Abstract
Importance
High-sensitivity cardiac troponin-T (hs-cTnT) is a biomarker of cardiovascular risk and could be approved in the US for clinical use soon. However, data linking long-term temporal change in hs-cTnT to outcomes are limited, particularly in primary prevention settings.
Objective
To examine the association of 6-year change in hs-cTnT with incident coronary heart disease (CHD), heart failure (HF; further sub-classified into reduced [HFrEF] and preserved ejection-fraction [HFpEF]), and all-cause mortality.
Design
The Atherosclerosis Risk in Communities (ARIC) prospective observational cohort.
Setting
General community US study with biracial representation.
Participants
8,838 participants (mean age 56 years, 59% female, 21% Black), initially free of CHD and HF, who had hs-cTnT measured twice, 6 years apart.
Main Outcome and Measures
Risk factor and temporal hs-cTnT data were collected. Using Cox regression, we examined the association of hs-cTnT change with subsequent CHD, HF, and death over a maximum of 16 years. Improvement in discrimination was determined by Harrell’s C-statistic.
Results
There were 1,157 CHD events, 965 HF events, and 1,813 deaths overall. Incident detectable hs-cTnT (baseline<5ng/L, follow-up≥5ng/L) was independently associated with subsequent CHD (HR 1.4, 95%CI 1.2–1.6), HF (HR 2.0, 95%CI 1.6–2.4) and death (HR 1.5, 95%CI 1.3–1.7), relative to hs-cTnT <5ng/L at both visits. In addition, HRs as high as 4 for CHD and death and 8 for HF were recorded among subjects with the most marked hs-cTnT increases (e.g. baseline<5ng/L, follow-up≥14ng/L). Risk for subsequent outcomes was lower among those with relative hs-cTnT reductions >50% from baseline. Furthermore, information on hs-cTnT change improved discrimination for HF and death when added to a model that included traditional risk-factors, NT-proBNP and baseline hs-cTnT. Among subjects with adjudicated HF hospitalizations, hs-cTnT change appeared to be similarly associated with both HFrEF and HFpEF.
Conclusions and Relevance
Temporal increases in hs-cTnT, suggestive of progressive myocardial damage, are independently associated with incident CHD, death and, above all, with heart failure. Serial determination of hs-cTnT trajectory adds clinically relevant information to baseline testing and may be useful in prognostic assessments and in targeting prevention strategies to high-risk individuals, especially among persons with Stage A or B heart failure.
Keywords: High-sensitivity Troponin, Serial Measurement, Heart Failure, Events
Introduction
Cardiac troponin is critical to the clinical diagnosis of myocardial infarction, particularly among symptomatic persons with chest pain.1 However, with the advent of new high-sensitivity assays, the utility of this biomarker may extend to the prognostic evaluation of adults at risk for coronary heart disease (CHD) or heart failure (HF).2,3
Detectable concentrations of high-sensitivity troponin, which indicate cardiomyocyte cell damage or death, are known to be present in a substantial proportion of asymptomatic adults without any history of cardiovascular disease.4 In this context, prior studies have found that single measurements of high sensitivity cardiac troponin-T (hs-cTnT) are independently associated with a range of adverse outcomes; including CHD, HF, and all-cause mortality.5–9
However, little is known about the implications of temporal change in hs-cTnT. The few studies to date among primary prevention cohorts were conducted exclusively in elderly individuals.10,11 Furthermore, while studies have consistently demonstrated hs-cTnT to be strongly associated with HF5,12–14, much less is known about the extent to which hs-cTnT change is a risk marker for HF with reduced ejection fraction (HFrEF) versus HF with preserved ejection fraction (HFpEF). A biomarker with the capacity to stratify risk for HFpEF could inform the phenotyping, and, ultimately, the prevention, of this vexing clinical syndrome.15
Thus, the goal of this study was to determine, among participants in the Atherosclerosis Risk in Communities (ARIC) Study who were initially free of CHD or HF, the association between 6-year change in serial hs-cTnT values and the following outcomes of interest: incident CHD, incident HF, and death. In addition, among those with an adjudicated HF hospitalization, we conducted secondary analyses evaluating the association between hs-cTnT change and incident HFrEF versus HFpEF.
Methods
Study Population
The ARIC Study is a community-based prospective cohort of 15,792 adult participants sampled from four U.S. communities (Forsyth Country, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland). Details of the study design have been published elsewhere.16 Institutional review boards at each study site reviewed and approved the study. Written informed consent was obtained from all participants. Of the 11,656 persons who had both a first (visit 2, 1990–1992) and a second (visit 4, 1996–1998) hs-cTnT measurement, we excluded those who had CHD or HF at or prior to visit 4 (n=1,308) and those who were missing other variables of interest (n=1,510). Thus, 8,838 persons were included in our main analytic sample (eTable 1).
Measurement of hs-cTnT and other exposure variables
Measurement of hs-cTnT occurred at two time points, 6 years apart. The measurement range of the assay is 3–100,000 ng/L with a limit of blank of 3 ng/L.17 Of note, hs-cTnT values between 3 and 5 ng/L are measurable, but with lower precision than values ≥5 ng/L.5 Thus, 5 ng/L is the limit of detection of this assay and was used as the cut-off for our ‘detectable’ hs-cTnT category. Values ≥14ng/L represent the 90th percentile in the ARIC sample and the 99th percentile value for a “healthy” reference group aged 20–70 years.18
The first hs-cTnT level was measured in stored serum samples from visit 2 at the University of Minnesota in 2012–2013 using a sandwich immunoassay method with a Roche Elecsys 2010 Analyzer (Roche Diagnostics, Indianapolis, Indiana). Intra-assay coefficients of variation (CVs) were 2.1% at a mean hs-cTnT concentration of 26 ng/L and 1.0% at 1990 ng/L. Inter-assay CVs were 6.0% at a mean hs-cTnT concentration of 25 ng/L and 3.7% at 1940 ng/L.
The second hs-cTnT level was measured in stored supernatant plasma samples from visit 4 at Baylor College of Medicine in 2010 using an electrochemiluminescence immunoassay implemented on a Roche Cobas e411 analyzer. Intra-assay coefficients of variation (CVs) were 2.1% at a mean hs-cTnT concentration of 29ng/L and 0.76% at 2378ng/L. Inter-assay CVs were 6.9% and 2.6% at mean cTnT concentrations of 29 ng/L and 2378 ng/L. A formal calibration study evaluating heterogeneity in hs-cTnT across specimen type and laboratory has been conducted and no significant differences were observed.19
Demographic and cardiac risk factor values from ARIC visit 4 (the baseline for outcomes assessment) were used as covariates for the analysis. All measurements were obtained using standardized protocols. Participants self-reported medications, alcohol use, smoking status and race (with options for the latter defined by the investigator). Body mass index (BMI) was calculated from measured weight and height. Blood pressure (BP) was recorded as the mean of 2 seated measurements. Hypertension was defined as systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg, or the use of anti-hypertensive medications. Left ventricular hypertrophy (LVH) was assessed using resting 12-lead electrocardiograms and defined by Cornell criteria.20 Diagnosed diabetes was defined as a self-reported physician diagnosis of diabetes or current use of diabetic medications.
Highly-sensitive C-reactive protein (hs-CRP) was measured in 2010 from stored plasma collected at visit 4 (Siemens Dade Behring BN II). Glomerular filtration rate was estimated (eGFR) using visit 4 serum creatinine and the CKD-EPI 2009 equation.21 N-terminal pro-brain natriuretic peptide (NT-proBNP) was measured in stored serum samples from visit 2 on a Roche Elecsys 2010 analyzer and in stored plasma from visit 4 using a Cobas e411 analyzer.
Follow-up for outcomes of interest
The baseline for events is visit 4 (1996–1998), which is when the second hs-cTnT measurement was obtained. The ascertainment of deaths and classification of CHD and HF events in ARIC have been described previously.22,23 Briefly, hospitalizations were reported annually by study participants or their proxy and also identified through surveillance of hospitals in each ARIC community. CHD events were adjudicated by an ARIC end points committee and were defined as a definite or probable myocardial infarction, death from CHD, or cardiac procedure.22 Heart failure hospitalization cases were identified from diagnosis codes and HF death (as well as all-cause mortality) from hospital discharge records for inpatient deaths and death certificates for deaths occurring outside the hospital.23
Beginning in 2005, the ARIC study conducted retrospective surveillance and adjudication of hospitalized HF events. Hospitalized medical records indicating signs or symptoms of HF were fully abstracted and reviewed.23 While the primary definition of HF hospitalization (defined above) did not change before or after 2005, we were able to determine HF subtype in cases occurring after 2005 using the abstracted ejection fraction, from either inpatient diagnostic tests (91% of these were based on trans-thoracic echocardiograms) or, when absent, preadmission imaging studies within 90 days. HFpEF was classified by normal systolic function (ejection fraction ≥50%), whereas persons admitted with HF and an ejection fraction <50% were considered to have HFrEF.24,25 Of the 965 incident HF hospitalizations overall, a total of 563 occurred after 2005 and were adjudicated. Of these, there were 306 HFrEF cases, 225 HFpEF cases, and 32 HF events with missing ejection fraction values.
Statistical Analyses
Visit 4 characteristics of the participants were compared across categories of 6-year change in hs-cTnT. To create these categories, hs-cTnT concentrations at each time point (visits 2 and 4) were grouped as: “undetectable” (hs-cTnT <5 ng/L), “detectable” (hs-cTnT ≥5 and <14 ng/L), or “elevated” (hs-cTnT ≥14ng/L).6,7 Temporal change across these groups was then categorized using each participant’s baseline and follow-up concentration. We conducted comparisons using: one-way analysis of variance for Gaussian continuous variables, Wilcoxon rank-sum test for non-normally distributed continuous variables, and chi-square test for proportions.
To model the association between absolute 6-year hs-cTnT change and subsequent events occurring after visit 4, we defined the following binary exposure categories; a) “Incident detectable" (hs-cTnT progression from <5 ng/L at visit 2 to ≥5 ng/L at visit 4 six years later); and b) “Incident elevation" (hs-cTnT progression from <14 ng/L at visit 2 to ≥14 ng/L at visit 4). Among all participants who had measurable hs-cTnT above the limit of the blank (≥3 ng/L, N=3,448), we also modeled the association of percent relative change [visit 4 hs-cTnT minus visit 2 hs-cTnT, divided by visit 2 hs-cTnT] with events. For this, we modeled the following exposure categories proposed by deFilippi et al.: relative hs-cTnT change <50%, relative increase ≥50%, and relative decrease ≥50% from the visit 2 level.10,26 We also evaluated 25% relative change cut-points.27
Cumulative incidence of CHD, HF and death among categories of hs-cTnT change were graphed using the Kaplan-Meier method. Multivariable analyses were performed using Cox models with follow-up through December 31, 2011 (median follow-up of ≈14 years, maximum ≈16 years). All models were adjusted for: age (years), race-center, gender, body mass index in kg/m2, C-reactive protein in mg/L, smoking (current; former; never), alcohol intake (current; former; never), systolic BP (in mmHg), hypertension medication use (yes, no), LVH by electrocardiogram (yes, no), diagnosed diabetes (yes, no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes, no), and estimated glomerular filtration rate in mL/min/1.73m2. We verified the proportionality of the hazards visually and with Schoenfeld residuals. We tested for statistical interaction by age, race, or gender. In models evaluating categories of relative change in hs-cTnT as the exposure, we further adjusted for visit 2 hs-cTnT (in addition to conducting sensitivity analyses adjusting instead for visit 4 hs-cTnT). Furthermore, in models evaluating absolute hs-cTNT change categories, sensitivity analyses were conducted with NT-proBNP change values between visits 2 and 4 (in pg/mL) added to the full model.
Among persons with measurable hs-cTnT at both visits (≥3 ng/L), we also graphed the hazard ratio (HR) for events using restricted cubic splines that were centered at the median of hs-cTnT change and truncated at the 1st and 99th percentiles. These spline models were adjusted using the variables in the main model described above (with the further addition of ba seline visit 2 hs-cTnT). Model discrimination was assessed using the Harrell C-statistic,28 and we estimated improvement in the C-statistic and net reclassification improvement (NRI29) for the addition of, first, visit 4 hs-cTnT, and, second, visit 2 hs-cTnT, to adjusted models (note that persons with hs-cTnT <3ng/L had values imputed as 1.5 ng/L in discrimination and NRI models).
Finally, our secondary analysis consisted of both Cox models and restricted cubic splines evaluating the adjusted association between categories of hs-cTnT change and either incident HFpEF or HFrEF. Given HF adjudication commenced in 2005, which was after our baseline of visit 4 (1996–1998), subjects at risk for either HF subtype were only under observation for these outcomes commencing 2005 and, thus, were treated as late entries for this analysis (i.e. follow-up time was left truncated at 2005). All analyses were conducted with Stata 13 (StataCorp). The nominal level of significance was defined as p<0.05 (2-sided).
Results
Differences in demographics and cardiac risk factors, according to 6-year temporal change in hs-cTnT, are shown in Table 1. In the sample overall, the mean age at visit 2 was 56 and at visit 4 was 62 years, 59% were female and 21% were black. In general, persons who had hs-cTnT <5 ng/L at both visits were younger, more likely to be female or white, and more likely to have a lower burden of cardiac risk factors.
Table 1.
Characteristics of the Study Population (ARIC participants without coronary heart disease or heart failure) at Visit 4: Overall and According to categories of 6-year change in high sensitivity cardiac troponin T (ng/L) between Visit 2 (1990–1992) and Visit 4 (1996–1998), n=8838
| Overall | According to categories of 6 year hs-cTNT change | p-value† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 2 hs-cTNT Level | <5 ng/L | 5–13 ng/L | ≥14 ng/L | ||||||
| Visit 4 follow-up hs-cTnT | <5 ng/L |
≥5 ng/L |
<5 ng/L |
5–13 ng/L |
≥14 ng/L |
<14 ng/L |
≥14 ng/L |
||
| Number (%) | 8838 | 3888 (44.0%) |
2152 (24.3%) |
574 (6.5%) |
1624 (18.4%) |
360 (4.1%) |
65 (0.7%) |
175 (2.0%) |
|
| Baseline Age at visit 2 | 56.5 (5.6) |
54.9 (5.1) |
57.1 (5.6) |
56.5 (5.5) |
58.7 (5.6) |
59.3 (5.8) |
58.0 (5.4) |
59.8 (5.4) |
<0.001 |
| Age at visit 4 (years) | 62.5 (5.6) |
60.9 (5.1) |
63.1 (5.6) |
62.5 (5.5) |
64.7 (5.6) |
65.3 (5.8) |
64.0 (5.4) |
65.8 (5.4) |
<0.001 |
| Female, % | 59 | 76.6 | 52.7 | 62.9 | 36.8 | 21.9 | 36.9 | 23.4 | <0.001 |
| Black, % | 21.4 | 20 | 19.4 | 24.2 | 23.2 | 25.6 | 23.1 | 40.6 | <0.001 |
| Smoking status | <0.001 | ||||||||
| Current, % | 14.6 | 19.2 | 11.6 | 14.1 | 8.9 | 10 | 12.3 | 13.1 | |
| Former, % | 41.9 | 37.6 | 44.5 | 36.6 | 47.5 | 50 | 50.8 | 49.1 | |
| Never, % | 43.6 | 43.2 | 44 | 49.3 | 43.5 | 40 | 36.9 | 37.7 | |
| Alcohol Intake status | <0.001 | ||||||||
| Current, % | 51 | 53.8 | 50.6 | 47.6 | 49.3 | 45.3 | 47.7 | 35.4 | |
| Former, % | 28.3 | 26 | 29.7 | 28.2 | 28.4 | 35.6 | 36.9 | 45.1 | |
| Never, % | 20.6 | 20.2 | 19.7 | 24.2 | 22.3 | 19.2 | 15.4 | 19.4 | |
| Systolic BP, mm Hg | 126.8 (18.7) |
124.1 (17.7) |
127.2 (18.5) |
126.9 (17.8) |
131.0 (20.0) |
131.5 (20.7) |
127.1 (19.1) |
132.9 (18.3) |
<0.001 |
| Antihypertensive Medication, % |
38.2 | 31.8 | 38.4 | 39.9 | 45 | 58.3 | 43.1 | 65.7 | <0.001 |
| Hypertension, % | 44.2 | 37.2 | 45.2 | 43.2 | 52.3 | 62.8 | 53.8 | 66.3 | <0.001 |
| LVH, % | 2.2 | 1.3 | 2.2 | 2.6 | 3 | 5 | 3.1 | 4 | <0.001 |
| BMI, % | <0.001 | ||||||||
| Normal weight (<25 kg/m2) | 51 | 53.8 | 50.6 | 47.6 | 49.3 | 45.3 | 47.7 | 35.4 | |
| Overweight (25–30 kg/m2) | 28.3 | 26 | 29.7 | 28.2 | 28.4 | 35.6 | 36.9 | 45.1 | |
| Obese (>30 kg/m2) | 20.6 | 20.2 | 19.7 | 24.2 | 22.3 | 19.2 | 15.4 | 19.4 | |
| LDL-cholesterol, mg/dL | 123.3 (33.2) |
123.9 (33.6) |
121.8 (32.9) |
125.2 (32.1) |
124.2 (33.2) |
117.8 (33.2) |
128.6 (35.3) |
120.9 (30.2) |
0.009 |
| HDL-cholesterol, mg/dL | 51.0 (16.6) |
53.7 (16.5) |
50.3 (17.2) |
51.2 (16.1) |
47.4 (15.3) |
44.8 (14.6) |
49.4 (18.2) |
45.6 (14.6) |
<0.001 |
| Triglyceride, mg/dL * | 120.0 (82.0) |
121.0 (80.5) |
120.0 (83.0) |
122.0 (76.0) |
119.0 (84.0) |
122.0 (90.5) |
124.0 (84.0) |
117.0 (96.0) |
0.867 |
| Lipid Medicines % | 2.3 | 2.1 | 2.6 | 3 | 1.8 | 3.9 | 3.1 | 1.1 | 0.143 |
| Diagnosed diabetes, % | 10.5 | 6.7 | 10.7 | 8.5 | 13.8 | 26.1 | 18.5 | 30.9 | <0.001 |
| eGFR, mL/min/1.73 m2 | 86.8 (15.4) |
89.6 (14.1) |
86.0 (15.2) |
87.6 (14.4) |
83.3 (15.3) |
79.0 (18.7) |
86.4 (15.4) |
77.8 (22.6) |
<0.001 |
| hs-CRP, mg/L * | 2.3 (4.2) |
2.5 (4.4) |
2.2 (3.9) |
2.4 (3.9) |
1.9 (3.9) |
2.6 (4.9) |
1.9 (3.7) |
2.4 (4.8) |
<0.001 |
| 6-year change in NT-proBNP, pg/mL |
+58.4 (2001.6) |
+19.0 (79.0) |
+48.3 (183.0) |
+23.2 (112.7) |
+40.0 (158.4) |
+124.2 (336.0) |
+13.8 (153.1) |
+1224.0 (14160.0) |
<0.001 |
Estimates are mean (SD) or %, unless otherwise indicated. Hs-cTNT= high-sensitivity Troponin-T, BP= Blood Pressure, LVH= left ventricular hypertrophy, BMI= body mass index, LDL= Low Density Lipoprotein, HDL= High Density Lipoprotein, eGFR= estimated Glomerular Filtration Rate, hs-CRP= highly sensitive C-Reactive Protein, NT-proBNP= N-terminus pro-Brain Natriuretic Protein. Hypertension was defined as being on anti-hypertensive medication, a systolic BP ≥140mmHg, or a diastolic BP ≥90mmHg. Diagnosed diabetes was defined by medication use or a self-reported physician diagnosis.
Median (IQR);
P values are for overall differences by one-way analysis of variance for Gaussian continuous variables, Wilcoxon rank-sum test for non-normally distributed continuous variables, and chi-square test for proportions
Over a median of 14 years of follow-up, there were 1157 CHD events, 965 HF hospitalizations, and 1813 deaths overall. Crude incidence rates (IRs, per 1,000 person years) for all three events were higher among persons with 6-year temporal increases in hs-cTnT (Table 2). Crude IRs were lower among those with a greater than 50% decrease in follow-up hs-cTnT. Kaplan-Meier cumulative survival curves were consistent with these IR findings; both by binary categories of absolute temporal change and by categories of percent relative temporal change (eFigure 1).
Table 2.
Crude incidence rates (per 1,000 person years) and adjusted* hazard ratios (95% confidence intervals) for incident coronary heart disease, heart failure hospitalization, or death; according to categories of 6-year change in high sensitivity cardiac troponin T (hs-cTNT), N=8838
| Outcomes | Absolute hs-cTNT change among all participants† | Relative change among persons with measurable hs-cTNT values‡ |
|||||
|---|---|---|---|---|---|---|---|
| Incident Detectable (Baseline <5ng/L, Follow-up ≥5ng/L) |
Incident Elevated (Baseline <14ng/L, Follow-up ≥14ng/L) |
>50% Decrease |
Change ≤50% |
>50% Increase |
|||
| No (N=3888) |
Yes (N=2152) |
No (N=8155) |
Yes (N=443) |
(N=74) | (N=2227) | (N=1147) | |
| Heart Failure | n=235 | n=260 | n=761 | n=134 | n=10 | n=309 | n=234 |
| Incidence Rate | 4.5 | 9.7 | 7.2 | 30.5 | 10.7 | 11.1 | 18.0 |
| Demographic Model* |
1 (ref) | 2.05 (1.70–2.47) |
1 (ref) | 3.88 (3.20–4.71) |
1.06 (0.57–2.00) |
1 (ref) | 1.64 (1.39–1.95) |
| Full Model* | 1 (ref) | 1.96 (1.62–2.37) |
1 (ref) | 2.78 (2.27–3.40) |
0.49 (0.23–1.01) |
1 (ref) | 1.60 (1.35–1.91) |
|
Coronary Heart Disease |
n=330 | n=301 | n=969 | n=119 | n=9 | n=374 | n=239 |
| Incidence Rate | 6.4 | 11.6 | 9.4 | 28.3 | 10.0 | 13.9 | 19.1 |
| Demographic Model* |
1 (ref) | 1.36 (1.15–1.61) |
1 (ref) | 2.09 (1.72–2.55) |
0.78 (0.40–1.52) |
1 (ref) | 1.31 (1.11–1.54) |
| Full Model* | 1 (ref) | 1.38 (1.16–1.63) |
1 (ref) | 1.75 (1.43–2.15) |
0.47 (0.22–1.03) |
1 (ref) | 1.28 (1.09–1.52) |
| Death | n=508 | n=486 | n=1496 | n=209 | n=17 | n=584 | n=392 |
| Incidence Rate | 9.5 | 17.5 | 13.7 | 42.8 | 17.7 | 20.1 | 28.2 |
| Demographic Model* |
1 (ref) | 1.45 (1.27–1.66) |
1 (ref) | 2.41 (2.08–2.80) |
1.00 (0.62–1.62) |
1 (ref) | 1.39 (1.22–1.58) |
| Full Model* | 1 (ref) | 1.50 (1.31–1.72) |
1 (ref) | 2.10 (1.80–2.46) |
0.57 (0.33–0.99) |
1 (ref) | 1.39 (1.22–1.59) |
Demographic Model- Adjusted for age, sex, race-center; Full Model- Adjusted for age, sex, race-center, body mass index (kg/m3), C-reactive protein (mg/L), smoking (current; former; never), drinking (current; former; never), systolic blood pressure (mmHg), current use of blood pressure-lowering medication (yes or no), diagnosed diabetes (yes or no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), and current use of cholesterol-lowering medication (yes or no), estimated GFR (mL/min/1.73m2), and left ventricular hypertrophy (yes or no, by Cornell criteria).
Absolute change categories are not mutually exclusive
Relative change sample (N=3448) only includes those who have measurable hs-cTnT values (≥3 ng/L) at both visits. Relative change is calculated as a percent of baseline hs-cTNT. The full model for this relative change exposure is further adjusted for visit 2 hs-cTNT.
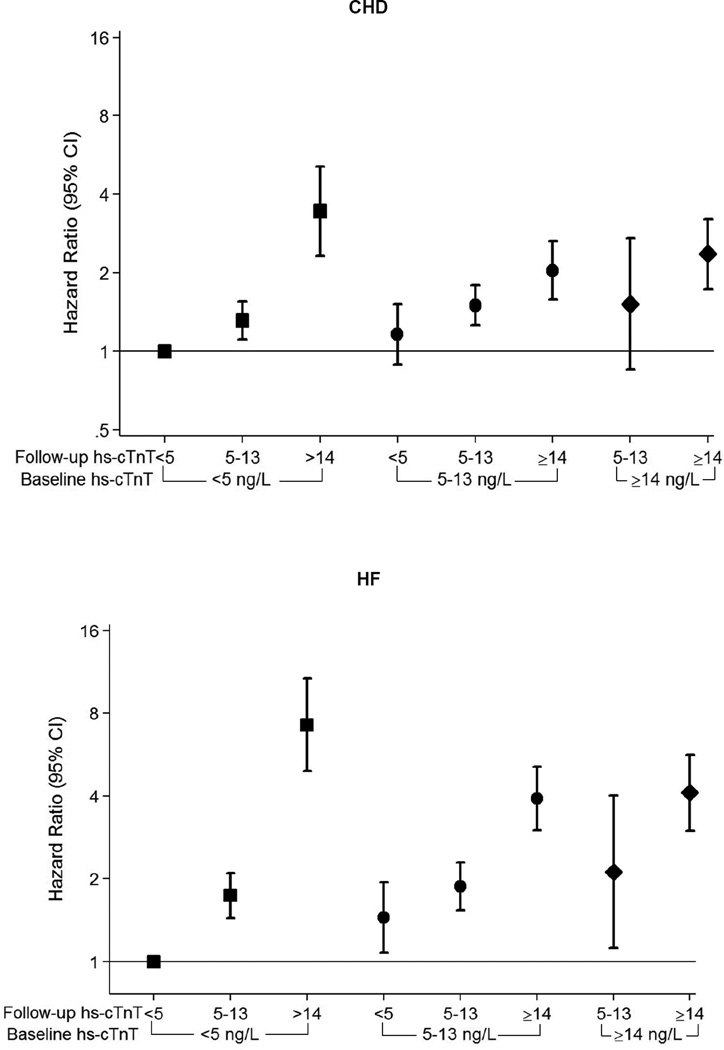
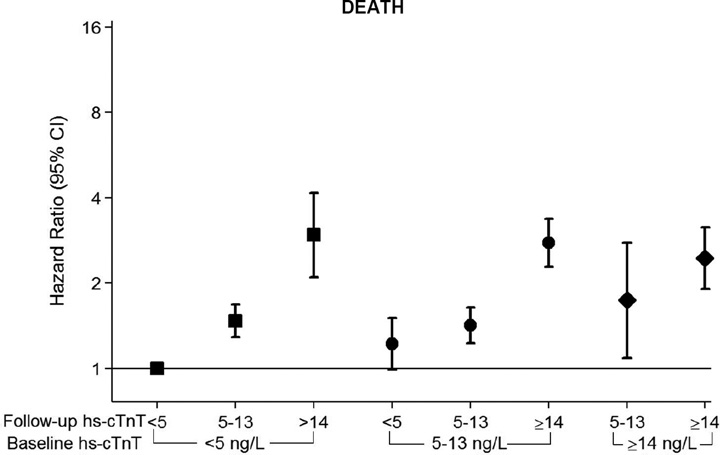
After full adjustment in our Cox proportional hazards models, increasing hs-cTnT remained a robust risk factor for all three events (Table 2). Of note, the association between categories of absolute 6-year hs-cTnT change (e.g. incident detectable and incident elevated) and adverse events was strongest for HF, with more modest associations for CHD and death. Indeed, among those with the most marked increases in hs-cTnT over 6 years, we found HRs as high as 8 for HF and 4 for both CHD and death (Figure 1, eFigure 2). There was no interaction based on age, race, or gender. Findings were quantitatively and qualitatively similar using sex-specific cut-offs for incident elevated hs-cTnT (eTable 2) and also with further adjustment for absolute NT-proBNP change between visits 2 and 4 (eTable 3). The associations depicted in our restricted cubic splines were qualitatively similar after stratification by visit 2 hs-cTnT concentration (eFigure 3).
Figure 1. Relative Risk for CHD, Heart Failure, or Death; According to 6 year change in high-sensitivity Troponin-T.
Adjusted* hazard ratios (95% confidence intervals) for incident coronary heart disease (CHD), heart failure hospitalization (HF), or death were incrementally higher according to categories of increasing 6-year change in high sensitivity cardiac troponin T (hs-cTNT) between ARIC Visit 2 (1990–1992) and Visit 4 (1996–1998)†
*Adjusted for age, sex, race-center, body mass index (kg/m3), C-reactive protein (mg/L), smoking (current; former; never), drinking (current; former; never), systolic blood pressure (mmHg), current use of blood pressure-lowering medication (yes or no), diagnosed diabetes (yes or no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), and current use of cholesterol-lowering medication (yes or no), estimated GFR (mL/min/1.73m2), and left ventricular hypertrophy (yes or no, by Cornell criteria).
† Note that the visit 2 <5ng/L and visit 4 <5ng/L category is the reference group throughout. Further, the visit 2 ≥14ng/L and visit 4 <5 ng/L category has been removed due to low numbers (6 participants).
Square Markers= Baseline hs-cTnT <5 ng/L, Round Markers= Baseline hs-cTnT 5–13 ng/L, Diamond Markers= Baseline hs-cTnT ≥14 ng/L
Analyses modeling the exposure of percent relative 6-year hs-cTnT change (which were further adjusted for visit 2 hs-cTnT) were consistent with the results for hs-cTnT change modeled in absolute terms, demonstrating that those individuals with a >50% increase in hs-cTnT had HRs (95% CIs) of 1.28 (1.09–1.52), 1.60 (1.35–1.91), and 1.39 (1.22–1.59); for CHD, HF and death respectively. Furthermore, those with a >50% decrease in hs-cTnT had generally lower risk for all 3 events (Table 2). Results were similar, but attenuated, both in sensitivity analyses modeling relative change after adjustment for visit 4 hs-cTnT (instead of visit 2 levels) and also in models using a 25% relative change cut-point27 (eTables 3 and 4).
The addition of both visit 4 hs-cTnT (testing the impact of baseline values) and visit 2 hs-cTnT (testing the impact of temporal change) significantly improved discrimination for both HF and death when added to fully adjusted base models that included NT-proBNP (Table 3). In contrast, while the most recent visit 4 hs-cTnT value improved discrimination for CHD events, information on hs-cTnT change did not add further prognostic value for CHD. Furthermore, the addition of both hs-cTnT values from visits 2 and 4 improved the continuous NRI for all three events, whereas the baseline visit 4 level alone did not (eTable 5).
Table 3.
C Statistics and differences in C Statistics for clinical events (Coronary Heart Disease, Heart Failure Hospitalization, and All-Cause Mortality) from models with and without both baseline hs-cTnT and hs-cTNT change in the Overall Population, N=8838
| C-statistic | Coronary heart disease | Heart failure | All-cause mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C- statistic |
Difference | p- value |
C- statistic |
Difference | p- value |
C- statistic |
Difference | p- value |
|
| Model 1* | 0.7217 | - | - | 0.7631 | - | - | 0.7256 | - | - |
| Model 1+ visit 4 hs-cTNT† (Model 2) |
0.7263 | 0.0046 | <0.001 | 0.7716 | 0.0085 | <0.001 | 0.7295 | 0.0039 | <0.001 |
| Model 2 +visit 2 hs-cTNT† (Model 3) |
0.7268 | 0.0004 | 0.104 | 0.7730 | 0.0014 | 0.016 | 0.7301 | 0.0007 | 0.043 |
Model 1 (fully adjusted base model) is adjusted for age, sex, race-center, body mass index (kg/m3), C-reactive protein (mg/L), smoking (current; former; never), drinking (current; former; never), systolic blood pressure (mmHg), current use of blood pressure-lowering medication (yes or no), diagnosed diabetes (yes or no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of cholesterol-lowering medication (yes or no), estimated GFR (mL/min/1.73m2), left ventricular hypertrophy (yes or no, by Cornell criteria), and change in NT-proBNP between visits 2 and 4 (pg/mL).
For this analysis persons with hs-cTNT values <3ng/L were imputed with a value of 1.5ng/L
In secondary analysis evaluating the association between 6-year hs-cTnT change and subsequent HF hospitalization subtype (occurring after 2005), we found that increasing hs-cTnT appears to be associated with both HFrEF and HFpEF outcomes (Table 4). These associations were grossly unchanged after further adjusting for NT-proBNP (eTable 6). The restricted cubic splines, modeling mean absolute hs-cTnT change as a continuous variable (per ng/L), were consistent with findings from the categorical analyses, suggesting increased risk for both HFrEF and HFpEF, particularly among those with absolute 6-year increases ranging from 1 to 10 ng/L (eFigure 4).
Table 4.
Crude incidence rates (per 1,000 person years) and adjusted* hazard ratios (95% confidence intervals) for heart failure subtypes (HFrEF and HFpEF) adjudicated after 2005; according to categories of 6-year change in high sensitivity cardiac troponin T (hs-cTNT)
| Heart Failure Subtypes |
Absolute hs-cTNT change among all participants | Relative change among persons with measurable hs-cTNT values† |
|||||
|---|---|---|---|---|---|---|---|
| Incident Detectable (Baseline <5ng/L, Follow-up ≥5ng/L) |
Incident Elevated (Baseline <14ng/L, Follow-up ≥14ng/L) |
>50% Decrease |
Change ≤50% |
>50% Increase |
|||
| No (n=3599) |
Yes (n=1867) |
No (n=7306) |
Yes (n=286) |
(n=71) | (n=2038) | (n=996) | |
| HFrEF | n=88 | n=72 | n=256 | n=30 | n=5 | n=121 | n=77 |
| Incidence Rate |
3.72 (3.02–4.58) |
6.10 (4.84–7.69) |
5.45 (4.82–6.15) |
18.77 (13.1–26.8) |
11.61 (4.83–27.90) |
9.57 (8.01–11.44) |
13.15 (10.5– 16.4) |
| Demographic Model* |
1 (ref) | 1.51 (1.09–2.10) |
1 (ref) | 2.77 (1.88–4.10) |
1.44 (0.59–3.52) |
1 (ref) | 1.31 (0.99–1.75) |
| Full Model* | 1 (ref) | 1.54 (1.10–2.16) |
1 (ref) | 2.43 (1.62–3.65) |
0.85 (0.31–2.31) |
1 (ref) | 1.26 (0.94–1.69) |
| HFpEF | n=69 | n=67 | n=199 | n=17 | n=3 | n=76 | n=46 |
| Incidence Rate |
2.91 (2.30–3.69) |
5.68 (4.47–7.22) |
4.23 (3.68–4.86) |
10.63 (6.61–17.11) |
6.97 (2.25–21.60) |
6.01 (4.80–7.53) |
7.86 (5.89–10.49) |
| Demographic Model* |
1 (ref) | 1.87 (1.31–2.66) |
1 (ref) | 2.58 (1.55–4.31) |
1.18 (0.37–3.74) |
1 (ref) | 1.31 (0.91–1.90) |
| Full Model* | 1 (ref) | 1.72 (1.20–2.47) |
1 (ref) | 1.80 (1.06–3.05) |
1.06 (0.32–3.51) |
1 (ref) | 1.16 (0.80–1.69) |
Demographic Model- Adjusted for age, sex, race-center; Full Model- Adjusted for age, sex, race-center, body mass index (kg/m3), C-reactive protein (mg/L), smoking (current; former; never), drinking (current; former; never), systolic blood pressure (mmHg), current use of blood pressure-lowering medication (yes or no), diagnosed diabetes (yes or no), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), and current use of cholesterol-lowering medication (yes or no), estimated GFR (mL/min/1.73m2), and left ventricular hypertrophy (yes or no, by Cornell criteria).
Sample (N=3448) includes only those who have measurable hs-cTnT values (≥3 ng/L) at both visits. Relative change is calculated as a percent of baseline hs-cTNT. The full model for this relative change exposure is further adjusted for visit 2 hs-cTNT.
Discussion
Temporal increases in hs-cTnT were independently associated with incident CHD, death, and, above all, HF events in this prospective biracial sample of middle-aged adults. In persons with decreasing hs-cTnT levels (e.g. 6-year reductions >50% from baseline), there was also evidence suggesting lower risk for outcomes compared to persons with stable or increasing concentrations.
To our knowledge, only two prior studies have examined the association between temporal change in hs-cTnT and subsequent events in primary prevention settings. The Cardiovascular Health Study (CHS) demonstrated that percent relative change in hs-cTnT is an independent risk factor for HF and death among elderly ambulatory adults.10,30 Despite some important differences (e.g. CHS evaluated the impact of 2–3 year change and was conducted in an older sample), our results from ARIC were generally consistent with those from CHS. Our results are also consistent with mortality data from asymptomatic elderly persons enrolled in the Prospective Investigation of the Vasculature in Uppsala Seniors study11, as well as findings from higher-risk persons with established cardiovascular disease.13,27,31
As such, our results extend prior research to include asymptomatic middle-aged adults and also demonstrate, for the first time, an association between temporal hs-cTnT change and incident CHD events. In addition, while our analysis is not designed to derive a risk prediction equation that incorporates hs-cTnT change, our discrimination and NRI results highlight the additive prognostic value of repeat hs-cTnT testing-which captures the trajectory of myocardial damage-over and above one-time measurements. Furthermore, subjects with the most marked increases in hs-cTnT were at highest risk for all three events, suggesting that accelerated progression of myocardial damage represents an extreme risk phenotype.
The association between hs-cTnT change and subsequent HF was most striking, raising the question of the impact of such change on HF subtypes. We found that, among those with adjudicated HF hospitalizations, hs-cTnT increases appear to be associated with both HFrEF and HFpEF. A prior analysis from CHS reported that serial increases in hs-cTnT over 2–3 years were associated with HFrEF, but not with HFpEF. However, a major limitation of this CHS analysis was that a significant proportion (43%) of HF hospitalizations did not have information on ejection fraction and were excluded.32 This contrasts with just 6% of our adjudicated HF cases being excluded due to lack of ejection fraction data.
Our results have a number of clinical implications. Together with the wealth of data supporting once-off baseline hs-cTnT measurements5,6, we demonstrate improved prognostic performance for serial measurement of hs-cTnT and add to a compelling argument that serial hs-cTnT monitoring, either alone or with other biomarkers, may identify high risk individuals and guide the prevention of either CHD or HF. Clinical trials are necessary to confirm this hypothesis. Our data also suggest that serial hs-cTnT monitoring could personalize the approach to HF prevention by stratifying risk for both HFrEF and HFpEF hospitalization in persons with stage A or B HF. Importantly, our results were independent of NT-proBNP, suggesting that hs-cTnT monitoring could add to an NT-proBNP based HF prevention strategy.33
While there are a number of established biomarkers of risk for HFrEF events, fewer biomarkers have been shown to increase risk for HFpEF events.34 Our results suggest that serial hs-cTnT monitoring, likely added to NT-proBNP, could allow future HFpEF trials to be enriched with individuals at high risk. Another potential clinical and research application of serial hs-cTNT measurement may be as a modifiable surrogate marker of treatment benefit on the myocardium. Indeed, significant reductions in hs-cTnT have been shown for sacubitril/valsartan relative to placebo in established HF.35
Our study has some limitations. These data are observational in nature and despite rigorous adjustment for confounding, residual confounding may be present. HF hospitalization was determined by diagnosis codes, which may misclassify some cases, and our outcome may not capture the impact of hs-cTnT change on outpatient HF. Furthermore, HF adjudication only began in 2005. As such, it is possible that HF events occurring after visit 4, but prior to 2005, may have differed in their HF subtype distribution with respect to the hs-cTnT change exposure. Because we only have hs-cTnT data from 2 time-points, 6 years apart, this analysis cannot define the optimal interval or number of serial hs-cTnT measurements necessary for assessing risk. While high-sensitivity Troponin assays are commercially available for clinical use in many countries, they have yet to be approved in the U.S. by the Food and Drug Administration.
In conclusion, our results demonstrate that two measurements of hs-cTnT appear to be better than one for characterizing risk, and that large increases in hs-cTnT are particularly deleterious. Temporal change in hs-cTnT may help guide the preventive management of asymptomatic persons at risk for CHD as well as adults with stage A or B heart failure (both HFrEF and HFpEF subtypes).
Supplementary Material
Acknowledgments
Dr Ballantyne has received grant support from Roche Diagnostics (and the National Institutes of Health). Drs. Ballantyne and Nambi are co-investigators on a provisional patent filed by Roche for use of biomarkers in heart failure prediction. Drs. Ballantyne and Selvin have served on an advisory board for Roche Diagnostics. Reagents for the high-sensitivity cardiac troponin-T and C-reactive protein assays were donated by Roche Diagnostics.
Funding: This research was supported by NIH/NIDDK grants R01DK089174 and K24DK106414 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr McEvoy is supported by the PJ Schafer fund for early career investigators and the Magic That Matters Fund at Johns Hopkins.
Role of the Funder/Sponsor: The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases funded the collection of data used in the study but had no role in the design, management, or conduct of the analysis, interpretation of the results, or the writing of the manuscript.
Additional Contributions: The authors thank the staff and participants of the ARIC study for their important contributions. The authors also thank Dr. Michael Steffes from the University of Minnesota Medical School for his significant contributions to this paper.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities Study
- CHD
Coronary Heart Disease
- eGFR
estimated Glomerular Filtration Rate
- HDLc
High Density Lipoprotein cholesterol
- HF
Heart Failure
- HFpEF
Heart Failure with Preserved Ejection Fraction
- HFrEF
Heart Failure with Reduced Ejection Fraction
- Hs-CRP
High sensitivity C-Reactive Protein
- Hs-cTnT
High sensitivity cardiac Troponin-T
- LVH
Left Ventricular Hypertrophy
- LDLc
Low Density Lipoprotein cholesterol
- NRI
Net Reclassification Improvement
- NT-proBNP
N-terminus pro Brain Natriuretic Peptide
Footnotes
Supplementary information is provided in an Online Appendix.
Drs McEvoy and Selvin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. McEvoy and Miss Chen performed the statistical analyses.
Conflict of Interest Disclosures: The other authors declare no commercial conflicts of interest (but receive National Institutes of Health grant funding).
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012 Oct 16;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 2.Apple FS. High-sensitivity cardiac troponin for screening large populations of healthy people: is there risk? Clinical chemistry. 2011 Apr;57(4):537–539. doi: 10.1373/clinchem.2011.162875. [DOI] [PubMed] [Google Scholar]
- 3.Giannitsis E, Katus HA. Highly sensitive troponins knocking at the door of primary prevention. European heart journal. 2014 Feb;35(5):268–270. doi: 10.1093/eurheartj/eht479. [DOI] [PubMed] [Google Scholar]
- 4.Sherwood MW, Kristin Newby L. High-sensitivity troponin assays: evidence, indications, and reasonable use. Journal of the American Heart Association. 2014 Feb;3(1):e000403. doi: 10.1161/JAHA.113.000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011 Apr 5;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA : the journal of the American Medical Association. 2010 Dec 8;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggers KM, Al-Shakarchi J, Berglund L, et al. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. American heart journal. 2013 Sep;166(3):541–548. doi: 10.1016/j.ahj.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM Investigators AS. Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Annals of epidemiology. 2013 Feb;23(2):66–73. doi: 10.1016/j.annepidem.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012 Sep 25;126(13):1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA : the journal of the American Medical Association. 2010 Dec 8;304(22):2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high-sensitive assay increase over time and are strong predictors of mortality in an elderly population. Journal of the American College of Cardiology. 2013 May 7;61(18):1906–1913. doi: 10.1016/j.jacc.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Selvin E, Lazo M, Chen Y, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014 Oct 14;130(16):1374–1382. doi: 10.1161/CIRCULATIONAHA.114.010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012 Jan 17;125(2):280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 14.Omland T, Rosjo H, Giannitsis E, Agewall S. Troponins in heart failure. Clinica chimica acta; international journal of clinical chemistry. 2015 Mar 30;443:78–84. doi: 10.1016/j.cca.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. European heart journal. 2011 Mar;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 17.Agarwal SK, Avery CL, Ballantyne CM, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clinical chemistry. 2011 Jun;57(6):891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clinical chemistry. 2010 Feb;56(2):254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 19.Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clinical chemistry. 2015 Jul;61(7):938–947. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987 Mar;75(3):565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern. Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. Journal of clinical epidemiology. 1996 Feb;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circulation. Heart failure. 2012 Mar 1;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heart Failure Society of, A. Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010 Jun;16(6):e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JP, Mentz RJ, Mebazaa A, et al. Patient Selection in Heart Failure With Preserved Ejection Fraction Clinical Trials. Journal of the American College of Cardiology. 2015 Apr 28;65(16):1668–1682. doi: 10.1016/j.jacc.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu AH, Lu QA, Todd J, Moecks J, Wians F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clinical chemistry. 2009 Jan;55(1):52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 27.Everett BM, Brooks MM, Vlachos HE, et al. Troponin and Cardiac Events in Stable Ischemic Heart Disease and Diabetes. The New England journal of medicine. 2015 Aug 13;373(7):610–620. doi: 10.1056/NEJMoa1415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996 Feb 28;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011 Jan 15;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glick D, DeFilippi CR, Christenson R, Gottdiener JS, Seliger SL. Long-term trajectory of two unique cardiac biomarkers and subsequent left ventricular structural pathology and risk of incident heart failure in community-dwelling older adults at low baseline risk. JACC. Heart failure. 2013 Aug;1(4):353–360. doi: 10.1016/j.jchf.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White HD, Tonkin A, Simes J, et al. Association of contemporary sensitive troponin I levels at baseline and change at 1 year with long-term coronary events following myocardial infarction or unstable angina: results from the LIPID Study (Long-Term Intervention With Pravastatin in Ischaemic Disease) Journal of the American College of Cardiology. 2014 Feb 4;63(4):345–354. doi: 10.1016/j.jacc.2013.08.1643. [DOI] [PubMed] [Google Scholar]
- 32.Seliger SL, de Lemos J, Neeland IJ, et al. Older Adults, "Malignant" Left Ventricular Hypertrophy, and Associated Cardiac-Specific Biomarker Phenotypes to Identify the Differential Risk of New-Onset Reduced Versus Preserved Ejection Fraction Heart Failure: CHS (Cardiovascular Health Study) JACC. Heart failure. 2015 Apr 28; doi: 10.1016/j.jchf.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA : the journal of the American Medical Association. 2013 Jul 3;310(1):66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 34.O'Meara E, de Denus S, Rouleau JL, Desai A. Circulating biomarkers in patients with heart failure and preserved ejection fraction. Current heart failure reports. 2013 Dec;10(4):350–358. doi: 10.1007/s11897-013-0160-x. [DOI] [PubMed] [Google Scholar]
- 35.Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015 Jan 6;131(1):54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.