Abstract
Adaptive features of innate immunity, also termed ‘trained immunity’, have recently been shown to characterize monocytes of BCG vaccinated healthy volunteers. Trained immunity leads to increased cytokine production in response to non-related pathogens via epigenetic reprogramming of monocytes. Recently, memory-like properties were also observed in NK cells during viral infections, but nothing is know whether memory properties of NK cells contribute to trained immunity due to BCG vaccination.
Here we show that BCG vaccination induced a significant increase in proinflammatory cytokine production by NK cells in response to both mycobacteria and unrelated pathogens in healthy volunteers. In addition, in a murine model of disseminated candidiasis, BCG vaccination led to an increased survival in SCID mice, which was partially dependent on NK cells.
So, BCG vaccination induces an increased responsiveness of NK cells to non-related microbial stimuli. These effects may contribute to the non-specific (heterologous) beneficial effects of BCG vaccination.
Keywords: trained immunity, BCG, innate immunity, vaccination
1. Introduction
The traditional paradigm in immunology is that innate immunity - as opposed to adaptive immunity - is static, and does not adapt after encountering an external stimulus to an enhanced functional state. However, we have recently shown that Bacillus Calmette-Guerin (BCG) (1) and Candida albicans (2) can induce enhanced non-specific protection to infections though epigenetic programming of monocytes, and we proposed the term ‘trained immunity’ for this effect (3).
Natural killer (NK) cells are an important cellular component of innate immune system. Interestingly, recent studies have shown that mouse and human NK cells exhibit adaptive memory-like properties, as they can be primed for enhanced IFNγ production upon restimulation (4-7). O'Leary et al. were the first to report that mouse NK cells may have recall responses to haptens during a delayed hypersensitivity response, a phenomenon previously attributed to T cells (4). Later it was shown that initial stimulation of murine and human NK cells with interleukin 12 and 18 (IL12 and IL18) leads to increased IFNγ production after restimulation with cytokines or activating receptor ligation up to three weeks afterwards (5, 6). In addition, Sun et al. described murine ‘memory’ NK cells with enhanced reaction upon restimulation, which are present after an initial infection with murine Cytomegalovirus (MCMV) (7).
These studies have shown adaptive characteristics of NK cells, with increased IFNƔ production upon re-exposure to cells with the same stimulus. In trained immunity, however, we observe that re-exposure to both the same or unrelated stimuli induces an enhanced secondary response, due to the non-specific nature of epigenetic priming of the cells (1, 2). Moreover, we have recently shown that trained immunity of monocytes induced by BCG vaccination lasts up to one year after the vaccination (8), and it is tempting to regard this effect as a mechanism for the non-specific (heterologous) protective effects of BCG vaccination (9). Considering the memory-like characteristics described for NK cells, in the present study we tested the hypothesis that NK cells also contribute to trained immunity after BCG vaccination in both human volunteers and in experimental murine disseminated candidiasis.
2. Methods
2.1. Subjects
Twenty-nine individuals (age range 20-36 years) scheduled to receive a BCG vaccination at the public health service because of travel or work in TB-endemic countries (starting after the study was finished) were included in the study. Blood was drawn before BCG vaccination, as well as two weeks and three months afterwards. In the first twenty volunteers the cytokine measurements were done on total PBMCs, therefore an extra nine volunteers were recruited to determine the role of NK cells. There are no demographic differences between the groups. The study was approved by the Arnhem-Nijmegen Ethical Committee, and written informed consent was given by all the participants.
2.2. Cellular stimulation assays
The mononuclear cell fraction was isolated from blood by density centrifugation of blood, diluted 1:1 in pyrogen-free saline over Ficoll-Paque (Pharmacia Biotech, PA, USA). Cells were washed twice in saline and resuspended in culture medium (RPMI, Invitrogen, CA, USA) supplemented with gentamicin 10 μg/ml, L-glutamine 10 mM, and pyruvate 10 mM. CD14+ (monocytes) and CD56+ (NK cells) subsets were purified from freshly isolated PBMCs using MACS microbeads, according to the instructions of the manufacturer (Miltenyi Biotec). Purity was checked with FACS and was >90%. Cells were counted in a Coulter counter (Coulter Electronics) and the number was adjusted to 5×105 cells/ml. A total of 1 × 105 monocytes or NK cells in a 100μl volume was added to round-bottom 96-wells plates (Greiner) with RPMI (with a additions as previously mentioned) or with sonicated MTB H37Rv (1μg/ml final concentration), heat-killed Candida albicans (1×106 microorganisms/ml, strain UC820), Staphylococcus aureus (1×106 microorganisms/ml), or E. coli lipopolysaccharide 1ng/ml (LPS, Sigma-Aldrich, 1ng/ml). After 48 hours supernatants were stored at −20°C. Cytokine concentrations were assessed in the supernatants using enzyme-linked immunosorbent assay (ELISA).
2.3. Cytokine measurements
Cytokine measurements of TNFα, IL-1β, IL-6, IFNβ and IFNγ were performed in the supernatants using commercial ELISA kits from R&D Systems, MN, USA (TNFα and IL-1β) PBL assay science, Piscataway, USA (IFNβ) or Sanquin, Amsterdam, The Netherlands (IL-6 and IFNγ).
2.4. Flow cytometric analysis
Cells were phenotypically analyzed using a Navios™ instrument with 10-color PMTs and three solid-state lasers (Beckman Coulter, Fullerton, FL). The list mode data files were further analyzed using Kaluza™ software (Beckman Coulter). In order to guarantee that the optics, laser, fluidics and fluorescence intensity were stable during all measurements calibration was performed using Flow Check Pro Fluorospheres (Beckman Coulter) and Cyto-Cal Multifluor + Violet Fluorescence Alignment Beads (Thermo Scientific, Fremont, CA). Cells were washed with PBS with 1% bovine serum albumin before being labelled with fluorochrome-conjugated mAbs. After incubation for 30 min at 4°C in the dark, cells were washed twice to remove unbound antibodies and analyzed. For cell surface staining, the following mAbs were used: CD3-ECD (A07748), CD16-FITC (IM0814U), CD45-Krome Orange (A96416), CD56-APC-Alexa Fluor750 (custom made), CD158a-APC-Alexa Fluor700 (custom made), CD158b-PC7 (A66901), CD158e1/e2-APC (A60795), CD159a-PC5.5 (custom made) (all from Beckman Coulter, Marseille, France) and CD159c-PE (FAB138P; R&D).
3.5. Mouse experiments
Non-obese diabetic (abbreviated NOD) –Prkdcscid mice (abbreviated SCID) lacking functional B and T cells, and NOD-PrkdcscidIL2rgtm1Wjll (abbreviated NOD/SCID/IL2Rγ, NSG) mice lacking B, T and NK cell, were obtained from Charles River Wiga (Sulzfeld, Germany). Female mice between 6 and 8 weeks of age were used. The mice were housed in a pathogen-free facility and were fed with sterilized laboratory chow (Hope Farms, Woerden, The Netherlands) and water ad libitum. The experiments were approved by the Ethics Committee for Animal Experiments of the Radboud University, Nijmegen. Mice were first injected with BCG vaccine SSI (750 μg/mouse) in a 100μL volume of sterile pyrogen-free phosphate-buffered saline (PBS) or with PBS alone. Two weeks later, mice were infected intravenously with a lethal dose of C. albicans blastoconidia (2×106 CFU/mouse). Survival was monitored for 28 days after the C. albicans injection.
2.6. Statistical analysis
Differences were analyzed using the Wilcoxon signed rank test or Friedman test for paired samples. P<0.05 was considered statistically significant. Otherwise stated, data are shown as cumulative results of level obtained in all volunteers (means + SEM).
3. Results
3.1. BCG does not change the proportion of NK cell subsets
In a first set of experiments, we examined possible changes in NK cell subsets following BCG vaccination. So far, no specific phenotypic marker has been described for memory or memory-like NK cells. For increased IFNγ production there are some candidate markers that have an established correlation with human NK cell IFNγ production, such as CD94, CD69, CD159a and CD159c (6). We decided to include some of these markers and in addition some novel activity and inhibitory markers of NK cells. NK cells can be divided into CD56dimCD16+ and CD56brightCD16− cell subsets, which differ in their homing properties. Because approximately 90% of peripheral blood NK cells are CD56dimCD16+, we decided to assess the differential expression of activation or inhibitory markers in this most prominent subset. The CD56brightCD16− NK cells dwell mainly in the lymph nodes tonsils; hence their number in our samples is too low to observe changes in activity and inhibitory cell markers.
Significant changes were noted neither in the number and distribution of NK cell subsets following BCG vaccination (Table 1), nor in the mean fluorescence indexes of these markers (MFI; Table 2).
Table 1.
Percentage of total cells of 20 volunteers before and after BCG vaccination.
| Before BCG | 2 weeks | 3 months | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | ||
| CD56dimCD16+ | 2.25 | 1.56 | 2.24 | 1.01 | 2.3 | 1.4 | 0.97 |
| CD56brightCD16− | 0.07 | 0.06 | 0.07 | 0.04 | 0.05 | 0.04 | 0.09 |
| CD158a+ (KIR2DL1)a | 25.8 | 6.91 | 24.9 | 5.86 | 25 | 6.92 | 0.76 |
| CD158b+ (KIR2DL2)a | 32.5 | 9.76 | 31.1 | 8.15 | 31.7 | 10.1 | 0.25 |
| CD158e+ (KIR3DL1/S1)a | 15.7 | 12.2 | 14.1 | 10.9 | 15.6 | 11.1 | 0.76 |
| CD159a+ (NKG2A)a | 46.1 | 14.1 | 46.9 | 13.9 | 45.2 | 13.3 | 0.07 |
| CD159c+ (NKG2C)a | 4.4 | 2.72 | 4.3 | 2.6 | 4.25 | 2.57 | 0.83 |
Table 2.
Mean fluorescence indexes of 20 volunteers before and after BCG vaccination.
| Before BCG | 2 weeks | 3 months | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | ||
| CD56dimCD16+a | 51.1 | 8.3 | 50 | 9.7 | 49.3 | 10.3 | 0.35 |
| CD56brightCD16–b | 30 | 5.55 | 30.6 | 5.37 | 28.5 | 5.43 | 0.25 |
| CD158a+ (KIR2DL1)c | 13.5 | 3.28 | 13.4 | 3.59 | 12.9 | 4.08 | 0.31 |
| CD158b+ (KIR2DL2)c | 8.66 | 1.89 | 8.52 | 2.06 | 7.95 | 2.58 | 0.45 |
| CD158e+ (KIR3DL1/S1)c | 8.56 | 5.85 | 10 | 7.51 | 9.81 | 6.96 | 0.35 |
| CD159a+ (NKG2A)c | 10.3 | 2.58 | 10.3 | 2.46 | 9.73 | 2.23 | 0.21 |
| CD159c+ (NKG2C)c | 6.56 | 3.31 | 5.99 | 1.22 | 5.89 | 1.26 | 0.12 |
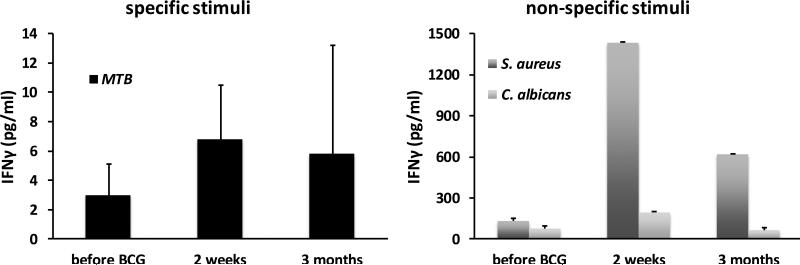
3.2. BCG vaccination does not increase interferon-gamma production by NK cells
Two weeks and three months after BCG vaccination, isolated monocytes displayed slightly increased IFNγ production in response to unrelated stimuli including S. aureus and C. albicans (Supplementary Figure 1), in accordance with previous observations (1). In contrast, isolated NK cells did not display an increase in IFNγ production following BCG vaccination (figure 1). IFNβ concentrations were below detection limits in stimulated NK cells and in monocytes in these experiments (data not shown).
Figure 1. BCG vaccination does not increase interferon-gamma production by NK cells.
NK cells isolated from naïve (non-exposed) volunteers, before and after (2 weeks and 3 months) vaccination with BCG were stimulated in vitro with sonicated M. tuberculosis, heat-killed S. aureus and C. albicans blastoconidia. INFγ production was assessed by ELISA in the supernatants. Data are presented as mean ± SEM (n = 9).
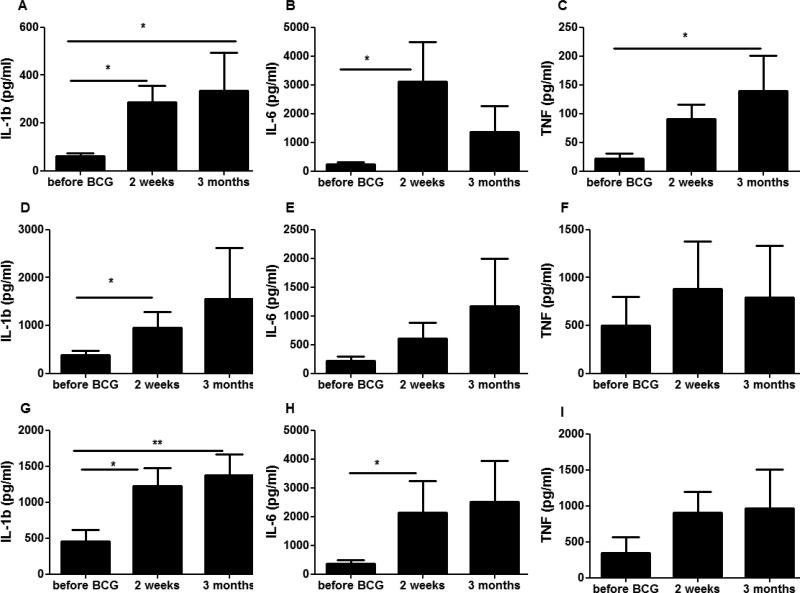
3.3. BCG enhances NK cell production of proinflammatory cytokines
BCG induces trained immunity in monocytes resulting in an increased production of proinflammatory cytokines (1). We examined whether BCG induces similar changes in NK cells. Indeed, NK cells isolated 2 weeks and 3 months after BCG vaccination produced more pro-inflammatory cytokines upon stimulation (Figure 2). This was especially true for IL1β, which showed after BCG vaccination a marked increase after stimulation with either MTB, C. albicans or S. aureus (figure 2A, D, G, respectively). Similar changes were noted, although not significantly in all cases, for IL6 and TNFα (Figure 2 B, E, H and Figure 2 C, F, I, respectively).
Figure 2. BCG vaccination increased the non-specific production of pro-inflammatory cytokines.
NK cells isolated from naïve (non-exposed) volunteers before and after (2 weeks and 3 months) vaccination were stimulated in vitro with sonicated M. tuberculosis (A-C), C. albicans blastoconidia (D-F) and heat-killed S. aureus (G-I). Pro-inflammatory cytokine production (IL-1β (A,D,G), IL-6 (B,E,H) and TNFα (C,F,I) was assessed by ELISA in the supernatants. *p<0.05, **p<0.01, ***p<0.005. Data are presented as mean ± SEM (n = 9). Friedman test was used to detect significant differences.
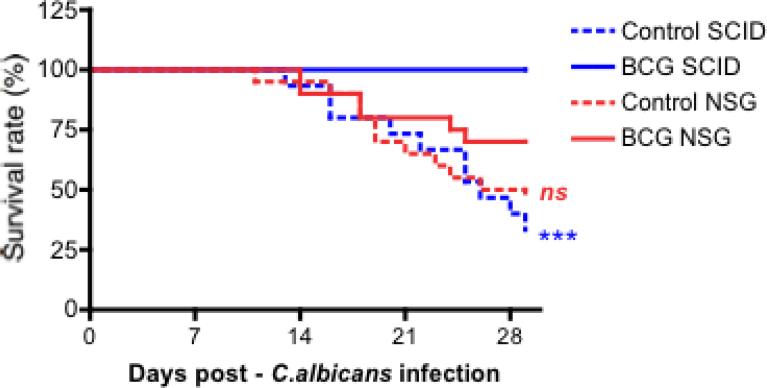
3.4. BCG-induced protection against disseminated Candida albicans infection is partially NK cell dependent
We have previously demonstrated that BCG vaccination protects SCID mice, which lack functional T and B cells, against systemic lethal candidiasis (1). We examined the role of NK cells for this BCG-induced protective effect, by comparing SCID mice with NOD/SCID /IL2Rγ (NSG) mice that both lack functional T and B cells, the latter lacking functional NK cells as well. In two separate experiments, we injected i.v. NSG and SCID mice with either BCG or saline. Two weeks later, all groups of mice were challenged with a lethal intravenous inoculum of C. albicans. Saline-injected SCID and NSG animals were similarly susceptible to the lethal infection, succumbing at the same rate to the candidiasis (Figure 3). As previously demonstrated, all BCG vaccinated SCID mice survived. This protective effect was partially lost in NSG mice, suggesting a role for NK cells in the protection conferred by BCG (Figure 3).
Figure 3. BCG-induced protection against disseminated Candida albicans infection is partially NK cell dependent.
(A) Survival rate of SCID and NSG mice to an infection with live C. albicans (2×106 CFU/mouse) injected intravenously. Mice were either vaccinated intravenously with PBS (Control) or BCG, 14 days prior to inoculation of lethal C. albicans dose (n≥15 per group, 2 independent experiments). ***p<0.005. vs control (PBS) animals.
4. Discussion
In the present study we show that BCG vaccination in healthy volunteers leads to an increased proinflammatory cytokine production by NK cells in response to mycobacteria, as well as to unrelated bacterial and fungal pathogens. This effect, most profoundly seen with IL1β, lasts for at least three months, in contrast to classical non-specific activation of innate immunity, but is not accompanied by changes in NK cell subset distribution or expression of cell surface markers. These effects of BCG on NK-cells are biologically relevant, as we demonstrate a functional role for the trained NK cells in a murine model of lethal systemic candidiasis, in which the protection induced by BCG vaccination in SCID mice seems partially dependent on NK cells. Surprisingly, this improved response does not involve an increase of IFNγ production, which is described in literature as an important memory-like property of NK cells (4-7).
Several recent studies have provided arguments for the existence of adaptive characteristics of NK cells, implying a paradigm shift in our understanding of the function of this innate cell population (4-7, 10). Several pathways have been described that may lead to functional reprogramming of NK cells. Firstly, infection with specific pathogens such as the virus MCMV results in an antigen-specific recall response of memory NK cells after reinfection. This process results in increased IFNγ production and enhanced cytotoxicity (7). Secondly, proinflammatory cytokines such as IL12, IL15 and/or IL18 can induce memory characteristics in NK cells. These NK cells do not possess a distinct surface phenotype, nor does restimulation result in enhanced cytotoxicity (5, 6). Thirdly, hapten or viral antigens can induce liver-restricted memory NK cells with increased cytotoxicity, an effect that is dependent on CXCR6 (4, 11). In the present study we uncover an additional dimension to the adaptive capabilities of NK cells in contrast with memory in the classical sense what is antigen specific: we demonstrate that a vaccination such as BCG induces non-specific priming of NK cells, resulting in an increased inflammatory cytokine response to unrelated stimuli, as well as enhanced resistance against an unrelated fungal infection.
The mechanisms that mediate the protective effects induced by NK cells during reinfection vary between the different models. Murine CMV infection induces NK cell expansion and contraction, reminiscent of T cell activation, and the protection depends on increased expression of the Ly49H NK cell receptor, on enhanced degranulation, and on increased production of IFNγ (7). Interestingly, as we show here, BCG-induced activation results in an improved production of proinflammatory cytokines such as IL-1β and TNF, rather than IFNγ, regardless of the stimulus. Despite the relatively small number of individuals who participated in the study, and the known biological variability in cytokine production, a large number of the parameters describing the proinflammatory cytokine production capacity differed significantly before and after BCG vaccination. Altogether, these studies suggest that NK cells have the capacity to react differently to various stimuli, and to adapt their memory function depending on the infectious agent they encounter. Whether epigenetic changes are at the basis of the increased expression of Ly49H after MCMV or the increased cytokine production after BCG, similarly to the trained immunity in monocytes, remains to be investigated.
The low IFNγ-production capacity reported in our experiments after stimulation with microbial stimuli is in line with earlier data in literature (12), which showed that NK cell production of IFNγ is obtained only by co-stimulation with a viral or mycobacterial stimulus and a stimulatory cytokine (IL2, IL12 or IL15 and IL18) (13-15). Another explanation for the lack of IFNγ production in our experiments could be the fact that mainly the CD56brightCD16− NK cell subset produces IFNγ, which is a minority in human peripheral blood.
Considering the memory-like features of NK cells described in the literature and in this study, one could reasonably hypothesize that the differences in protection induced by BCG between SCID and NSG mice are due to the NK cells. However, defects of differentiation and function of antigen presenting cells (APCs) have been also reported in NSG mice (16), and therefore we cannot exclude that the BCG effects observed here are due to combined effects of NK cells and APCs such as macrophages and/or dendritic cells.
An important observation of the present study is the long-term effect on NK cell function exerted by BCG vaccination. Long-lasting effects have been previously reported in studies showing that pre-activated NK cells that underwent cell divisions had similar degrees of enhanced IFNγ production, indicating heritable memory-like properties (5, 6). Others have also shown that in mice memory-like NK cell responses persist for at least one month in vivo after adoptive transfer (4, 5). Similarly, epigenetic reprogramming leads to enhanced inflammatory properties in monocytes for up to 3 months (1) or even one year (8) after BCG vaccination. One could speculate on the mechanisms inducing these effects. The process of trained immunity in monocytes is mediated by epigenetic regulation (1, 2), and similar processes have been reported to mediate at least some aspects of NK cell maturation and adaptive change (17). Although with the currently available technology it is not yet possible to investigate epigenetic profiles of circulating NK cells, given the low numbers of circulating NK cells in normal individuals, studies using an improved methodology in the near future are warranted.
In conclusion, we demonstrate that BCG vaccination enhances the cytokine production by human NK cells after re-challenge with an unrelated microbial stimulus, a process earlier termed ‘trained immunity’. This effect is long lasting, and demonstrates important adaptive characteristics of NK cells, a prototype innate immune population. These findings have important consequences on the one hand for our better understanding of innate immunity, and on the other hand, to explain and better exploit the effects of vaccines. Non-specific effects of BCG vaccination are widely known in literature (18) and our results with NK cells may explain some of these non-specific protective effects of BCG. Better insights into the role of trained immunity may thus have important consequences for vaccine design, more specifically with regard to selection of antigens and the development of new adjuvants that boost both specific immunity and the adaptive properties of innate immune cells.
Supplementary Material
Acknowledgments
Funding
This work was supported by a Vici Grant of the Netherlands Organization for Scientific Research [to M.G.N.], a Vidi Grant of the Netherlands Organization for Scientific Research [to R.v.C] and by grants from the US National Institutes of Health; and the Helmsley Trust [AI 062773, DK 043351 DK 83756 to R. J. X.].
References
- 1.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Sadia S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci, U S A. 2012;109(1091-6490 (Electronic)):17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cel Host Microbe. 2012;12(2):223–32. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–61. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 5.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama W. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci, U S A. 2009;106:1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–60. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(1476-4687 (Electronic)):557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LAB, Jacobs C, et al. Long-Lasting Effects of BCG Vaccination on Both Heterologous Th1/Th17 Responses and Innate Trained Immunity. J Innate Immunol. [published online ahead of print October 30, 2013] (doi:10.1159/000355628) [Google Scholar]
- 9.Flanagan KL, van Crevel R, Curtis N, Shann F, Levy O. Heterologous (“nonspecific”) and sex-differential effects of vaccines: epidemiology, clinical trials, and emerging immunologic mechanisms. Clin Infect Dis. 2013;57(2):283–9. doi: 10.1093/cid/cit209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34(6):251–8. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brill KJ, Li Q, Larkin R, Canaday DH, Kaplan DR, Boom WH, et al. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immun. 2001;69(3):1755–66. doi: 10.1128/IAI.69.3.1755-1765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portevin D, Young D. Natural killer cell cytokine response to M. bovis BCG Is associated with inhibited proliferation, increased apoptosis and ultimate depletion of NKp44(+)CD56(bright) cells. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esin S, Counoupas C, Aulicino A, Brancatisano F, Maisetta G, Bottai D, et al. Interaction of Mycobacterium tuberculosis cell wall components with the human natural killer cell receptors NKp44 and Toll-like receptor 2. Scand J Immunol. 2013;77:460–9. doi: 10.1111/sji.12052. [DOI] [PubMed] [Google Scholar]
- 15.Marcenaro E, Ferranti B, Falco M, Moretta L, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int Immunol. 2008;20(9):1155–67. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- 16.Serreze DV, Gaskins Hr Fau - Leiter EH, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150(0022-1767 (Print)) [PubMed] [Google Scholar]
- 17.Cichocki F, Miller JS, Anderson SK, Bryceson YT. Epigenetic regulation of NK cell differentiation and effector functions. Front Immunol. 2013;4(55) doi: 10.3389/fimmu.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaby P, Benn C. Non-specific and sex-differential effects of routine vaccines: what evidence is needed to take these effects into consideration in low-income countries? Hum Vaccin. 2011;7(1):120–4. doi: 10.4161/hv.7.1.13848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.