participants who had at least two viral load measurements during the study period and were antiretroviral (ARV) treatment experienced.
Housing, insurance status, alcohol abuse, substance abuse, hepatitis B, hepatitis C, and mental health/depression were measured at the time of study enrollment. Hepatitis B, C, and mental health/depression were based on ICD9 coding.
Chi-square or Wilcoxon test; significant p-values <0.05 are bolded.
Other race includes mixed race individuals, Asians, Alaska Natives, American Indians, Native Hawaiians, and Pacific Islanders.
When VS was measured over a maximum of a three-year observation period, high proportions of participants were on ART and suppressed at each time interval (Figure 1). For each six-month interval, 94.4% to 97.6% of participants were on ART with 81.2% to 83.8% of participants suppressed at each time point. Among those participants not virally suppressed, the median VL ranged between 2,262 copies/ml to 4,611 copies/ml with as many as 21.3% having VL results at or above 100,000 copies/ml. Further, among those with VL of 100,000 copies/ml or more, 86.0–99.0% were prescribed ART at the time of their high VL test, indicative of poorly controlled HIV replication, potential treatment failure, and/or poor medication adherence.
Figure 1. Trends in Antiretroviral Use and Viral Suppression among DC Cohort participants, January 2011–June 2014.
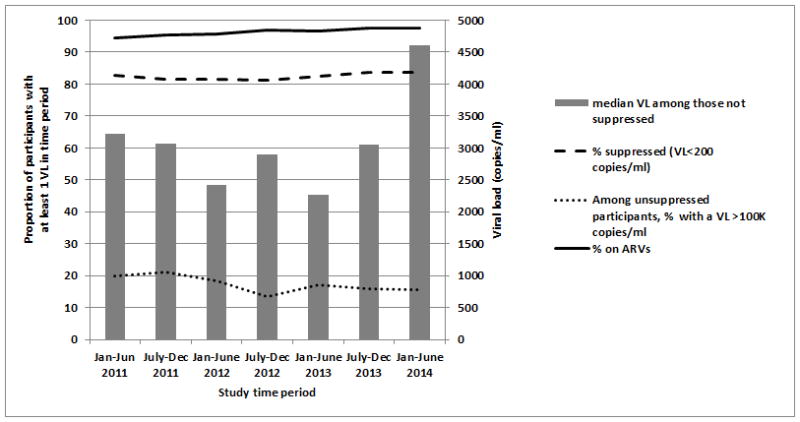
This figure presents the proportion of participants who were on antiretroviral (ARV) treatment, had at least one VL test result, and were suppressed (VL<200 copies/ml) at each 6-month interval between January 2011 and June 2014. Among those who were not suppressed, the median VL and percentage of participants who had a VL over 100,000 copies/ml are also shown.
In the unadjusted Cox proportional hazards model, among participants with baseline CD4 count and known years since HIV diagnosis (n=4,141), older age, male sex, non-Hispanic white race/ethnicity, private insurance, MSM, and higher CD4 count were all significantly associated with earlier time to achieving VS (p<0.05) (Table 2). However, in the adjusted multivariate model, perinatal infection was significantly associated with longer time to VS and only older age and higher CD4 count at enrollment remained significantly associated with earlier achievement of VS. After adjustment for other factors, for each five-year increase in age, there was a 4% increase in achieving VS and for every 100-cell/μl increase in CD4 count, there was a 5% increase in achieving VS.
Table 2.
Factors Associated with Achieving Viral Suppression (N=4,141).1
| Characteristic | HR (95%CI) | aHR (95%CI)2 |
|---|---|---|
| Age (per 5 yrs) | 1.05 (1.04, 1.06) | 1.04 (1.03, 1.06) |
| Sex at birth | ||
| Male | ref | ref |
| Female | 0.88 (0.81, 0.94) | 0.95 (0.87, 1.03) |
| Race/Ethnicity | ||
| Non-Hispanic black | 0.82 (0.75, 0.89) | 0.93 (0.84, 1.02) |
| Non-Hispanic white | ref | ref |
| Hispanic | 0.96 (0.81, 1.13) | 1.08 (0.91, 1.28) |
| Other | 0.86 (0.67, 1.10) | 0.96 (0.75, 1.24) |
| Unknown | 1.01 (0.85, 1.21) | 1.05 (0.88, 1.26) |
| Housing status | ||
| Permanent/stable | ref | ref |
| Temporary/unstable | 0.91 (0.81, 1.02) | 0.97 (0.86, 1.09) |
| Homeless | 0.89 (0.68, 1.16) | 0.93 (0.70, 1.22) |
| Other/Unknown | 0.99 (0.87, 1.14) | 0.99 (0.85, 1.14) |
| Insurance status | ||
| Public | 0.87 (0.82, 0.94) | 0.94 (0.87, 1.02) |
| Private | ref | ref |
| Other | 0.85 (0.69, 1.04) | 0.90, 0.73, 1.11) |
| Unknown | 0.91 (0.75, 1.11) | 0.91 (0.74, 1.12) |
| Mode of transmission | ||
| MSM | ref | ref |
| High risk heterosexual | 0.91 (0.84, 0.98) | 0.93 (0.84, 1.02) |
| IDU | 0.99 (0.87, 1.12) | 0.98 (0.84, 1.13) |
| Perinatal | 0.53 (0.44, 0.64) | 0.63 (0.51, 0.79) |
| MSM/IDU | 1.09 (0.83, 1.43) | 1.10 (0.84, 1.45) |
| Other | 0.98 (0.77, 1.23) | 0.98 (0.78, 1.25) |
| Unknown | 0.97 (0.89, 1.06) | 0.96 (0.88, 1.06) |
| Years HIV positive (per 5 years) | 1.01 (0.99, 1.03) | 0.98 (0.96, 1.01) |
| CD4 at enrollment (per 100 cells/microliter) | 1.05 (1.04, 1.06) | 1.05 (1.04, 1.06) |
| Alcohol abuse | ||
| No | ref | ref |
| Yes | 0.98 (0.89, 1.09) | 0.99 (0.88, 1.11) |
| Unknown | 0.98 (0.91, 1.05) | 1.00 (0.90, 1.10) |
| History of substance abuse | ||
| No | ref | ref |
| Yes | 0.97 (0.89, 1.06) | 1.00 (0.91, 1.09) |
| Unknown | 1.01 (0.94, 1.09) | 1.02 (0.93, 1.12) |
| Hepatitis C status | ||
| Negative | ref | ref |
| Positive | 1.05 (0.96, 1.15) | 0.99 (0.89, 1.10) |
| Hepatitis B status | ||
| Negative | ref | ref |
| Positive | 1.03 (0.86, 1.23) | 1.04 (0.87, 1.25) |
| Mental Health/Depression | ||
| No | ref | ref |
| Yes | 1.03 (0.96, 1.10) | 1.00 (0.93, 1.07) |
Excludes participants with missing baseline CD4 or years HIV-diagnosed. Clinic type (hospital vs. CBO) was adjusted for as a random effect in the model.
Adjusted for all other variables in the model. Significant hazard ratios and 95% confidence intervals are bolded.
Among participants whose VL was suppressed at enrollment or who achieved suppression during observation, 4,024 had at least one subsequent VL test result after VS. Participants who sustained VS had an average of 6.47 VLs recorded after suppression with a mean testing rate of 3.33 tests per person-year (95%CI:3.28–3.37); those who did not sustain VS had an average of 3.99 VLs recorded with a mean testing rate of 3.83 tests per person-year (95%CI:3.71–3.95). Using Kaplan-Meier analysis, most participants (n=2,994; 74.4%) remained VS throughout the study period (Figure 2a); 29.0% of black participants were unable to sustain VS, with a mean time to VF of 31.5 months compared to those of other races (15.7%, with mean time to failure of 34.9 months) (log rank chi-square, p<0.0001). When stratified by mode of transmission, time to VF was lowest for perinatally infected participants (mean time to failure of 18.7 months with 38.8% experiencing failure), followed by those infected through other modes of transmission and heterosexuals. MSM had the longest mean time to failure of 32.9 months with only 22.2% experiencing VF (log rank chi-square, p<0.001) (Figure 2b).
Figure 2. Kaplan-Meier Curves of Sustained Viral Suppression by Race and Mode of Transmission.
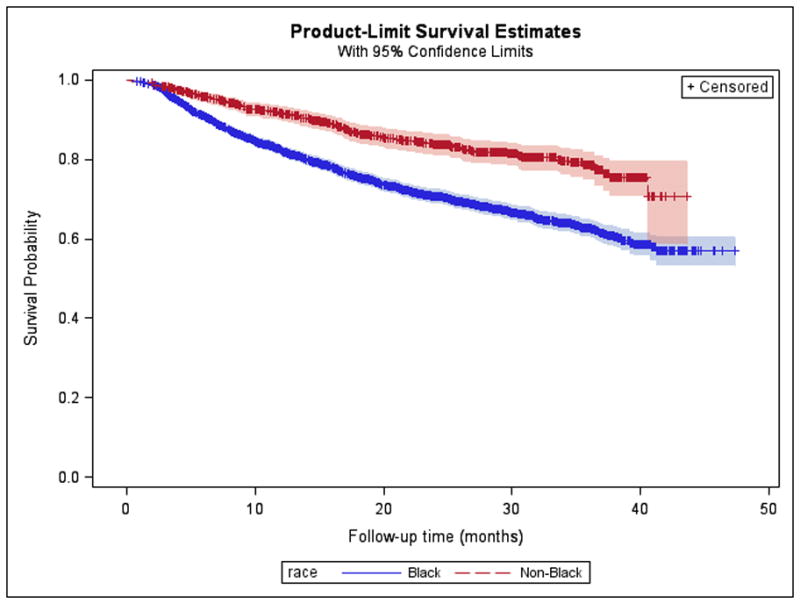
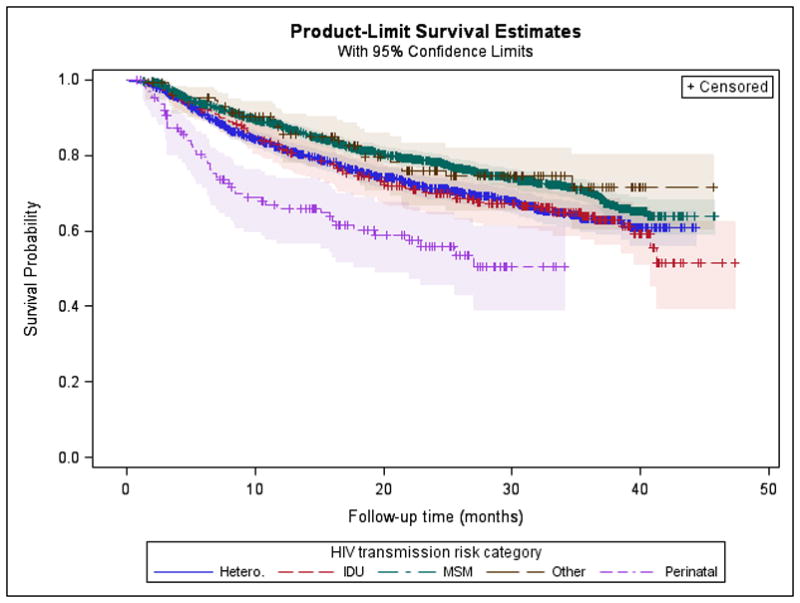
2A. The mean time to virologic failure (defined as a VL>200 copies/ml after achieving suppression) among black participants was 31.5 months compared to those of other races (34.9 months) (log rank chi-square, p<0.0001). 2B. The mean time to virologic failure for participants infected through perinatal transmission was 18.7 months, and for those infected through high-risk heterosexual contact it was 29.6 months compared to participants infected through MSM (32.9 months) (log rank chi-square, p<0.001).
In univariate Cox proportional hazards models among patients VS during observation with known CD4 count and years HIV-diagnosed who had at least one subsequent VL recorded (n=3,921), older age and higher CD4 count at the time of suppression were associated with longer times to VF, while female sex, non-Hispanic black and race/ethnicity, temporary or no housing, public insurance, heterosexual, injection drug use, or perinatal mode of HIV transmission, greater number of years HIV-diagnosed, alcohol use, substance abuse, and mental health disorders, were all associated with earlier time to VF (Table 3). After adjustment for other factors, age, sex, race/ethnicity, insurance, mode of HIV transmission, years HIV-diagnosed, CD4 count, and mental health/depression remained significantly associated with time to VF.
Table 3.
Factors Associated with Earlier Time to Virologic Failure (N=3,921).1
| Characteristic | HR (95%CI) | aHR (95%CI)2 |
|---|---|---|
| Age (per 5 yrs) | 0.93 (0.91, 0.96) | 0.92 (0.89, 0.95) |
| Sex at birth | ||
| Male | ref | ref |
| Female | 1.52 (1.33, 1.75) | 1.32 (1.12, 1.56) |
| Race/Ethnicity | ||
| Non-Hispanic black | 2.18 (1.77, 2.69) | 1.75 (1.40, 2.19) |
| Non-Hispanic white | ref | ref |
| Hispanic | 1.42 (0.95, 2.12) | 1.26 (0.83, 1.89) |
| Other | 1.34 (0.74, 2.44) | 1.21 (0.66, 2.21) |
| Unknown | 1.54 (1.03, 2.31) | 1.69 (1.12, 2.55) |
| Housing status | ||
| Permanent/stable | ref | ref |
| Temporary/unstable | 1.53 (1.18, 1.99) | 1.19 (0.91, 1.57) |
| Homeless | 1.76 (1.10, 2.81) | 1.58 (0.97, 2.56) |
| Other/Unknown | 0.71 (0.62, 0.81) | 0.87 (0.71, 1.07) |
| Insurance status | ||
| Public | 1.91 (1.59, 2.29) | 1.31 (1.07, 1.60) |
| Private | ref | ref |
| Other | 0.96 (0.79, 1.16) | 0.85 (0.65, 1.10) |
| Unknown | 1.24 (0.61, 2.52) | 0.84 (0.41, 1.74) |
| Mode of transmission | ||
| MSM | ref | ref |
| High risk heterosexual | 1.31 (1.12, 1.52) | 1.07 (0.88, 1.29) |
| IDU | 1.36 (1.09, 1.70) | 1.17 (0.90, 1.53) |
| MSM/IDU | 0.75 (0.40, 1.40) | 0.64 (0.34, 1.21) |
| Perinatal | 2.55 (1.87, 3.47) | 1.65 (1.11, 2.44) |
| Other | 1.16 (0.72, 1.87) | 1.10 (0.68, 1.77) |
| Unknown | 1.16 (0.97, 1.40) | 1.07 (0.87, 1.30) |
| Years HIV positive (per 5 years) | 1.06 (1.02, 1.11) | 1.12 (1.07, 1.17) |
| CD4 at viral suppression (per 100 cells/μL) | 0.90 (0.88, 0.92) | 0.89 (0.87, 0.91) |
| Alcohol abuse | ||
| No | ref | ref |
| Yes | 1.36 (1.12, 1.66) | 1.17 (0.93, 1.46) |
| Unknown | 1.30 (1.13, 1.49) | 1.25 (1.03, 1.51) |
| History of substance abuse | ||
| No | ref | ref |
| Yes | 1.36 (1.15, 1.60) | 1.11 (0.93, 1.33) |
| Unknown | 1.01 (0.87, 1.18) | 0.88 (0.73, 1.07) |
| Hepatitis C status | ||
| Negative | ref | ref |
| Positive | 1.11 (0.90, 1.37) | 0.82 (0.64, 1.04) |
| Hepatitis B status | ||
| Negative | ref | ref |
| Positive | 1.04 (0.69, 1.55) | 0.92 (0.61, 1.39) |
| Mental Health/Depression | ||
| No | ref | ref |
| Yes | 1.47 (1.28, 1.70) | 1.24 (1.06, 1.45) |
Includes participants who were suppressed at study enrollment or achieved suppression during observation; excludes participants with unknown CD4 cell count or years HIV-diagnosed. Clinic type (hospital vs. CBO) was adjusted for as a random effect in the model.
Adjusted for all other variables in the model. Significant hazard ratios and 95% confidence intervals are bolded.
Discussion
Among a large urban cohort of HIV-infected persons in care, the majority of patients were able to achieve and maintain VS. The rates of VS observed among DC Cohort participants were higher than those observed in other national studies (Hall et al., 2013; Muthulingam, Chin, Hsu, Scheer, & Schwarcz, 2013) as well as those measured using local surveillance data.(District of Columbia HIV/AIDS, Hepatitis, STD, TB Administration, 2014) This may be explained in part by the fact that DC Cohort participants may reflect a subset of HIV-infected persons who are more fully engaged in HIV care and have been living with HIV for a relatively long period of time. Earlier diagnosis of HIV and subsequent treatment initiation may also explain the high rates of suppression.(Silverberg et al., 2006) This may be particularly true in Washington, DC where access to HIV care and treatment is facilitated by high rates of insurance coverage, DC Department of Health initiatives such as “treatment on demand”, and an AIDS Drug Assistance Program that has never had a waiting list.(District of Columbia Department of Health, 2014, personal communication May 26, 2015) Observed high rates of VS coupled with reductions in HIV deaths and declines in incident infections (District of Columbia HIV/AIDS, Hepatitis, STD, TB Administration, 2014) may be reflective of the impact of these city-wide public health initiatives.
However, consistent with previous studies, in our study population blacks, younger persons, and participants infected perinatally or through heterosexual contact were less likely to, or took longer to achieve VS, and were more likely to experience VF. Younger age is a well-documented risk factor for poor engagement in care and subsequently poorer outcomes including VS. Adolescents and young adults have been found to have steep drop offs along the care continuum and lower rates of VS compared to their older counterparts.(Adeyemi, Livak, McLoyd, Smith, & French, 2013; Hall et al., 2013; Whiteside et al., 2014; Yehia, Fleishman, Metlay, Moore, & Gebo, 2012; Zanoni & Mayer, 2014) Issues such as treatment fatigue, disclosure, lack of social support, and stigmatization may lead to periods of non-adherence and may help explain this finding, particularly among those perinatally infected, given the long duration of infection.(Abramowitz et al., 2009; Giannattasio et al., 2011; Reisner et al., 2009; Williams et al., 2006) Additionally, older persons may be more engaged in care due to the management of other chronic health conditions perhaps resulting in a more regular relationship with their HIV provider and may subsequently be more adherent. (Crawford, Sanderson, Breheny, Fleming & Thornton, 2014; Yehia et al., 2015)
While race/ethnicity did not remain significantly associated with achieving VS after adjusting for other factors, it was associated with higher rates of VF. Lower rates of achieved and sustained VS, as well as higher rates of VF, have also been observed among blacks in other HIV cohorts. (Adeyemi, Livak, McLoyd, Smith, & French, 2013; Anastos et al., 2000; Beer, Oster, Mattson, Skarbinski, for the Medical Monitoring Monitoring Project, 2014; Gant et al., 2014; Gifford et al., 2000; Gulick et al., 2006; Hall et al., 2013; Hartzell, Spooner, Howard, Wegner, & Wortmann, 2007; Lucas, Chaisson, & Moore, 1999; Lucas, Chaisson, & Moore, 2003; McFall et al., 2013; Pence et al., 2008; Yehia et al., 2012) In contrast, in other studies, no major differences by race were observed with respect to virologic or immunologic outcomes; however, racial minorities did have a longer time to treatment initiation than whites.(Jensen-Fangel et al., 2002) Explanations for these observed disparities have been attributed to cultural factors (Gifford et al., 2000), health literacy (Beer et al., 2014), and provider cultural competency (Saha et al., 2013). Higher rates of VF among blacks may be due to lower visit adherence, (Howe et al., 2014) poor ART adherence,(Beer et al., 2014; Giordano et al., 2010; Mugavero et al., 2009; Schackman et al., 2007) depression, (McFall et al., 2013) and lower socioeconomic status.(McFall et al., 2013) Thus, interventions addressing these factors may result in improved ART initiation, adherence, and clinical outcomes among racial and ethnic minorities.(Howe et al., 2014)
Although our results indicate that heterosexually infected persons were less likely to sustain VS compared to MSM, there is less consensus as to the effect of mode of transmission on VS. Heterosexually infected males have been less likely to achieve VS compared to infected MSM. (Hall et al., 2013) However, other studies have found no difference in VS rates by transmission category in multivariate analysis. (Hanna et al., 2013; Muthulingam, Chin, Hsu, Scheer, & Schwarcz, 2013) While many successful interventions exist focused on HIV prevention, engagement and retention in care among HIV-infected MSM (Bouris et al., 2013; Hightow-Weideman, Smith, Valera, Matthews, Lyons, 2011; Maulsby et al., 2013), fewer interventions focus specifically on heterosexuals. Identifying individual, social, or structural factors such as increasing patient contact, medical case management, or testing technological interventions, may help to improve engagement, retention, and VS among heterosexuals and other HIV-infected persons. (Gardner et al., 2014; Higa, Marks, Crepaz, Liau, Lyles, 2012; IAPAC, 2015; Ko, Liu, Lai, Pai, Ko, 2013; Thompson et al., 2012)
While the majority of DC Cohort participants were able to achieve VS, intermittent episodes of high viremia (at least one VL greater than 100,000 copies/ml) among those unable to maintain VS were observed among 24% of participants during the study period. Persons with persistently and intermittently high VLs are at higher risk for developing resistant virus (Lucas et al., 1999) and transmitting virus.(Terzian et al., 2012) In this analysis, unsustained virologic control was associated with structural factors including insurance, and unstable housing. These data thus can inform public health decisions regarding strategies to reduce the costs associated with HIV care and reduce HIV associated morbidities. In Washington, DC successful interventions to address these structural barriers have included implementation of the Affordable Care Act, inclusive of early Medicaid expansion, as well as the availability of programs such as the Housing Opportunities for Persons Living with HIV/AIDS (HOPWA). Integration of mental health services with HIV care programs has also been shown to improve retention in care among persons with mental health and depression diagnoses (Sin & DiMatteo, 2014; Pyne et al., 2011; Safren et al., 2009) Further analysis of factors such as ART regimens, retention in care, medication adherence, and the development of drug resistance, as well as clinic-level factors such as the availability of supportive ancillary services, may also provide further insight into understanding these observed treatment failures.
There are several limitations in our analysis. The data presented here only reflect those HIV-infected persons in care and who agreed to participate in the DC Cohort study. Furthermore, the disparities in VS identified are representative of a cohort of HIV-infected individuals who seek care regularly and are most likely highly engaged in care, treatment experienced, and therefore more likely to achieve suppression. Data on persons who were lost to follow-up, died, transferred care to a non-DC Cohort clinic or did not have laboratory testing were censored at the time of these events; however, additional analyses found that these participants accounted for less than 3 percent of participants (data not shown). Although data on ART prescription was provided, the accuracy of ART start dates prior to study enrollment was limited hence we did not include time on ART as a covariate in our analysis. We did not have ART adherence data to further explain some of the outcomes observed with respect to suppression and virologic failure. Finally, this paper presents data on only the first three and a half years of follow up.
Despite these limitations, strengths of this analysis include that it provides a longitudinal representation of HIV care in a major urban area on more than one third of persons living with HIV in Washington, DC. Additionally, the DC Cohort includes data on a diverse group of persons receiving HIV care in a variety of clinical settings. Through the DC Cohort, HIV providers receive data in aggregate form that can more readily be analyzed thereby allowing clinicians to take more timely action to improve the quality of patient care. In the future, sites that are deemed as underperforming may be able to work with the District of Columbia Department of Health to receive additional support and resources through the development and implementation of targeted individual, structural, and population-level interventions. The ability to maintain suppression over long periods of time will require careful population-based monitoring, which the DC Cohort study is uniquely designed to provide. While this current analysis includes only the first 4,311 patients for whom longitudinal data were available, this population represents 85% of the patients at participating clinics who were approached.
In conclusion, high percentages of individuals in this observational cohort of HIV-infected persons receiving care achieved and sustained VS. We identified disparities in VS and identified subcategories of persons who remain under-treated and are potentially contributing to the ongoing spread of HIV in the city. Efforts to identify persons with disparate outcomes will allow for appropriate targeting of resources to improve VS and achieve national goals aimed at reducing health disparities and maximizing outcomes along the care continuum.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [UO1 AI69503-03S2]. Data in this manuscript were collected by the DC Cohort investigators and research staff located at: Cerner Corporation (Darlene Hankerson, Dana Franklin); Children’s National Medical Center Adolescent and Pediatric clinics; Family and Medical Counseling Service; Georgetown University (Mary Young); George Washington Medical Faculty Associates; George Washington University Department of Epidemiology and Biostatistics (Maria Jaurretche, Sally Behan); Howard University (Saumil Doshi); La Clinica Del Pueblo; Metro Health; National Institutes of Health (Carl Dieffenbach); Unity Health Care; Veterans Affairs Medical Center; Washington Hospital Center; Whitman-Walker Health. We would also like to acknowledge the Research Assistants at the participating sites, the DC Cohort Community Advisory Board, and the DC Cohort participants.
Footnotes
Conferences
Portions of these data were presented in poster format at the Conference on Retroviruses and Opportunistic Infections Conference in Boston, MA, in February 2014, Abstract 993.
Conflict of Interest
The authors have no conflicts to declare.
Contributor Information
Amanda D. Castel, Email: acastel@gwu.edu, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health, 950 New Hampshire Ave NW 5th flr, Washington, DC 20052, 202-994-8325.
Mariah M. Kalmin, Email: mariah.kalmin@gmail.com, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health, 950 New Hampshire Ave NW 5th flr, Washington, DC 20052, 202-994-8325
Rachel L.D. Hart, Email: rachel.hart@cerner.com, 2800 Rockcreek Parkway, North Kansas City, MO 64117, 816-201-1138
Heather A. Young, Email: youngh@gwu.edu, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health, 950 New Hampshire Ave NW 5th flr, Washington, DC 20052, 202-994-6518
Harlen Hays, Email: Harlen.Hays@cerner.com, 2800 Rockcreek Parkway, North Kansas City, MO 64117, 816-446-1592.
Debra Benator, Email: Debra.Benator@va.gov, Veterans Affairs Medical Center, 50 Irving St. NW, Washington, DC 20422, 202-745-830l.
Princy Kumar, Email: kumarp@gunet.georgetown.edu, Georgetown University, 110 Kober Cogan Building, 3800 Reservoir Rd. NW, Washington, DC 20007, 202-687-6845.
Richard Elion, Email: RElion@wwc.org, Whitman-Walker Health, 1701 14th St. NW, Washington, DC 20009, 202-745-6142.
David Parenti, Email: dparenti@mfa.gwu.edu, Division of Infectious Disease, George Washington Medical Faculty Associates, ACC 5-411, 2150 Pennsylvania Ave. NW, Washington, DC 20037, 202-741-2234.
Maria Elena Ruiz, Email: MariaElena.Ruiz@medstar.net, Washington Hospital Center, 110 Irving St. NW, Suite 2A-38C, Washington, DC 20010, 202-877-7164.
Angela Wood, Email: afulwood@fmcsinc.org, Family and Medical Counseling Service, 2041 Martin Luther King, Jr. Ave. SE, Suite 300, Washington, DC 20020, 202-889-7900.
Lawrence D’Angelo, Email: ldangelo@cnmc.org, Burgess Adolescent Clinic, Children’s National Medical Center, 111 Michigan Ave. NW, Washington, DC 20010, 202-884-3068.
Natella Rakhmanina, Email: nrakhman@cnmc.org, Special Immunology Service Pediatric Clinic Children’s National Medical Center, 111 Michigan Ave. NW, Washington, DC 20010, 202-476-2083.
Sohail Rana, Email: srana@howard.edu, Howard University Hospital Pediatric HIV Clinic, 2041 Georgia Ave. NW Suite 3300, Washington, DC 20060, 202-865-6498.
Maya Bryant, Email: maya.bryant@howard.edu, Howard University Hospital Adult Infectious Disease Clinic, 2041 Georgia Ave. NW, Washington DC 20059, 202-865-1877.
Annick Hebou, Email: ahebou@metrohealthdc.org, MetroHealth, 1012 14th St. NW, Suite 700, Washington, DC 20005, 202-638-0750.
Ricardo Fernández, Email: Rfernandez@lcdp.org, La Clinica Del Pueblo, 2831 15th St. NW, Washington, DC 20009, 202-462-4788.
Stephen Abbott, Email: SAbbott@unityhealthcare.org, Unity Health Care, 3924 Minnesota Ave. NE, Washington, DC 20019, 202-398-8683.
James Peterson, Email: jpeterso@gwu.edu, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health, 950 New Hampshire Ave NW 5th flr, Washington, DC 20052, 202-994-2852.
Kathy Wood, Email: kwood@cerner.com, 1953 Gallows Rd., Suite 500, Vienna, VA 22182, 703-245-8146.
Thilakavathy Subramanian, Email: thila.subramanian@cerner.com, 2800 Rockcreek Parkway, North Kansas City MO, 64117, 816-888-2674.
Jeffrey Binkley, Email: Jeff.Binkley@cerner.com, 600 Corporate Pointe, Suite 320, Culver City, CA 90230, 310-598-4467.
Lindsey Powers Happ, Email: lpowers@gwu.edu, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health, 950 New Hampshire Ave NW 5th flr, Washington, DC 20052, 202-994-3340.
Michael Kharfen, Email: Michael.kharfen@dc.gov, HIV/AIDS, Hepatitis, Sexually Transmitted Diseases, Tuberculosis Administration (HAHSTA), District of Columbia Department of Health, 899b North Capitol Street NE, 4th flr, Washington, District of Columbia, 20002, 202-671-4900.
Henry Masur, Email: hmasur@cc.nih.gov, Department of Critical Care Medicine, National Institutes of Health, 10 Center Drive, Room 2C145, Bethesda, Maryland 20892, 301-496-9320.
Alan E. Greenberg, Email: aeg1@gwu.edu, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health, 950 New Hampshire Ave NW 5th flr, Washington, DC 20052, 202-994-0612
References
- Abramowitz S, Koenig LJ, Chandwani S, Orban L, Stein R, Lagrange R, Barnes W. Characterizing social support: global and specific social support experiences of HIV-infected youth. AIDS Patient Care STDS. 2009;23(5):323–330. doi: 10.1089/apc.2008.0194. [DOI] [PubMed] [Google Scholar]
- Adeyemi OM, Livak B, McLoyd P, Smith KY, French AL. Racial/ethnic disparities in engagement in care and viral suppression in a large urban HIV clinic. Clin Infect Dis. 2013;56(10):1512–1514. doi: 10.1093/cid/cit063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV, … Greenblatt RM. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24(3):218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- Beer L, Oster AM, Mattson CL, Skarbinski J Medical Monitoring Project. Disparities in HIV transmission risk among HIV-infected black and white men who have sex with men, United States, 2009. AIDS. 2014;28(1):105. doi: 10.1097/QAD.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benator DA, Elmi A, Rodriguez MD, Gale HB, Kan VL, Hoffman HJ, … Squires L. True Durability: HIV Virologic Suppression in an Urban Clinic and Implications for Timing of Intensive Adherence Efforts and Viral Load Monitoring. AIDS Behav. 2014 doi: 10.1007/s10461-014-0917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouris A, Voisin D, Pilloton M, Flatt N, Eavou R, Hampton K, Kuhns LM, … Schneider JA. Project nGage: Network Supported HIV Care Engagement for Younger Black Men Who Have Sex with Men and Transgender Persons. J AIDS Clin Res, Aug. 2013;31:4. doi: 10.4172/2155-6113.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD on behalf of the DC Cohort Executive Committee. Implementation of a City-Wide Cohort HIV-Infected Persons in Care in Washington DC: The DC Cohort; Washington, D.C. July 2012; Poster presentation at the XIX International AIDS Conference.2012. Abstract A-452-0146-08025. [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N … HIV Prevention Trials Network (HPTN) 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TN, Sanderson WT, Breheny P, Fleming ST, Thornton A. Impact of non-HIV related comorbidities on retention in HIV medical care: does retention improve over time? AIDS Behav. 2014;18:617–624. doi: 10.1007/s10461-013-0524-y. [DOI] [PubMed] [Google Scholar]
- District of Columbia HIV/AIDS, Hepatitis, STD, TB Administration. District of Columbia HIV/AIDS, Hepatitis, STD, and TB Administration Epidemiology and Surveillance Report: Surveillance Data Through December 2012. 2014 Retrieved from http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/HAHSTA%20Annual%20Report2014_06232015_FINAL.pdf.
- Gant Z, Bradley H, Hu X, Skarbinski J, Hall HI, Lansky A. Hispanics or Latinos living with diagnosed HIV: progress along the continuum of HIV care - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):886–890. [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Giordano TP, Marks G, Wilson TE, Craw JA, Drainoni M, Keruly JC … for the Retention in Care Study Group. Enhanced Personal Contact With HIV Patients Improves Retention in Primary Care: A Randomized Trial in 6 US HIV Clinics. Clin Infect Dis. 2014;59(5):725–734. doi: 10.1093/cid/ciu357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio A, Officioso A, Continisio GI, Griso G, Storace C, Coppini S, … Pisacane A. Psychosocial issues in children and adolescents with HIV infection evaluated with a World Health Organization age-specific descriptor system. J Dev Behav Pediatr. 2011;32(1):52–55. doi: 10.1097/DBP.0b013e3181f51907. [DOI] [PubMed] [Google Scholar]
- Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. JAIDS J Acquired Immune Defic Syndromes. 2000;23(5):386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Visnegarwala F, White AC, Jr, Troisi CL, Frankowski RF, Hartman CM, Grimes RM. Patients referred to an urban HIV clinic frequently fail to establish care: factors predicting failure. AIDS Care. 2005;17(6):773–783. doi: 10.1080/09540120412331336652. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of Retention in HIV Care Among a National Cohort of US Veterans. HIV clinical trials. 2009;10(5):299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Bartsch G, Zhang Y, Tedaldi E, Absalon J, Mannheimer S, … MacArthur RD. Disparities in outcomes for African American and Latino subjects in the Flexible Initial Retrovirus Suppressive Therapies (FIRST) trial. AIDS Patient Care and STDs. 2010;24(5):287–295. doi: 10.1089/apc.2009.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AE, Hays H, Castel AD, Subramanian T, Happ LP, Jaurretche M … DC Cohort Executive Committee. Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: technical challenges and innovative solutions. J Am Med Inform Assoc. 2015 Dec 31; doi: 10.1093/jamia/ocv176. pii: ocv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunlock RM, Pilcher CD, Murphy RL, Koletar SL, Carlson M, Reichman RC … AIDS Clinical Trials Group (ACTG) A5095 Study Team. Three- vs Four-Drug Antiretroviral Regimens for the Initial Treatment of HIV-1 Infection: A Randomized Controlled Trial. JAMA: The Journal of the American Medical Association. 2006;296(7):769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents Living With HIV in 13 US Areas. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, … Skarbinski J. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, Jacobson LP, … Gange SJ. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009. Clinical Infectious Diseases. 2013;56(8):1174–1182. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell JD, Spooner K, Howard R, Wegner S, Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. Journal of acquired immune deficiency syndromes (1999) 2007;44(4):411–416. doi: 10.1097/QAI.0b013e31802f83a6. [DOI] [PubMed] [Google Scholar]
- Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to Improve Retention in HIV Primary Care: A Systematic Review of U.S. Studies. Current HIV/AIDS Reports. 2012;9(4):313–325. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping Them in “STYLE”: Finding, Linking, and Retaining Young HIV-Positive Black and Latino Men Who Have Sex with Men in Care. AIDS Patient Care STDS. 2011;25(1):37–45. doi: 10.1089/apc.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, Napravnik S, Cole SR, Kaufman JS, Adimora AA, Elston B, … Mugavero MJ. African American race and HIV virological suppression: beyond disparities in clinic attendance. Am J Epidemiol. 2014;179(12):1484–1492. doi: 10.1093/aje/kwu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Advisory Panel on HIV Care Continuum Optimization. IAPAC Guidelines for Optimizing the HIV Care Continuum for Adults and Adolescents. Journal of the International Association of Providers of AIDS Care. 2015 doi: 10.1177/2325957415613442. 2325957415613442. [DOI] [PubMed] [Google Scholar]
- Jensen-Fangel S, Pedersen L, Pedersen C, Larsen CS, Tauris P, Moller A, … Obel N. The effect of race/ethnicity on the outcome of highly active antiretroviral therapy for human immunodeficiency virus type 1-infected patients. Clin Infect Dis. 2002;35(12):1541–1548. doi: 10.1086/344769. [DOI] [PubMed] [Google Scholar]
- Ko N, Liu H, Lai Y, Pai Y, Ko W. Case Management Interventions for HIV-Infected Individuals. Current HIV/AIDS Reports. 10(4):390–397. doi: 10.1007/s11904-013-0183-7. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Annals of Internal Medicine. 1999;131(2):81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Chaisson RE, Moore RD. Survival in an urban HIV-1 clinic in the era of highly active antiretroviral therapy: a 5-year cohort study. Journal of acquired immune deficiency syndromes (1999) 2003;33(3):321–328. doi: 10.1097/00126334-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Maulsby C, Millett G, Lindsey K, Kelley R, Johnson K, Montoya D, Holtgrave D. A systematic review of HIV interventions for black men who have sex with men (MSM) BMC Public Health. 2013;13:625. doi: 10.1186/1471-2458-13-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall AM, Dowdy DW, Zelaya CE, Murphy K, Wilson TE, Young MA … Women’s Interagency, H. I. V. S. Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity. J Acquir Immune Defic Syndr. 2013;64(3):289–298. doi: 10.1097/QAI.0b013e3182a095e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Allison JJ, Giordano TP, Willig JH, Raper JL, Wray NP, … Saag MS. Racial Disparities in HIV virologic failure: Do missed visits matter? Journal of acquired immune deficiency syndromes (1999) 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthulingam D, Chin J, Hsu L, Scheer S, Schwarcz S. Disparities in Engagement in Care and Viral Suppression Among Persons With HIV. Journal of acquired immune deficiency syndromes (1999) 2013;63(1):112–119. doi: 10.1097/QAI.0b013e3182894555. [DOI] [PubMed] [Google Scholar]
- Olatosi BA, Probst JC, Stoskopf CH, Martin AB, Duffus WA. Patterns of engagement in care by HIV-infected adults: South Carolina, 2004–2006. AIDS (London, England) 2009;23(6):725–730. doi: 10.1097/QAD.0b013e328326f546. [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Washington, DC: 2014. [Google Scholar]
- Pence BW, Ostermann J, Kumar V, Whetten K, Thielman N, Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47(2):194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- Pyne JM, Fortney JC, Curran GM, Tripathi S, Atkinson JH, Kilbourne AM, Hagedorn HJ, … Gifford AL. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171(1):23–31. doi: 10.1001/archinternmed.2010.395. [DOI] [PubMed] [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, Mayer KH. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Korthuis PT, Cohn JA, Sharp VL, Moore RD, Beach MC. Primary care provider cultural competence and racial disparities in HIV care and outcomes. Journal of general internal medicine. 2013;28(5):622–629. doi: 10.1007/s11606-012-2298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackman BR, Ribaudo HJ, Krambrink A, Hughes V, Kuritzkes DR, Gunlock RM. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy: results of ACTG A5095. J Acquir Immune Defic Syndr. 2007;46(5):547–554. doi: 10.1097/QAI.0b013e31815ac499. [DOI] [PubMed] [Google Scholar]
- Silverberg MJ, Wegner SA, Milazzo MJ, McKaig RG, Williams CF, Agan BK … Tri-Service AIDS Clinical Consortium Natural History Study Group. Effectiveness of highly-active antiretroviral therapy by race/ethnicity. AIDS. 2006;20(11):1531–1538. doi: 10.1097/01.aids.0000237369.41617. [DOI] [PubMed] [Google Scholar]
- Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med. 2014;47(3):259–269. doi: 10.1007/s12160-013-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzian AS, Bodach SD, Wiewel EW, Sepkowitz K, Bernard MA, Braunstein SL, Shepard CW. Novel use of surveillance data to detect HIV-infected persons with sustained high viral load and durable virologic suppression in New York City. Plos One. 2012;7(1):e29679–e29679. doi: 10.1371/journal.pone.0029679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Orrell C, … Nachega JB. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012;156(11):817–294. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005–2009: Do patients who initiate care stay in care? AIDS Patient Care and STDs. 2011;25(2):79–88. doi: 10.1089/apc.2010.0151. doi: http://dx.doi.org/10.1089/apc.2010.0151. [DOI] [PubMed] [Google Scholar]
- United States Centers for Disease Control and Prevention. Vital Signs: HIV Diagnosis, Care, and Treatment Among Persons Living with HIV – United States, 2011. MMWR Morb Mortal Wkly Rep. 2014a;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- United States Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. Vol. 19. Atlanta, GA: Centers for Disease Control and Prevention; 2014b. [Google Scholar]
- United States Department of Health and Human Services. DHHS Common Core Indicators. Retrieved March 12, 2015, from https://www.aids.gov/pdf/hhs-common-hiv-indicators.pdf.
- United States Office of National AIDS Policy. National HIV/AIDS Strategy: Federal Implementation Plan. 2010. Washington, D.C: White House Office of National AIDS Policy; 2010. [Google Scholar]
- Whiteside YO, Cohen SM, Bradley H, Skarbinski J, Hall HI, Lansky A … Centers for Disease Control and Prevention. Progress along the continuum of HIV care among blacks with diagnosed HIV- United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(5):85–89. [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Storm D, Montepiedra G, Nichols S, Kammerer B, Sirois PA … Pediatric AIDS Clinical Trials Group (PACTG) 219C Team. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics. 2006;118(6):e1745–1757. doi: 10.1542/peds.2006-0493. [DOI] [PubMed] [Google Scholar]
- Yehia BR, Fleishman JA, Metlay JP, Moore RD, Gebo KA. Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. JAMA. 2012;308(4):339–342. doi: 10.1001/jama.2012.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Rebeiro P, Althoff KN, Agwu AL, Horberg MA, Samji H … North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr. 2015;68(4):413–419. doi: 10.1097/QAI.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28(3):128–135. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]


