Abstract
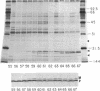
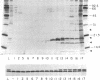
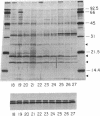
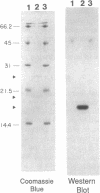
Partially purified yeast microsomal signal peptidase appears to be a complex of four polypeptides of 13, 18, 20, and 25 kDa. The 18-kDa chain is the product of the Sec11 gene, which is necessary for signal peptidase activity. The 25-kDa subunit is a glycoprotein that binds Con A. Two related methods for purification of the enzyme are presented; the first includes removal of peripheral membrane proteins from microsomes by alkali extraction, solubilization of the enzyme by nonionic detergent and high salt, and four different chromatographic procedures. An alternative method was developed based on lectin-affinity chromatography.
Full text
PDF


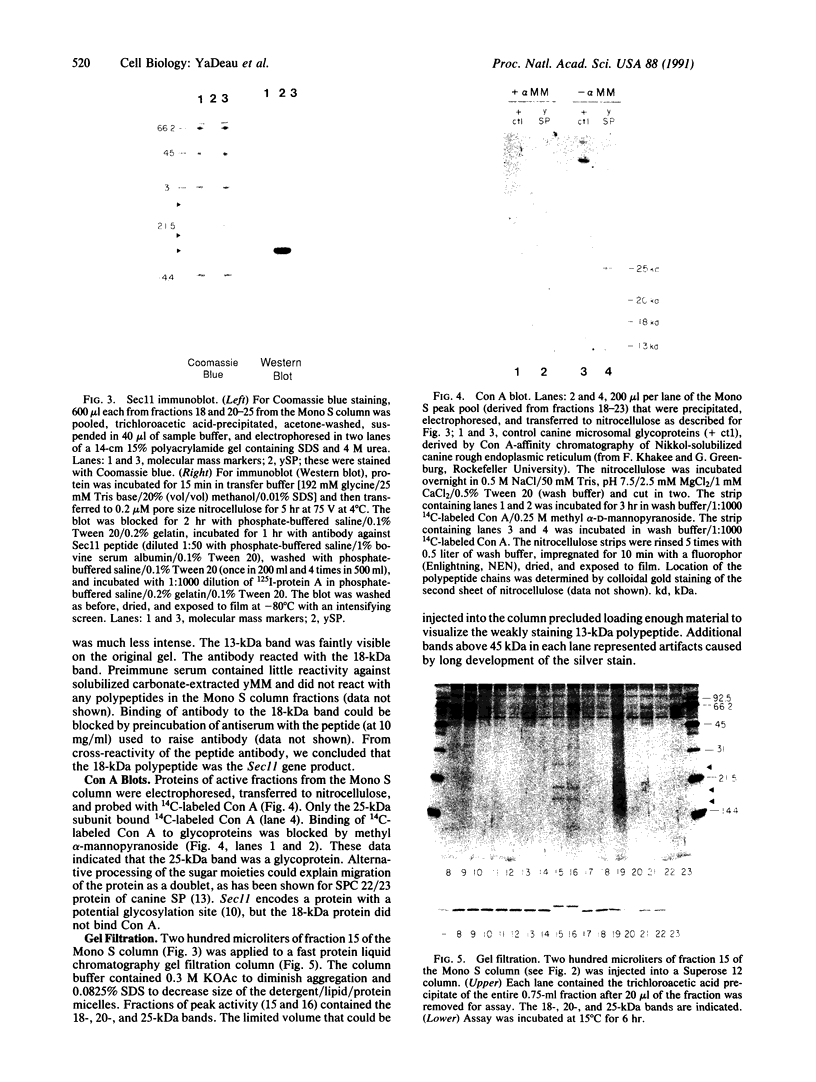
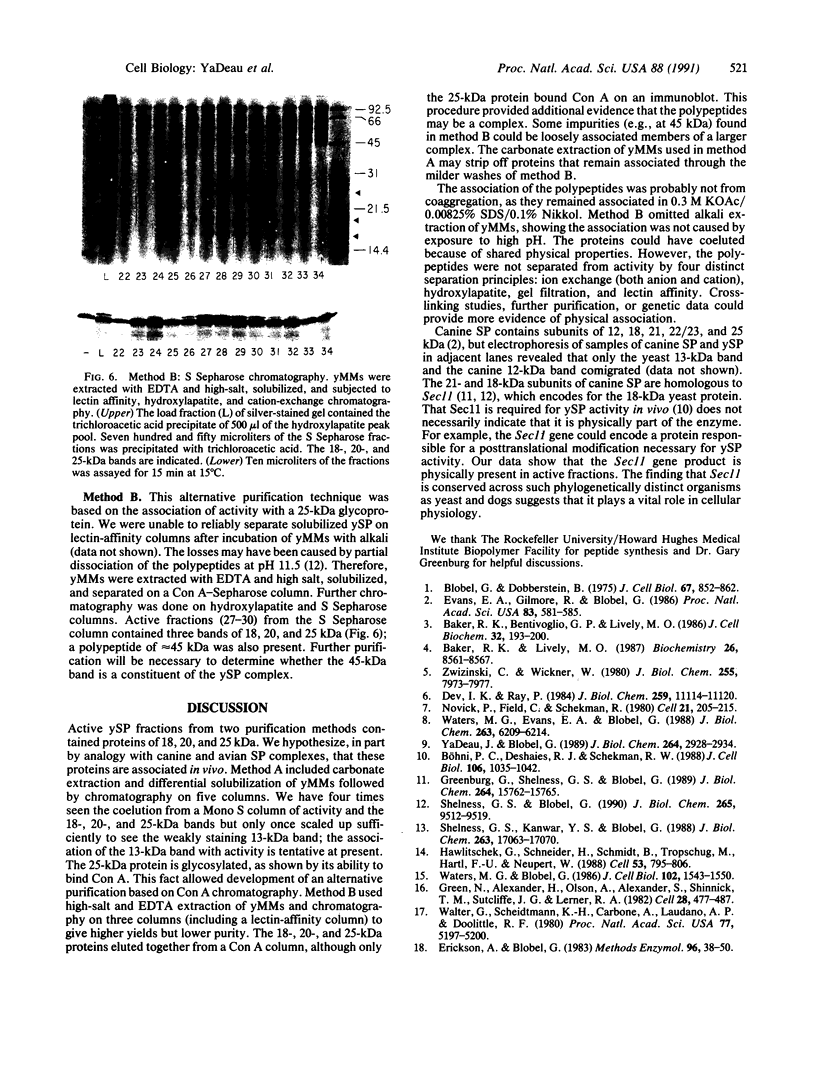
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. K., Bentivoglio G. P., Lively M. O. Partial purification of microsomal signal peptidase from hen oviduct. J Cell Biochem. 1986;32(3):193–200. doi: 10.1002/jcb.240320305. [DOI] [PubMed] [Google Scholar]
- Baker R. K., Lively M. O. Purification and characterization of hen oviduct microsomal signal peptidase. Biochemistry. 1987 Dec 29;26(26):8561–8567. doi: 10.1021/bi00400a010. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhni P. C., Deshaies R. J., Schekman R. W. SEC11 is required for signal peptide processing and yeast cell growth. J Cell Biol. 1988 Apr;106(4):1035–1042. doi: 10.1083/jcb.106.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. K., Ray P. H. Rapid assay and purification of a unique signal peptidase that processes the prolipoprotein from Escherichia coli B. J Biol Chem. 1984 Sep 10;259(17):11114–11120. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Gilmore R., Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A. 1986 Feb;83(3):581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Greenburg G., Shelness G. S., Blobel G. A subunit of mammalian signal peptidase is homologous to yeast SEC11 protein. J Biol Chem. 1989 Sep 25;264(27):15762–15765. [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Shelness G. S., Blobel G. Two subunits of the canine signal peptidase complex are homologous to yeast SEC11 protein. J Biol Chem. 1990 Jun 5;265(16):9512–9519. [PubMed] [Google Scholar]
- Shelness G. S., Kanwar Y. S., Blobel G. cDNA-derived primary structure of the glycoprotein component of canine microsomal signal peptidase complex. J Biol Chem. 1988 Nov 15;263(32):17063–17070. [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986 May;102(5):1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Evans E. A., Blobel G. Prepro-alpha-factor has a cleavable signal sequence. J Biol Chem. 1988 May 5;263(13):6209–6214. [PubMed] [Google Scholar]
- YaDeau J. T., Blobel G. Solubilization and characterization of yeast signal peptidase. J Biol Chem. 1989 Feb 15;264(5):2928–2934. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]