Abstract
The Sds3 transcriptional corepressor facilitates the assembly of the 1-2 megadalton histone deacetylase (HDAC)-associated Sin3L/Rpd3L complex by providing a crucial homo-dimerization activity. Sds3 engages the scaffolding protein Sin3A, via a bipartite motif within the Sin3 interaction domain (SID) comprising a helix and an extended segment. Here we show that the SID samples two discrete, substantially populated conformations with lifetimes in the tens of milliseconds range. The two conformations differ via a translation of the main chain and the corresponding side chains in the 5 to 7 Å range. Given the close proximity of the SID to other functional motifs in Sds3 at the sequence level, the conformational exchange has the potential to regulate these activities.
Keywords: conformational exchange, conformational heterogeneity, multiple binding modes, NMR spectroscopy, protein-protein interaction
Introduction
Biological macromolecules are not static entities, belying the views implied by atomic-resolution structures determined by crystallographic, NMR, or cryoEM approaches. Advances in NMR instrumentation and methodology over the past couple of decades have shaped our views regarding the amplitudes and timescales of motion in macromolecules and their impact on biological function.1-3 A recurring theme to emerge from these studies is the propensity of these molecules to sample high energy states, in addition to a dominant ground-state conformation. These poorly populated and so-called ‘hidden’, ‘dark’, or ‘invisible’ states often resemble functionally-relevant conformations such as target/substrate-bound states in the apo-protein. Here, we describe how a short polypeptide segment adopts two distinct conformations that are significantly populated in slow-to-intermediate exchange on the timescale of NMR chemical shift measurements when bound to its protein target, even though this feature is not evident from 1H-15N correlated spectra.
The histone deacetylase-associated Sin3L/Rpd3L corepressor complex is a 1.2-2 megadalton, evolutionarily conserved, multi-subunit chromatin-modifying complex that exerts its negative effects on gene transcription by reducing acetylation levels at specific genomic loci.4-8 The mammalian complex comprising ∼10 subunits, six of which are conserved from yeast to human, regulates a broad range of genes and plays important roles in growth, development and homeostasis. Five conserved subunits within this complex including Sin3A/B, SAP30/SAP30L, Sds3/BRMS1/BRMS1L, HDAC1/2, and RbAp46/48 (in this notation, paralogous subunits are indicated by “/”) comprise the core complex and the subunits harbor similar as well as disparate functions including scaffolding, dimerization, nucleic acid binding, histone binding, and HDAC recruitment.9-14 We recently showed that the Sds3 subunit harbors dimerization and nucleic acid-binding functions, in addition to its previously reported activity of directly engaging the scaffolding protein Sin3A/B; we also clarified the structural bases for the Sds3 dimerization and Sin3-binding functions.14
Multiple, Distinct Sds3 Conformers Interact with Sin3A
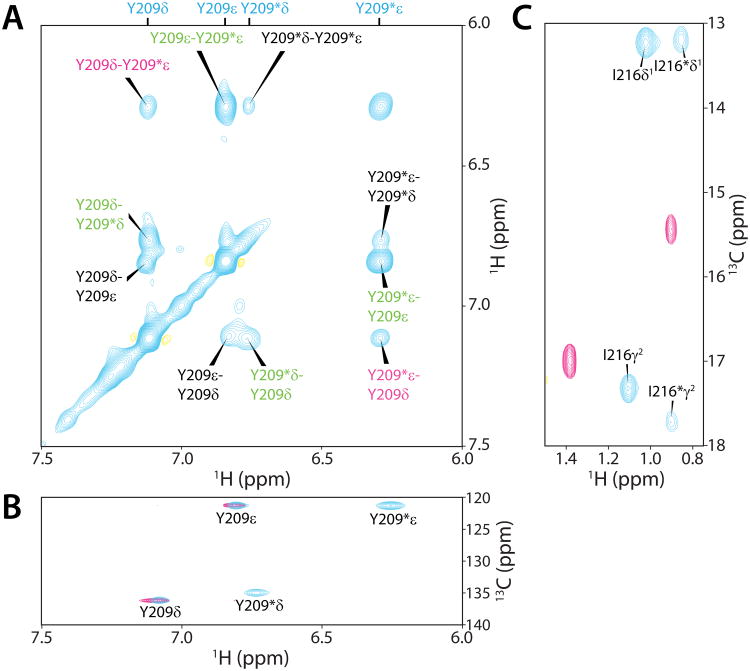
The 1H-15N correlated spectra of both Sin3A HID (HDAC interaction domain) and Sds3 SID (Sin3 interaction domain) in complex with Sds3 SID and Sin3A HID, respectively, were characterized by a single set of correlations that in turn implied a dominant single conformation for the respective polypeptides (Supplementary Figure S1). Unexpectedly, 2D 1H-13C correlated and 3D 13C-edited spectra of Sds3 SID recorded in complex with Sin3A HID were characterized by two sets of resonances for a subset of nuclei, especially for side chains at the protein-protein interface such as Tyr209 and Ile216 (Figure 1; Supplementary Table S1). The chemical shifts for the two conformers were distinct from those of the apo-SID (Figure 1B & 1C), implying two different modes of HID-binding. Virtually all of the detectable resonances belonging to the second conformer exhibited poor relaxation properties which precluded complete resonance assignments of the second conformer via through-bond approaches. However, exchange peaks were readily detected between the two conformers in the NOESY spectra, which facilitated assignment of key resonances at the protein-protein interface for the second conformer including the methyl bearing side chains of Leu207, Leu211, Ile216, Leu220, and Thr222, the Hα/Hβ resonances of Asp219, the Hδ/Hε resonances of Tyr209, among others (Supplementary Table S1). This approach is vividly illustrated for the Tyr209 Hδ/Hε resonances in 2D 15N,13C double half-filtered 1H-1H NOESY spectra, where exchange peaks between the two sets of Hδ/Hε resonances could be readily observed (Figure 1A).
Figure 1.
Detection of two discrete sets of resonances for a subset of aliphatic and aromatic nuclei in Sds3 SID NMR spectra recorded in the presence of Sin3A HID. Expanded plots of (A) 15N,13C double half-filtered 1H-1H NOESY (mixing time, τm = 140 ms),17 (B) aromatic 1H-13C HSQC, and (C) aliphatic 1H-13C HSQC spectra. NMR spectra were recorded on an Agilent DD2 600 MHz spectrometer at 30 °C; samples were prepared as previously described.14 1H-13C spectra for the apo-SID are shown as overlays (magenta) for comparison and to verify these resonances were distinct from those of the second conformer. The double half-filtered spectra were recorded for a 15N,13C-labeled sample of Sin3A HID in complex with unlabeled Sds3 SID. The peaks in all the spectra are annotated with assignments and the peaks belonging to the second conformer are denoted by asterisks; direct intramolecular 1H-1H NOEs, exchange peaks, and exchanged-relayed NOEs are colored black, green, and magenta, respectively.
In direct contrast to the Sds3 SID, only a single set of resonances was detected for the Sin3A HID in 1H-15N and 1H-13C correlated spectra, although main chain and side chain resonances for a few residues at or near the HID-SID interface including Arg699 and Lys703 were significantly broadened. Although the origin of these line broadening effects is unknown, such effects are not uncommon at protein-ligand interfaces and could, in part, be due to multiple modes of ligand binding.15,16
Insights into the Sin3A Surface Targeted by the Second Sds3 SID Conformer
We had previously described the solution structure of Sin3A HID in complex with the Sds3 SID conformer that was not only amenable to sequence-specific resonance assignments but also yielded a self-consistent set of both intra- and inter-molecular 1H-1H NOEs.14 In this structure, the SID engages the HID via a bipartite structural motif comprising a segment spanning from Ala205 to Thr212 in an extended conformation followed immediately by a helix extending from Asp213 to Lys225 (Figure 2A).
Figure 2.

Intermolecular 1H-1H NOEs indicate a significantly altered conformation for Sds3 SID in the Sin3A HID-Sds3 SID complex. (A) Solution structure of the first conformer of Sds3 SID (magenta) in complex with Sin3A HID (cyan; PDB ID: 2N2H).14 Interacting residues are annotated (top panel). A schematic depiction of the alternative locations on the Sin3A HID surface sampled by the highlighted side chains in the second conformer of Sds3 SID (middle panel). The alternative conformation sampled by these side chains would result in a unidirectional translation of the main chain by ∼5-7 ångstroms. A sequence alignment establishing the equivalency of key SID residues (shaded in magenta) in the two conformers at the protein-protein interface (bottom panel). (B) Expanded plots depicting direct and exchange-relayed, intermolecular 1H-1H NOEs involving the two SID conformers in the 13C-filtered,13C-edited NOESY spectrum (τm=140 ms)18 recorded for the 15N,13C-Sin3A HID in complex with unlabeled Sds3 SID. NOEs assignments are annotated with those NOEs involving the second conformer denoted by asterisks; exchange-relayed NOEs are underlined.
In the course of analyzing the 3D 13C-filtered,13C-edited NOESY spectra, it was apparent that there were a number of peaks that could not be explained by NOEs involving the first conformer, whereas, they could be readily explained by NOEs involving the second conformer (Figure 2B). For example, Tyr209 Hδ shows an unambiguous NOE to Met702 Hε, and similarly, Asp219 Hα and Hβ show NOEs to Ile695 Hδ1 while Leu220 Hδ2 shows NOEs to Ala692 Hβ and Ile695 Hδ1. Because exchange-relayed NOEs were also observed in this spectrum (e.g. involving Leu620 Hδ1 and Tyr209 Hε in the second conformer and Met702 Hε and Tyr209 Hδ in the first conformer, both of which were due to direct NOEs involving the same SID protons in the second conformer; Figure 2B), we exercised extreme caution in interpreting NOEs. The aforementioned direct NOEs involving the second conformer served to anchor the assignments for the other NOEs involving this conformer, and collectively, they formed a self-consistent set of NOEs (Figure 2B; Supplementary Table S2).
The widespread lack of chemical shift information at the backbone level coupled with the general lack of intramolecular NOEs involving the second conformer precluded computational modeling of this conformation using the relatively small number of intermolecular NOEs. However, the unambiguous NOE between Met702 Hε in the HID and Tyr209 Hδ in the SID indicates that the Tyr209 side chain engages the pocket occupied by Leu211 in the first conformer (Figure 2A, middle panel); likewise, the unambiguous NOE between Ala205 Hβ in the SID and Leu620 Hδ1 in the HID indicates that this side chain engages the pocket occupied by Leu207 in the first conformer (Supplementary Table S2; Figure 2A, middle panel). These observations imply a translation by approximately two residues in the C-terminal direction at both the main chain and side chain levels in the second conformer relative to the first conformer within the extended segment of the SID bipartite motif (Figure 2A, bottom panel). Similarly, unambiguous NOEs involving Leu220 Hδ2 in the SID and Ala692 Hβ and Ile695 Hδ1 in the HID, places the Leu220 side chain in the second conformer in the pocket occupied by Leu223 in the first conformer; likewise, the NOEs involving Asp219 Hα and Hβ in the SID to Ile695 Hδ1 in the HID, places the Asp219 side chain in the second conformer in a similar location as Thr222 in the first conformer (Figure 2A, middle panel). These observations imply a translation by approximately three residues in the C-terminal direction at the main chain and side chain levels in the second conformer relative to the first conformer within the helical segment of the SID (Figure 2A, bottom panel). In our subjective model, the Leu211 side chain moves to a shallower pocket occupied by Ile216 while Thr222 moves to a position in the vicinity of Leu226 in the first conformer (Figure 2A, middle panel). Thus, the side chain of Asp219, which engages in salt-bridging interactions with Arg699 in the HID in the first conformer (Supplementary Figure 2B), is positioned slightly further from the arginine side chain in the second conformer, diminishing but perhaps not eliminating the electrostatic interaction.
The Same Sds3 Residues in Both Conformers are Critical for Engaging Sin3A HID
We sought to interrogate our model by mutating selected SID side chains and assaying for the presence (or absence) of both (or one) of the conformers by NMR. We specifically targeted those residues that have no significant role in the first conformer in engaging the HID but could play a role in stabilizing the interaction with the second conformer. We selected residues at or near the N-terminus of the helix, as this segment is largely devoid of any stabilizing intermolecular interactions in the first conformer (Supplementary Figure S2). Based on our model, we expected Asp213 and/or Glu214 in the second conformer to be in proximity to Lys677 in the HID, potentially engaging the latter in stabilizing electrostatic interactions. Although the Asp213Gln and Glu214Arg mutations produced a modest two- to three-fold decrease in affinity for the HID,14 neither mutation produced any dramatic reductions in the populations of either conformer (Supplementary Figure S3). We also tested the role of Gln215 because of its potential to directly engage Arg699 in the HID in the second conformer. However, this mutation also did not produce any significant changes to the populations of either conformer. Collectively, our results suggest that both conformers are immune to changes in this particular segment of SID or are immune to the specific types of mutations that were engineered.
We do note, however, that arginine mutations of Asp219 and Leu223 in the SID completely abolished Sin3A HID binding.14 This suggests that Asp219 likely engages in stabilizing electrostatic interactions with Arg699 in the second conformer. The complete abrogation of the HID-SID interaction for the Leu223Arg mutant was somewhat unexpected because, based on our model, this residue in the second conformer would be expected to reside near Leu226 at the periphery of the HID-SID interface. However, it is plausible that Leu223 targets a complementary surface in the HID that is untouched by the first conformer. Collectively, our results suggest that the same residues of SID in the first conformer that play vital roles for engaging HID are also important for interactions involving the second conformer.
Sds3 Conformers Exchange on the Millisecond Timescale
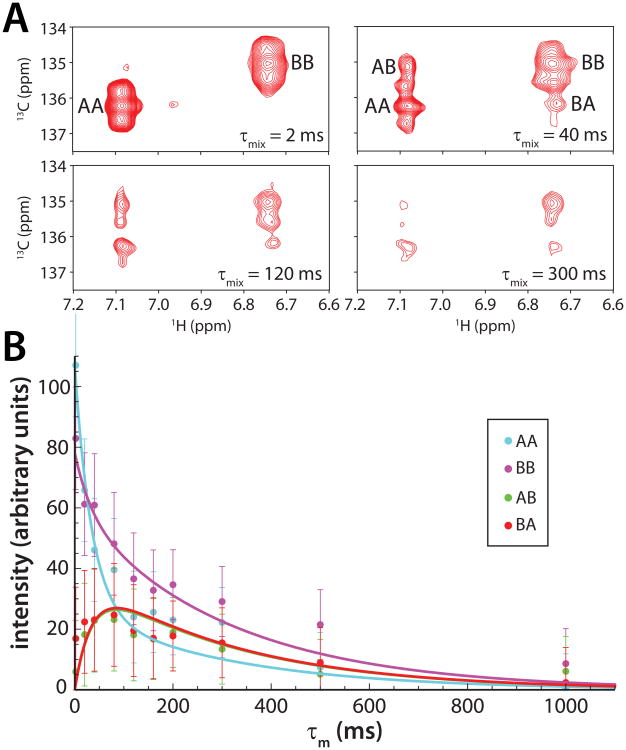
To characterize the exchange kinetics involving the two Sds3 conformers, a series of ZZ-exchange 1H-13C correlated spectra for aromatic resonances with mixing times ranging from 2 to 1000 ms were acquired. Although the 13Cε chemical shifts for the two conformers were similar (Figure 1B), the 1Hδ and 13Cδ chemical shifts were well-resolved to permit the intensities of the auto and exchange peaks to be monitored as a function of mixing time (Figure 3A). Non-linear least squares fitting that included error weighting and error propagation into the fitted values yielded a rate of chemical exchange (kex) of 37 ± 5 s-1 or conformer lifetimes in the ∼50 ms range. This puts the timescale of exchange in the realm of intermediate exchange, which could explain the absence of two sets of resonances for the HID at the protein-protein interface and for the HID and SID at the backbone level, especially if the difference in chemical shifts between the two states is small. It could also explain the line broadening effects witnessed for a subset of HID resonances at the interface. In the course of analyzing the exchange rates, we quantified the relative populations of the conformers and found that the second conformer comprised a substantial 42% of the total population.
Figure 3.
ZZ-exchange spectroscopy reveals exchange kinetics between the two conformers of Sin3A HID-bound Sds3 SID. (A) Changes in auto (AA/BB) and exchange (AB/BA) peak intensities monitored as a function of mixing time due to chemical exchange for the 1Hδ -13Cδ correlations of Tyr209 in Sds3 SID. A series of exchange spectra with the following mixing times: 2, 20, 40, 80, 120, 160, 200, 300, 500, and 1000 ms were acquired. Representative spectra are shown. (B) Peak volumes for the auto and exchange peaks were integrated using Felix 98.0 ((Felix NMR, San Diego, CA) and graphed as a function of mixing time. The data were fitted using Mathematica (Wolfram Research, Champaign, IL) following the non-linear least-squares fitting protocol described by Farrow et al.19; the solid lines represent fitted curves. Measurement errors were quantified for replicate data acquired with mixing times of 80 and 200 ms; the errors were interpolated or extrapolated for the other mixing times. All fitting parameters were fitted simultaneously and globally. In order to aid the fitting process, the rate constants kAB and kBA were constrained relative to one another, based on the knowledge of the equilibrium constant (Keq = kAB/kBA = IBB/IAA = 0.715, where IAA and IBB are the intensities of the auto peaks) derived from a 1H-13C HSQC acquired with a 7 s relaxation delay.19 In the notation above, the first conformer is A and the second conformer is B.
Conclusions
In summary, we have described a polypeptide segment that adopts two distinct conformations that are substantially populated when bound to its protein target. The conformations engage closely overlapping surfaces but differ from each other through a translation at the backbone and side chain levels ranging between 5 and 7 Å. We surmise that the hydrophobic pockets located on a relatively flat surface of the target and the presence of an imperfectly-repeating sequence motif within the polypeptide (Figure 2A, bottom panel) allows for multiple modes of target engagement. The biological implications of these conformational transitions are presently unknown, but we note that the segment that engages the protein target is only four residues removed from another highly-conserved segment that can bind nucleic acids non-specifically (Supplementary Figure S2). We propose that the conformational transitions involving the former segment could play a role in regulating the activity of the latter segment. Finally, we note that conformational homogeneity by NMR is commonly evaluated based on the characteristics of 1H-15N correlated spectra; studies of the Sds3-Sin3A interaction described herein serve a cautionary tale in that heterogeneity could be manifest in more subtle ways.
Supplementary Material
Acknowledgments
Funding for this work was provided by an American Heart Association Grant 14GRNT2017003 to I.R. We thank the Lurie Cancer Center at Northwestern for supporting structural biology research. M.D.C. was supported by Northwestern summer and academic year Undergraduate Research Grants.
Abbreviations
- HDAC
histone deacetylase
- HID
HDAC interaction domain
- HSQC
heteronuclear single quantum coherence
- NOE
nuclear Overhauser effect
- NOESY
NOE spectroscopy
- SID
Sin3 interaction domain
References
- 1.Baldwin AJ, Kay LE. NMR spectroscopy brings invisible protein states into focus. Nat Chem Biol. 2009;5:808–14. doi: 10.1038/nchembio.238. [DOI] [PubMed] [Google Scholar]
- 2.Kleckner IR, Foster MP. An introduction to NMR-based approaches for measuring protein dynamics. Biochim Biophys Acta. 2011;1814:942–68. doi: 10.1016/j.bbapap.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthis NJ, Clore GM. Visualizing transient dark states by NMR spectroscopy. Q Rev Biophys. 2015;48:35–116. doi: 10.1017/S0033583514000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrozza MJ, Florens L, Swanson SK, Shia WJ, Anderson S, Yates J, Washburn MP, Workman JL. Stable incorporation of sequence specific repressors Ash1 and Ume6 into the Rpd3L complex. Biochim Biophys Acta. 2005;1731:77–87. doi: 10.1016/j.bbaexp.2005.09.005. discussion 75-6. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein RA, Ekwall K. Sin3: a flexible regulator of global gene expression and genome stability. Curr Genet. 2005;47:1–17. doi: 10.1007/s00294-004-0541-5. [DOI] [PubMed] [Google Scholar]
- 6.Cunliffe VT. Eloquent silence: developmental functions of Class I histone deacetylases. Current Opinion in Genetics and Development. 2008;18:404–10. doi: 10.1016/j.gde.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonel P, Costello I, Hendrich B. Keeping things quiet: roles of NuRD and Sin3 co-repressor complexes during mammalian development. International Journal of Biochemistry and Cell Biology. 2009;41:108–16. doi: 10.1016/j.biocel.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith KT, Sardiu ME, Martin-Brown SA, Seidel C, Mushegian A, Egidy R, Florens L, Washburn MP, Workman JL. Human family with sequence similarity 60 member A (FAM60A) protein: a new subunit of the Sin3 deacetylase complex. Mol Cell Proteomics. 2012;11:1815–28. doi: 10.1074/mcp.M112.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayer DE, Lawrence QA, Eisenman RN. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–76. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 10.Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell. 1997;89:349–56. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 11.Alland L, David G, Shen-Li H, Potes J, Muhle R, Lee HC, Hou H, Jr, Chen K, DePinho RA. Identification of mammalian Sds3 as an integral component of the Sin3/histone deacetylase corepressor complex. Mol Cell Biol. 2002;22:2743–50. doi: 10.1128/MCB.22.8.2743-2750.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y, Imhoff R, Sahu A, Radhakrishnan I. Solution structure of a novel zinc finger motif in the SAP30 polypeptide of the Sin3 corepressor complex and its potential role in nucleic acid recognition. Nucleic Acids Res. 2009;37:2142–52. doi: 10.1093/nar/gkp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak AJ, Alfieri C, Stirnimann CU, Rybin V, Baudin F, Ly-Hartig N, Lindner D, Muller CW. Chromatin-modifying complex component Nurf55/p55 associates with histones H3 and H4 and polycomb repressive complex 2 subunit Su(z)12 through partially overlapping binding sites. J Biol Chem. 2011;286:23388–96. doi: 10.1074/jbc.M110.207407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark MD, Marcum R, Graveline R, Chan CW, Xie T, Chen Z, Ding Y, Zhang Y, Mondragon A, David G, Radhakrishnan I. Structural insights into the assembly of the histone deacetylase-associated Sin3L/Rpd3L corepressor complex. Proc Natl Acad Sci U S A. 2015;112:E3669–78. doi: 10.1073/pnas.1504021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster MP, Wuttke DS, Radhakrishnan I, Case DA, Gottesfeld JM, Wright PE. Domain packing and dynamics in the DNA complex of the N-terminal zinc fingers of TFIIIA. Nat Struct Biol. 1997;4:605–8. doi: 10.1038/nsb0897-605. [DOI] [PubMed] [Google Scholar]
- 16.Reibarkh M, Malia TJ, Wagner G. NMR distinction of single- and multiple-mode binding of small-molecule protein ligands. J Am Chem Soc. 2006;128:2160–1. doi: 10.1021/ja055971z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otting G, Wüthrich K. Heteronuclear filters in two-dimensional [1H,1H]-NMR spectroscopy: combined use with isotope labelling for studies of macromolecular conformation and intermolecular interactions. Q Rev Biophys. 1990;23:39–96. doi: 10.1017/s0033583500005412. [DOI] [PubMed] [Google Scholar]
- 18.Zwahlen C, Legault P, Vincent SJF, Greenblatt J, Konrat R, Kay LE. Methods for measurement of intermolecular NOEs by multinuclear NMR spectroscopy: Application to a bacteriophage lambda N-peptide/boxB RNA complex. J Am Chem Soc. 1997;119:6711–6721. [Google Scholar]
- 19.Farrow NA, Zhang O, Forman-Kay JD, Kay LE. A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J Biomol NMR. 1994;4:727–34. doi: 10.1007/BF00404280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.