Abstract
The tooth enamel organ (EO) is a complex epithelial cell assembly involved in multiple aspects of tooth development, including amelogenesis. The present study focuses on the role of the nonameloblast layers of the EO, the stratum intermedium, the stellate reticulum, and the outer enamel epithelium (OEE). The secretory stage stratum intermedium was distinguished by p63-positive epithelial stem cell marks, highly specific alkaline phosphatase labeling, as well as multiple desmosomes and gap junctions. At the location of the presecretory stage stellate reticulum, the pre-eruption EO prominently featured the papillary layer (PL) as a keratin immunopositive network of epithelial strands between tooth crowns and oral epithelium. PL cell strands contained numerous p63-positive epithelial stem cells, while BrdU proliferative cells were detected at the outer boundaries of the PL, suggesting that the stellate reticulum/PL epithelial cell sheath proliferated to facilitate an epithelial seal during tooth eruption. Comparative histology studies demonstrated continuity between the OEE and the general lamina of continuous tooth replacement in reptiles, and the outer layer of Hertwig's epithelial root sheath in humans, implicating the OEE as the formative layer for continuous tooth replacement and tooth root extension. Cell fate studies in organ culture verified that the cervical portion of the mouse molar EO gave rise to Malassez rest-like cell islands. Together, these studies indicate that the nonameloblast layers of the EO play multiple roles during odontogenesis, including the maintenance of several p63-positive stem cell reservoirs, a role during tooth root morphogenesis and tooth succession, a stabilizing function for the ameloblast layer, the facilitation of ion transport from the EO capillaries to the enamel layer, as well as safe and seamless tooth eruption.
Introduction
Epithelia are cohesive cell sheets that constitute selective barriers between the environment and the underlying cells of the body [1,2]. Many epithelial cells are polarized into an apical pole that provides an exchange interface with other parts of the body and a basal portion that interacts with surrounding extracellular matrices and adjacent neighboring cells [1]. As a result of their highly organized nature and their ability to coordinate movement and rearrangement of individual cells, epithelial sheets play key roles in morphogenesis and organogenesis [3]. Epithelial morphogenesis is a multistep process [4] that includes (1) cell fate specification via transcription factors [5], (2) induction of morphogenetic events by extracellular signals [6,7], (3) morphogenetic changes through coordinated cytoskeletal changes and cell adhesion dynamics [8], and (4) coordinated cell proliferation and apoptosis events that augment the morphogenetic cell sheet rearrangement process [9,10].
The tooth enamel organ (EO) is a typical example of a complex epithelial organ that through a series of transcriptional signals and extracellular matrix cues determines the shape of the developing tooth and greatly influences the development and fate of many related tissues that contribute to the formation of a fully erupted dentition [11]. The odontogenic epithelium is a highly multifaceted epithelial cell population that gives rise to multiple cell types, each of which are uniquely differentiated toward a specialized function related to the life of the tooth [12]. Some of the derivatives of the odontogenic epithelium undergo additional generational changes and develop into new cell types that hardly resemble their precursors in matters of function, shape, and biochemistry.
Early in its career, the odontogenic epithelium forms the EO, which then shapes the tooth crown and a cervical loop [13]. The cervical loop in turn develops into a potent cell sheath that prepatterns the shape of the tooth root [14,15]. Simultaneously, the coronal part of the EO differentiates into a four-layer cap featuring signaling preameloblasts at the odontoblast border [16]. Once dentin is secreted, a new chapter in the metamorphosis of the EO begins with the differentiation of preameloblasts into secretory ameloblasts and the onset of enamel matrix and mineral secretion [17]. Many of these secretory ameloblasts become resorptive ameloblasts following initial enamel secretion and resorb excess water and protein fragments from the enamel matrix to further facilitate the completion of the enamel layer [18]. The functions and the eventual fate of the remaining three layers of the EO, namely the stratum intermedium, the stellate reticulum, and the outer enamel epithelium (OEE), are little understood. It has been speculated that the nonameloblast components of the EO either function as aids during mineral transport, to provide stability for the secretory EO, or act as an epithelial stem cell niche [19,20].
In the present article, we have performed a number of cell labeling, cell tracking and, cell fate studies to identify individual functions of the three nonameloblast layers of the EO. These studies have revealed novel functions for these unusual cell layers as stem cell reservoirs, facilitators of tooth eruption, and members of Hertwig's epithelial root sheath (HERS) and defined their individual contributions. Together, these studies shed new light on the intriguing kaleidoscope of functions of the nonameloblast cell layers of the EO: stratum intermedium, stellate reticulum, and OEE.
Materials and Methods
Animals and tissue preparation
Three-, 6-, 9-, 12-, 15-, and 18-day-old mice served as the experimental model in the majority of the studies presented here. In addition, a young gecko (Coleonyx brevis) of undefined age was sacrificed for comparative studies. All animal experiments and procedures were conducted according to the guidelines of the University of Illinois at Chicago Animal Care Committee. For the present study, mice and the gecko were sacrificed and mandibles were fixed in 10% buffered formalin for 2 days, followed by decalcification for 2 weeks with 5% EDTA and 1% formalin. Following decalcification, specimens were dehydrated, embedded in paraffin, and cut into 5 μm sagittal sections along the long axis of the molar teeth. For periodic acid Schiff (PAS) staining, tissues were fixed in formalin, decalcified as described above, embedded in JB-4 (Polysciences), and sectioned using glass knifes. For semithin sections and electron microscopy, tooth organs were dissected and fixed in Karnovsky's fixative as previously described [21]. Following postfixation in osmium tetroxide, samples were processed for semithin sections.
Tissue layer identification using classic histologic stains
Three-day postnatal first mandibular molar tissue sections were stained using the Azan technique or Masson Goldner's technique as described [22]. Plastic sections were stained with PAS solution as published [23] and semithin sections of Epon blocks were contrasted with Paragon Epoxy stain (Polysciences) for 1 min on a 50°C hot plate.
Immunohistochemistry
Immunoreaction procedures were performed on paraffin sections as previously described [24]. The following reagents served as primary antibodies: (1) wide-spectrum screening cytokeratin antibody (Dako/Agilent), (2) goat anti-rabbit whole molecule alkaline phosphatase antibody (Sigma), and (3) anti-p63 antibody ab735 (Abcam). Briefly, sections were deparaffinized, rehydrated, and treated with 5% peroxide and methanol followed by a brief incubation in 10 mM sodium citrate buffer with 0.05% Tween 20 at pH 6.0 for antigen retrieval. Sections were then incubated with 1% bovine serum albumin (BSA) for 30 min at room temperature to block nonspecific binding of the antibody. After blocking, sections were incubated with primary antibody at a concentration of 1:100, washed three times in PBS, and immunolabeling was visualized using an ABC Peroxidase Standard Staining kit or a Histomouse Broad Spectrum AEC kit (both from Thermo Fisher Scientific) and Hematoxylin as a counterstain.
Electron microscopy
Sections were cut from Epon blocks using a Reichert-Jung ultramicrotome. Thickness of sections was determined by comparing the interference reflex colors of sections with a standard table. Sections were then contrasted in 1% uranyl acetate followed by Reynold's lead citrate for 15 min each. Observations were made on a JEOL 1220 TEM at 80 kV.
BrdU labeling for cell proliferation
To identify proliferating cells in the papillary layers (PLs) of pre-eruption molar teeth, 15- and 18-day-old mice were intraperitoneally injected with BrdU (100 mg/kg; Sigma) 1 h before euthanization to label the proliferating cells. Mandibles were dissected and processed for paraffin sections. Following deparaffinization, sections were stained using a BrdU staining kit (Thermo Fisher Scientific) as previously described [25].
Organ culture and DiI labeling
Our Trowell-type tooth organ procedure has been described previously [26,27]. In the present study, 3-day postnatal EOs were dissected and cultured at the liquid–air interface of a Trowell organ culture dish for 6 days using BGJb+ medium (Fitton-Jackson's modified BGJ) supplemented with 100 μg/mL l-ascorbic acid, 100 U/mL penicillin/streptomycin, and 5% fetal calf serum. Explanted tooth organs were cultured at 37.5°C with atmospheric conditions of 95% air and 5% CO2. Initial pH was adjusted to 7.4 and the medium was changed every other day. All experiments were done in triplicate.
Human specimens
The University of Illinois at Chicago was home to a number of large-scale sections through human jaws that were presumably brought to Chicago by the famed oral histopathologist Joseph-Peter Weinmann from the University of Vienna [28,29]. The micrographs presented in this article were generated from these sections.
Results
Stratum intermedium cells were rich in TNAP and inorganic phosphate while ameloblasts stained for glycogens and/or other polysaccharides
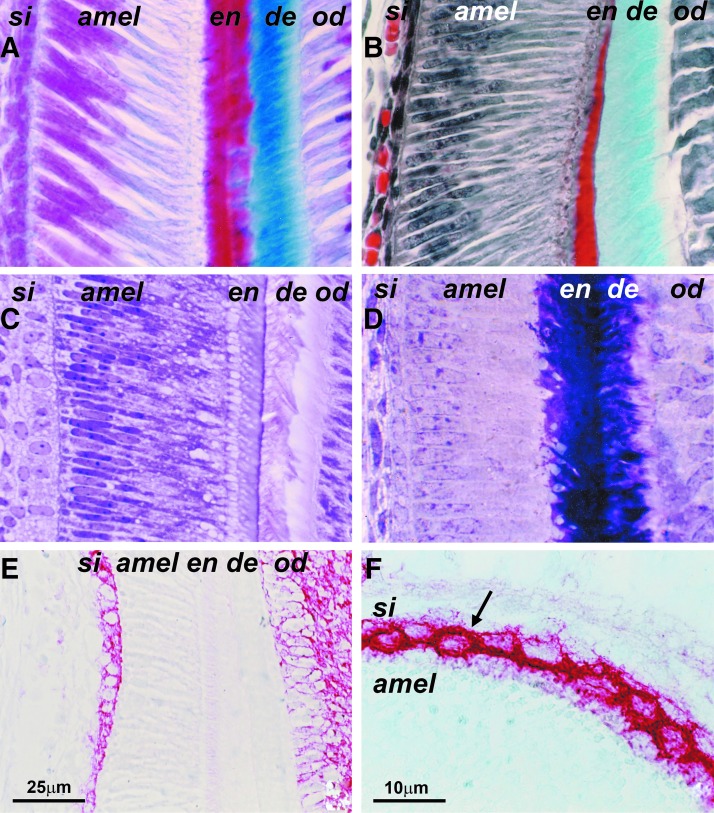
To reveal cytological details of the ameloblast and stratum intermedium cell layers, sections of EOs were subjected to a classic histological staining regimen (Fig. 1A–D). Micrographs from stained sections distinguished between an elongated and parallel arranged secretory ameloblast cell layer oriented perpendicular to the adjacent one- to three-layered row of cuboidal stratum intermedium cells (Fig. 1A–D). The PAS procedure demonstrated a positive reaction for glycogens and other polysaccharides in the ameloblast nuclei and cytoplasm and at the dentin/enamel junction, while PAS staining was absent in the stratum intermedium and in the ameloblast secretory vesicles (Fig. 1C). To reveal the mineralization potential of ameloblast and stratum intermedium cell layers, tissue sections were subjected to TNAP (tissue-nonspecific alkaline phosphatase) immunoreactions and von Kossa staining. The stratum intermedium layer was marked by a highly specific reaction for TNAP on the cell membranes and around a few vesicular structures in the cytosol, while there was no TNAP detected in the adjacent ameloblast and stellate reticulum layers (Fig. 1E–G). However, there was TNAP reactivity in the odontoblast/pulp complex (Fig. 1E).
FIG. 1.
Classic histology of the ameloblast/stratum intermedium complex. (A) Azan staining. Compare the perpendicular orientation of ameloblast (amel) and stratum intermedium (si) cell layers and the red color of the nuclei as revealed by Heidenhain's azan staining protocol. Enamel (en) appears in bright red and dentin (de) in bright blue. The odontoblasts (od) are located immediately adjacent to the pale blue predentin. (B) Masson–Goldner staining. Pierre Masson's connective tissue trichrome stain using Weigert's hematoxylin as a nuclear marker sharply distinguishes between elongated secretory ameloblasts (amel) and perpendicular stratum intermedium (si) cell orientation, while the enamel layer (en) is bright red and the dentin (de) appears in green color. (C) Periodic acid Schiff (PAS) staining of plastic sections. Note the identification of glycogens and other polysaccharides in the ameloblasts (amel) and at the dentin/enamel junction (en/de) and the absence of PAS staining in the stratum intermedium (si) and in the ameloblast secretory vesicles (arrow). (D) Polychromatic Paragon Epoxy stain to identify cellular details on semithin sections. Here the combination of toluidine blue and basic fuchsin resulted in intense dark violet staining of enamel (en) and dentin (de) mineralized tissues and high resolution at the interface between ameloblast (amel) and stratum intermedium (si) cell layers. (E, F) TNAP alkaline phosphatase enzyme immunohistochemistry. Note the strong staining for alkaline phosphatase in the stratum intermedium (si) and the absence thereof in the stellate reticulum (sr), ameloblast (amel), enamel (en), and dentin (de) cell layers (E). (F) is a higher magnification image.
Numerous desmosomes were indicative of strong adhesion between adjacent stratum intermedium/ameloblast cell layers
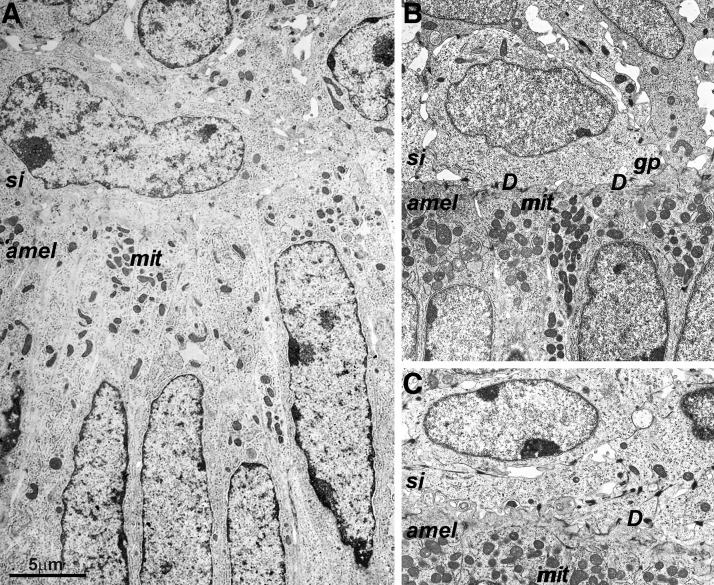
Electron microscopy of 3-day postnatal mouse molar EOs revealed numerous desmosomes and gap junctions at the interface between stratum intermedium and ameloblast cell layers (Fig. 2A–C).
FIG. 2.
Electron microscopy of the ameloblast/stratum intermedium interface. (A) Perpendicular orientation of ameloblast (amel) and stratum intermedium (si) nuclei). Multiple mitochrondria (mit) were located at the basal ameloblast pole. (B, C). Numerous desmosomes (des) and gap junctions (gj) at the interface between stratum intermedium and ameloblast cell layers.
P63-positive epithelial stem cells were initially restricted to the stratum intermedium and thereafter detected in ameloblasts, stellate reticulum, and HERS
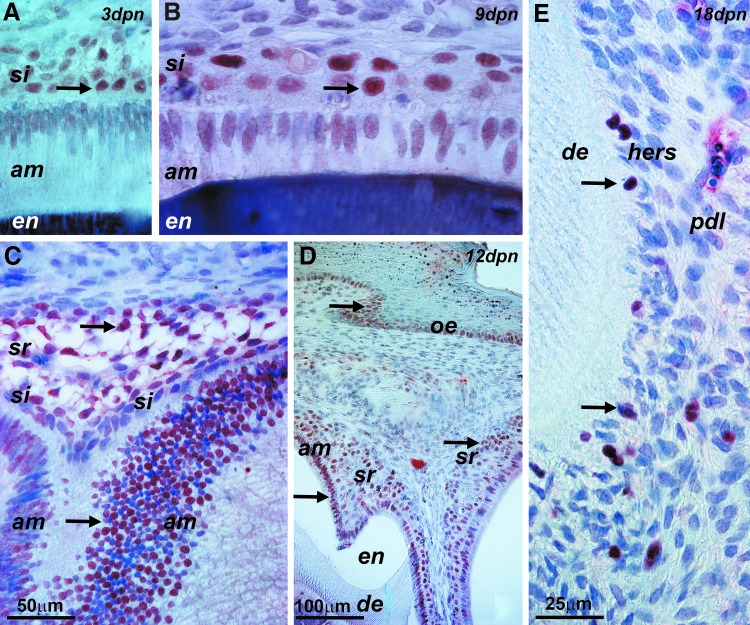
The epithelial stem cell marker p63 was used to identify epithelial progenitors throughout postnatal EO development. During the secretory stage (3 days postnatal), p63 specifically labeled stratum intermedium cell nuclei (Fig. 3A). During the resorptive stage of amelogenesis (9 days postnatal), there was strong immunoreactivity for p63 in the stratum intermedium nuclei and moderate staining for p63 in some ameloblast nuclei (Fig. 3B). Subsequently, during the protective phase (12 days postnatal), p63-labeled nuclei were identified in the ameloblast cell layer, stellate reticulum, and the basal layer of the oral epithelium (Fig. 3C, D). Following tooth eruption, p63-positive nuclei were detected in cells of HERS adjacent to the root surface of erupted teeth (Fig. 3E).
FIG. 3.
Epithelial stem cell marker p63 expression during postnatal mouse molar EO development. (A) Secretory stage 3-day postnatal mouse molar EO, including stratum intermedium (si) and ameloblast (amel) cell layers and the enamel mineral layer (en). The arrow points to p63-positive stratum intermedium cell nuclei. (B) EO at 9 days postnatal. Arrows point to p63 labels in the stratum intermedium (si) and ameloblast (am) cell layers. (C, D) Twelve-day postnatal EO. The arrows point to p63-labeled nuclei in the ameloblast cell layer (am), stellate reticulum (sr), and stratum basale of the oral epithelium (oe). (E) Root tip and adjacent periodontal tissue of an 18-day postnatal first mouse molar tooth root. Root dentin (de), periodontal ligament (pdl), and Hertwig's epithelial root sheath (hers) are labeled. The arrows point to p63-positive nuclei present in HERS cells. EO, enamel organ.
The epithelial continuity between ameloblasts, stratum intermedium, PL, and oral epithelium was visualized using a pan-keratin antibody
A pan-Keratin antibody was used as a marker to identify epithelial components at the interface between oral epithelium and pre-eruption EO. Our pan-Keratin antibody reacted with the EO PL, stellate reticulum epithelial strands, ameloblasts, and the oral epithelium (Fig. 4A). Pre-eruption EOs were characterized by a thickened stratum intermedium as the structural base for a network of epithelial strands in the location of the former stellate reticulum, the PL (Fig. 4B). The pan-keratin antibody also identified the continuous epithelial strands between ameloblasts, PL, and oral epithelium (Fig. C).
FIG. 4.
Eruption stage PL development. Pan-Keratin immunoreactions in 15-day postnatal pre-eruption mouse molars. (A) Third molar with incomplete connection between PL (sr, pap) and oral epithelium (oe). Dentin (de), ameloblasts (am), and pulp were labeled for orientation purposes. (B) The PL (pap) formed a network of epithelial strands at the location of the former stellate reticulum between the oral mucosa mesenchyme (mes) and the ameloblast layer (am). Note the thickened stratum intermedium (si). (C) PL immediately before eruption. Epithelial strands between the ameloblasts (am) and the oral epithelium (oe) were continuous at that stage. PL, papillary layer.
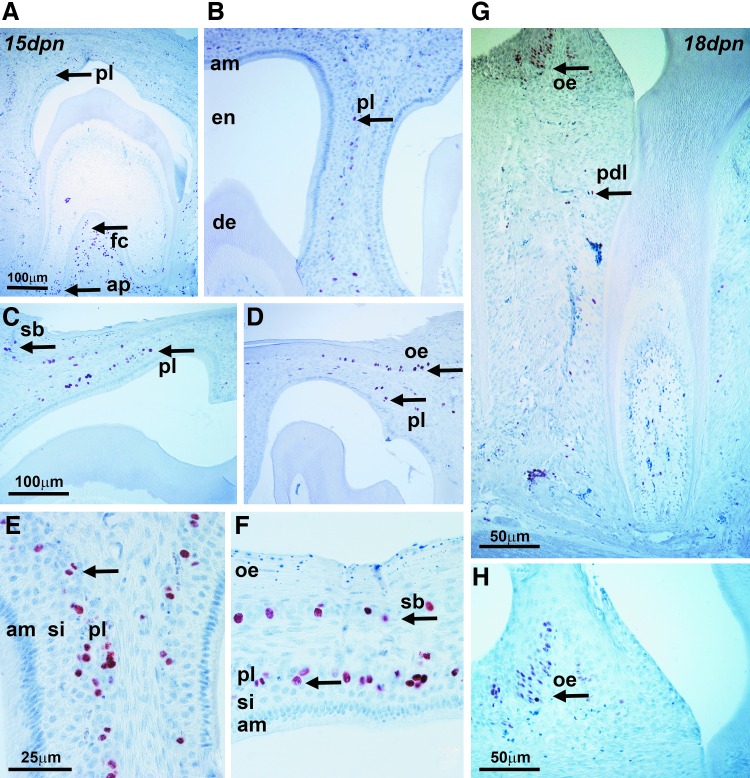
In pre-eruption EOs, proliferative cells were confined to the outer periphery of the PL and the basal layer of the oral epithelium
To identify proliferating cell populations in pre-eruption EOs, mice were injected with BrdU and proliferative cells were identified using an anti-BrdU antibody and 3,3′-diaminobenzidine staining. In 15-day postnatal EOs, BrdU-positive cells were detected in the outer periphery of the PL and in the stratum basale of the oral epithelium (Fig. 5A–F). BrdU-positive cells were also demonstrated in periodontal tissues, including the apical papilla and the furcation (Fig. 5A). BrdU staining was absent from ameloblasts, stratum intermedium, stellate reticulum proper, and enamel and dentin (Fig. 5A–F). In 18-day postnatal pre-eruption teeth, BrdU-positive cells were restricted to the cervical periodontal ligament and the oral epithelium (Fig. 5G).
FIG. 5.
Cell proliferation in eruption stage mouse molar tooth organs. Mice were injected with BrdU and proliferating cells were marked using an anti-BrdU antibody and 3,3′-diaminobenzidine staining. (A–F) Proliferating cells were detected at the apical papilla (ap) and at the furcation (fc) between roots (A, arrows for ap and fc), in the papillary layer (A–F, pl), and in the basal layer (sb) of the oral epithelium (oe). There was no BrdU staining in the ameloblast (am) and stratum intermedium (si) cell layers, and also enamel (en) and dentin (de) remained unstained. In the 18-day postnatal samples (G, H), BrdU-positive cells were restricted to the cervical periodontal ligament (pdl) and to the oral epithelium (oe).
Cell tracking with the fluorescent cell contact dye DiI suggested cell movements within the EO from the stellate reticulum to the stratum intermedium
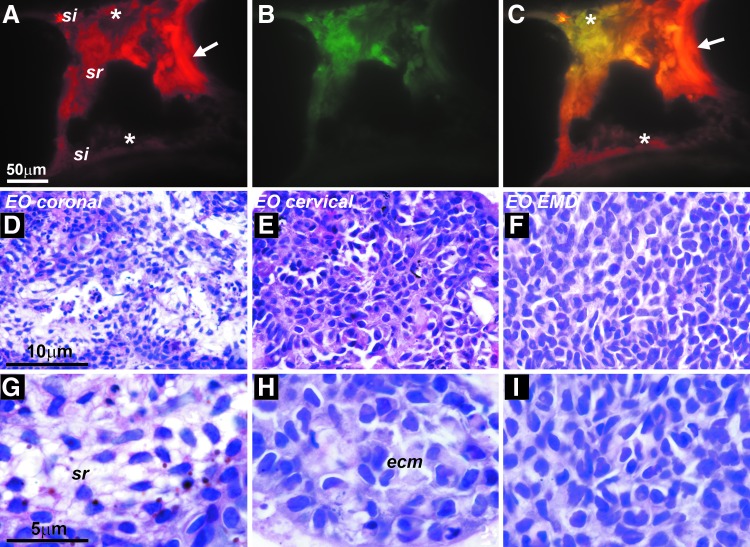
To identify possible cell movements during postnatal mouse molar EO development, DiI crystals were placed in the center of the stellate reticulum of first mandibular molar tooth organs, which were cultured on nitrocellulose filters in Trowell organ culture dishes. Following 6 days of culture, DiI fluorescence was recorded not only in the stellate reticulum but also in the stratum intermedium and a portion of the ameloblast layer (Fig. 6A, B).
FIG. 6.
Mouse molar organ culture studies for cell fate mapping. (A–C) Identification of DiI fluorescence 6 days after insertion of a DiI crystal into the center of the stellate reticulum. After 6 days of culture, DiI fluorescence was detected in the stellate reticulum (arrow, site of crystal insertion) and a portion of the stratum intermedium (asterisks). Fluorescence filter combinations were fluorescein (A), Texas Red (B), and merge (C). Note the DiI labeling in the stratum intermedium (asterisk, A, C). (D–G) Cell morphology of cultured epithelium organs after 6 days of culture on Trowell nitrocellulose discs. (D, G) Coronal EO culture revealed cell layers resembling stellate reticulum (sr) cell populations. (E, H) Cervical loop organ culture resulted in densely packed polygonal cells surrounded by substantial amounts of extracellular matrix (ecm). (F, I) Coronal EO culture on Emdogain-coated discs yielded dense, cuboidal cell assemblies resembling stratum intermedium (si) cells, while stratum reticulum-like cell networks were absent.
EO cell fate studies demonstrated that the coronal portion of the EO gave rise to stellate reticulum-like populations, while the apical portion of the EO generated epithelial rests of Malassez-like cell islands and EO loops
For this study, EOs were separated into coronal and apical portions (cervical loop), and EO subsets were cultured for 6 days on nitrocellulose discs in Trowell organ culture dishes. After 6 days of culture, coronal EO cultures in BGJb (Fitton-Jackson modification) revealed cell layers resembling ameloblasts (en), stratum intermedium, and stellate reticulum (Fig. 6D, G). When coronal EOs were cultured on Emdogain-coated discs, cell shapes became cuboidal and cell assemblies resembled stratum intermedium cell layers, while stratum reticulum-like cell networks were absent (Fig. 6F, I). Finally, cervical loop cell sheath cultures resulted in columnar ameloblast-like cell loops and polygonal polychromatic cells surrounded by substantial amounts of extracellular matrix, resembling epithelial rests of Malassez (ERM) (Fig. 6E, H).
Discussion
In the present study, we have performed a series of cell labeling, cell tracking, and cell fate studies to ask which of the nonameloblast layers of the EO are associated with which specific functions during tooth development. Specifically, to identify stratum intermedium cell function, EOs were subjected to light microscopic stains, alkaline phosphatase and stem cell marker immunoreactions, as well as electron microscopy. Pre-eruption tooth organs were labeled for epithelial cells, proliferation, and stem cell markers to determine the relationship between the stellate reticulum and the PL. Organ culture studies were conducted to distinguish EO progeny between the coronal and cervical portion of the EO and to evaluate the effect of enamel matrix derivative on EO cell behavior. As a final step, histological preparations from reptilian and human developing dentitions were used to localize the OEE in nonmurine specimen and to infer functional implications. Together, these studies shed new light on the individual function(s) of each of the three nonameloblast lineages of the EO.
P63 as a marker for epithelial stem cells in the enamel organ
In the present study, p63 was localized in the secretory stage stratum intermedium and postsecretory ameloblast cell populations. Prior to eruption, p63 was also detected in the stellate reticulum, and posteruption, p63 was recognized in cells of HERS. In recent years, p63 has become universally accepted as an epithelial stem cell marker [30]. P63 has been identified in adult epidermal stem cells, keratinocyte stem cells, thymus epithelial precursor cells, and prostate epithelial basal cells [31,32], while mice lacking p63 revealed a severely compromised epidermis [33–36]. P63 is the oldest conserved homologue of the p53 family of transcription factors and involved in all levels of epithelial stemness maintenance and regulation [30,37]. It has been suggested that p63 maintains epithelial stemness through Hedgehog, WNT, and Notch signaling, by controlling cell adhesion and by adversely affecting the polycomb protein CBX4 [38–42]. Hedgehog, WNT, and Notch signaling all play a role in tooth development [43], and it is likely that p63 contributes to the regulation of these signal pathways to maintain epithelial stemness in selected EO cell populations. Our data revealed a surprising degree of p63 upregulation in approximately half of the postsecretory ameloblasts, suggesting that some ameloblasts regain their stemness potential following enamel secretion. However, in the secretory stage EO, epithelial stem cell potential as identified by p63 was restricted to the stratum intermedium, suggesting that the stratum intermedium constitutes the presecretory EO stem cell layer during early amelogenesis.
The stratum intermedium: a multipotent stem cell layer involved in ion transport and enamel organ stability
As mentioned above, our immunoreactions established the stratum intermedium as the primary early stem cell layer of the EO. A putative role of the stratum intermedium as the presecretory EO stem cell layer is supported by earlier studies demonstrating Sonic hedgehog expression in the stratum intermedium [44]. The presence of stem cells in the EO has been previously postulated based on FGF 10-induced cell proliferation in the stratum intermedium [45,46]. The origin of stratum intermedium stem cells is not entirely clear. However, our DiI-labeling studies provide evidence for directed cell movements from the stellate reticulum to the stratum intermedium, opening up the possibility that stratum intermedium epithelial stem cells may originate from the stellate reticulum. Our studies also demonstrate that p63 stem cell labeling was present in secretory stage stratum intermedium and not in ameloblasts, while p63 was lost in the pre-eruption stage stratum intermedium, and p63 staining was found in approximately half of the ameloblasts at this stage. Moreover, p63 staining became prominent in the postsecretory and pre-eruptive stellate reticulum/PL. This oscillation of p63 levels between EO layers during development suggests that either cell populations migrate between those layers or that EO cells are capable of regaining their progenitor potential at later stages in development.
Our immunohistochemical studies revealed a stark contrast between high levels of alkaline phosphatase expression in the stratum intermedium and almost complete absence thereof in the neighboring stellate reticulum and ameloblast EO cell layers. Our finding confirms earlier reports of exceptionally high levels of alkaline phosphatase in the stratum intermedium [46,47]. Some authors have speculated that the main function of the alkaline phosphatase in the stratum intermedium is to transport phosphate from blood vessels into the EO [47]. Alternatively, high doses of alkaline phosphatase in the stratum intermedium may also facilitate the formation of phosphorylated macromolecules, as studies using the alkaline phosphatase inhibitor I-pBTM have demonstrated [47]. It is not clear whether those high levels of alkaline phosphatase in the stratum intermedium are exclusively due to their role in phosphate transport or in the generation of phosphorylated macromolecules as alkaline phosphatase also plays a role in stem cells and is often used as a stem cell marker [48].
Our electron microscopical studies provide evidence for multiple desmosomes and gap junctions at the interface between stratum intermedium and the proximal ameloblast pole. The presence of large numbers of desmosomes has been attributed to the function of the stratum intermedium as a stabilizer for the ameloblast layer [49,50]. Previous studies have noted a substantial increase in the number of gap junctions from presecretory to secretory stage and have associated the gap junctions with metabolic, mechanical, or ionic regulatory functions [51,52]. However, at this point, the specific role of the high number of junctional complexes at the ameloblast/stratum intermedium interface remains to be further determined.
The stellate reticulum as formative cell population for the papillary layer
Our data demonstrated formation of a PL of keratin-positive epithelial tissue strands at the interface between the tooth crowns and the oral epithelium. Individual cells within those epithelial cells resembled those of the stellate reticulum, and the keratin-positive cells formed a cellular network at the site of the former stellate reticulum. Moreover, our BrdU labeling experiments demonstrated cell proliferation within the outer surface layer of the PL, while the p63-positive epithelial stem cells were localized within the PL itself. Together, these data establish that the PL is a stellate reticulum derivative that proliferates during tooth eruption to establish an epithelial continuity during tooth eruption.
Tooth eruption is a key event in the life of organisms as the erupting tooth punctures the oral cavity and exposes the underlying connective tissues to germs and other pathogens of the oral cavity. Prolonged eruption as it occurs during wisdom tooth eruption results in delayed wound healing and an inflammatory process called pericoronitis. The epithelial integrity of the PL during tooth eruption prevents and reduces the possibility for oral pathogens to invade the oral connective tissues and cause inflammation of the jaws.
Earlier studies have identified typical extracellular matrix proteins such as fibronectin, syndecan 1, laminin 5, and the heparan sulfate perlecan in the stellate reticulum [53–56], suggesting that the stellate reticulum is a proteoglycan-rich cell layer that functions to protect the developing tooth organ prior to amelogenesis. However, during amelogenesis, the stellate reticulum becomes greatly reduced, prompting speculations that its function may be limited to the presecretory stage alone. Moreover, some authors have speculated that the PL does not form by mitotic division of progenitor cells, suggesting that the PL is formed through the alteration of cell shapes [57,58]. However, our keratin labeling studies demonstrate that the PL forms as a network of keratin-positive cells just adjacent to the stratum intermedium at the same location as the presecretory stratum intermedium, our BrdU labeling studies reveal that the PL grows through proliferation of its outermost cells, and our p63 marker studies have identified multiple epithelial stem cells inside of the PL. Together, these studies suggest that the stellate reticulum plays a key role in the establishment of the PL as an epithelial barrier that facilitates tooth eruption and that this PL is formed through cell proliferation.
The outer enamel epithelium: source of continuous tooth replacement in reptiles and outer cell layer of HERS
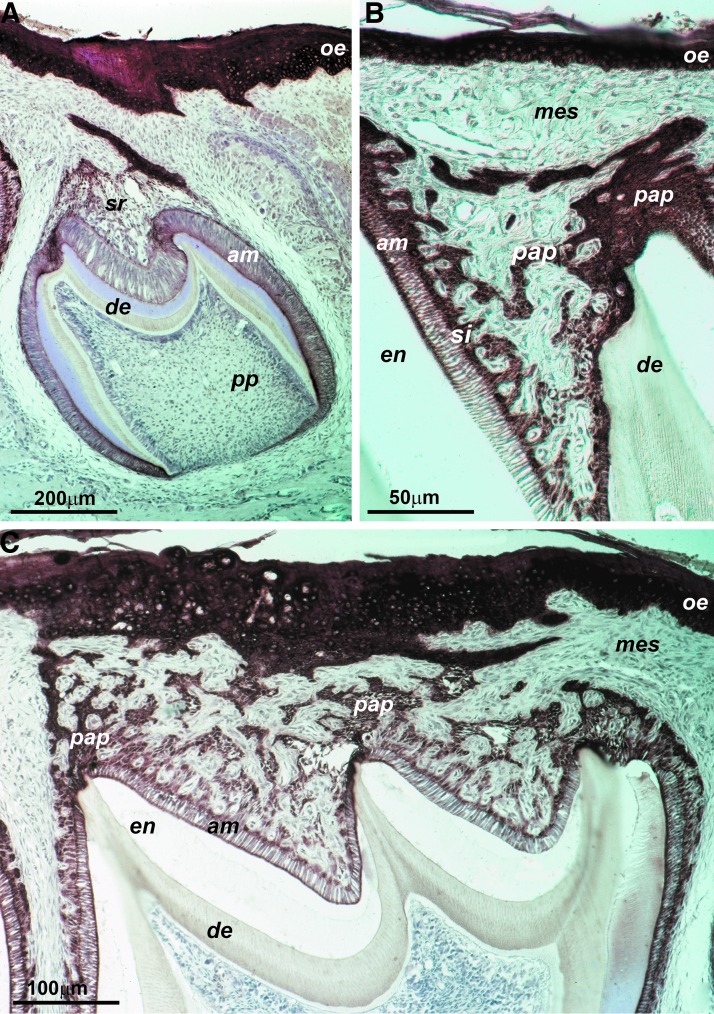
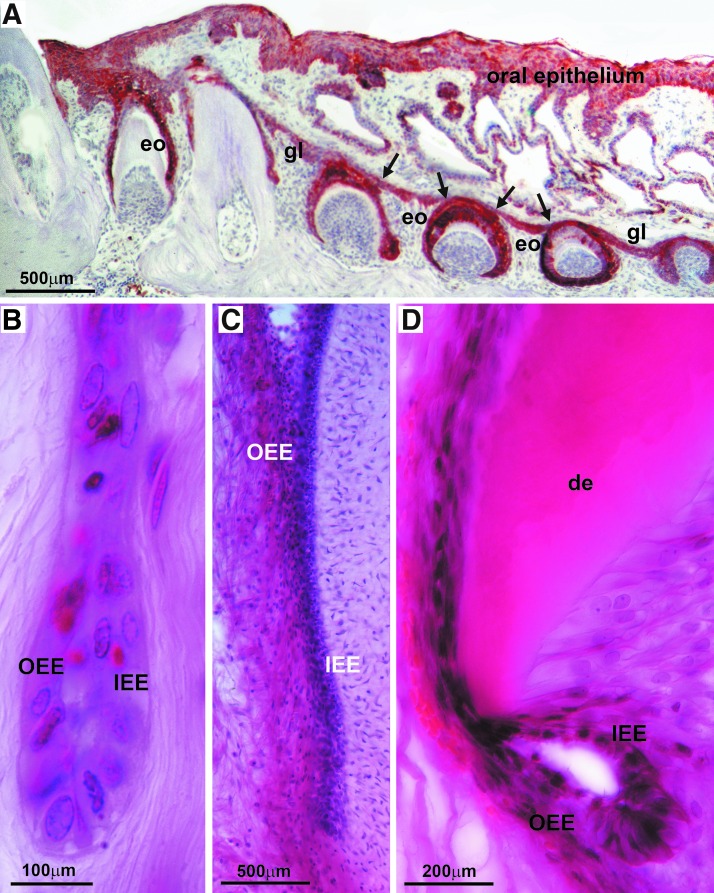
There is only little information available concerning the function of the OEE as it becomes disintegrated during mammalian amelogenesis. In general, textbooks note that the OEE connects with the inner enamel epithelium (IEE) at the cervical loop and that it consists of a layer of cuboidal epithelial cells. The connection between OEE and IEE became very obvious in our human specimen, in which the cervical portion of the OEE continued into the outer layer of HERS, the bilayer of epithelial cells that migrate in apical direction and determine the shape of the tooth root (Fig. 7). In addition, anti-keratin immunostainings of the row of successional EOs of a gecko dentition revealed that the OEE seamlessly interfaced with the general lamina (Fig. 7). The general lamina is an interconnected string of epithelial tissues that give rise to the successional teeth of the reptilian dentition, much like the successional dental lamina in humans. Finally, our organ culture studies demonstrated that the cervical rim of the EO gave rise to a unique population of cuboidal cells that were surrounded by a thick layer of matrix in all directions, resembling ERM cells on a light microscopic level. Together, these data establish the OEE as a morphogenetic epithelial tissue with highest regenerative potential as it gives rise to successional dental laminae and contributes to the root forming Hertwig's root sheath. Our study also suggests differences in developmental potential between the coronal and the cervical portion of the EO, with the coronal portion defaulting toward a stellate reticulum-type cell morphology, while the cervical loop portion gave rise to ERM-like cuboidal cells. These findings suggest that already in secretory stage EOs, the cervical and coronal portions of the EO contain different developmental programs.
FIG. 7.
Then, outer EO epithelium in nonmurine specimen. (A) Pan-keratin labeling of the general lamina of a gecko (Coleonyx brevis) dentition. The immunohistochemical reaction identified the oral epithelium (oe), the general lamina (gl), and the enamel organ (eo) for six successional teeth. The arrows point to the site at which the general lamina gives rise to individual enamel organs. (B–D) Outer (OEE) and inner (IEE) enamel epithelium contributing to HERS of human teeth. The dentin (de) layer is marked for orientation.
In summary, this study sheds light on the EO as a multipotential four-layered tissue complex that not only contributes to the secretion of tooth enamel but also contains several stem cell reservoirs, provides morphogenetic clues for tooth root morphology and tooth succession, maintains EO stability, enables ion transport, and facilitates safe and seamless tooth eruption. Perhaps the time has come that the lesser known daughter populations of the EO emerge from their hidden wildflower state and receive the well-deserved recognition as the quarterbacks of odontogenesis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roignot J, Peng X. and Mostov K. (2013). Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol 5:a013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterfield M, Du X, Schüpbach T, Wieschaus E. and Shvartsman SY. (2013). Three-dimensional epithelial morphogenesis in the developing Drosophila egg. Dev Cell 24:400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher AG, Osterfield M, Baker RE. and Shvartsman SY. (2014). Vertex models of epithelial morphogenesis. Biophys J 106:2291–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schock F. and Perrimon N. (2002). Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol 18:463–493 [DOI] [PubMed] [Google Scholar]
- 5.Leptin M. (1995). Drosophila gastrulation: from pattern formation to morphogenesis. Annu Rev Cell Dev Biol 11:189–212 [DOI] [PubMed] [Google Scholar]
- 6.Montell DJ. (2001). Command and control: regulatory pathways controlling invasive behavior of the border cells. Mech Dev 105:19–25 [DOI] [PubMed] [Google Scholar]
- 7.Oda H. and Tsukita S. (2001). Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci 114:493–501 [DOI] [PubMed] [Google Scholar]
- 8.Gumbiner BM. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357 [DOI] [PubMed] [Google Scholar]
- 9.Conlon I. and Raff M. (1999). Size control in animal development Cell 96:235–244 [DOI] [PubMed] [Google Scholar]
- 10.Vaux DL. and Korsmeyer SJ. (1999). Cell death in development Cell 96:245–254 [DOI] [PubMed] [Google Scholar]
- 11.Maas R. and Bei M. (1997). The genetic control of early tooth development. Crit Rev Oral Biol Med 8:4–39 [DOI] [PubMed] [Google Scholar]
- 12.Thesleff I, Vaahtokari A. and Partanen AM. (1995). Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol 39:35–50 [PubMed] [Google Scholar]
- 13.Zeichner-David M, Diekwisch T, Fincham A, Lau E, MacDougall M, Moradian-Oldak J, Simmer J, Snead M. and Slavkin HC. (1995). Control of ameloblast differentiation. Int J Dev Biol 39:69–92 [PubMed] [Google Scholar]
- 14.Diekwisch TG. (2001). The developmental biology of cementum. Int J Dev Biol 45:695–706 [PubMed] [Google Scholar]
- 15.Luan X, Ito Y. and Diekwisch TGH. (2006). Evolution and development of Hertwig's epithelial root sheath. Dev Dyn 235:1167–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CE. and Nanci A. (1995). Overview of morphological changes in enamel organ cells associated with major events in amelogenesis. Int J Dev Biol 39:153–161 [PubMed] [Google Scholar]
- 17.Diekwisch TGH, Berman BJ, Gentner S. and Slavkin HC. (1995). Initial enamel crystals are spatially not associated with mineralized dentine. Cell Tissue 279:149–167 [DOI] [PubMed] [Google Scholar]
- 18.Smith CE. (1979). Ameloblasts: secretory and resorptive functions. J Dent Res 58(Spec Issue B):695–707 [DOI] [PubMed] [Google Scholar]
- 19.Tummers M. and Thesleff I. (2003). Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development 130:1049–1057 [DOI] [PubMed] [Google Scholar]
- 20.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y. and De Bari C. (2007). Stem cell niches in mammals. Exp Cell Res 313:3377–3385 [DOI] [PubMed] [Google Scholar]
- 21.Diekwisch T, David S, Bringas P, Santos V. and Slavkin HC. (1993). Antisense inhibition of AMEL translation demonstrates supramolecular controls for enamel HAP crystal growth during embryonic mouse molar development. Development 117:471–482 [DOI] [PubMed] [Google Scholar]
- 22.Romeis B. (1989). Mikroskopische Technik. Urban und Schwarzenberg, Munchen [Google Scholar]
- 23.Van Goor H, Gerrits PO. and Grond J. (1986). The application of lipid-soluble stains in plastic-embedded sections. J Histochem 85:251–253 [DOI] [PubMed] [Google Scholar]
- 24.Diekwisch TG, Ware J, Fincham AG, and Zeichner-David M. (1997). Immunohistochemical similarities and differences between amelogenin and tuftelin gene products during tooth development. J Histochem Cytochem 45:859–866 [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Fukumoto S, Yamada Y, Evans CA, Diekwisch TG, and Luan X. (2016). Ameloblastin, an extracellular matrix protein, affects long bone growth and mineralization. J Bone Miner Res 31:1235–1246 [DOI] [PubMed] [Google Scholar]
- 26.Diekwisch TG. (1998). Subunit compartments of secretory stage enamel matrix. Connect Tissue Res 38:101–111 [DOI] [PubMed] [Google Scholar]
- 27.Diekwisch TG. (2002). Pathways and fate of migratory cells during late tooth organogenesis. Connect Tissue Res 43:245–256 [PubMed] [Google Scholar]
- 28.Luan X. and Diekwisch TG. (2007). Vienna-Chicago: the cultural transformation of the model system of the un-opposed molar. Bioessays 29:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diekwisch TG. (2016). Oral Biology and Chicago. Evolution and Development 18:3–6 [DOI] [PubMed] [Google Scholar]
- 30.Melino G, Memmi EM, Pelicci PG. and Bernassola F. (2015). Maintaining epithelial stemness with p63. Sci Signal 8:re9. [DOI] [PubMed] [Google Scholar]
- 31.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F. and Loda M. (2000). p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 157:1769–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F. and De Luca M. (2001). p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 98:3156–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills PR, Davies RJ. and Devalia JL. (1999). Airway epithelial cells, cytokines, and pollutants. Am J Respir Crit Care Med 160(5 Pt 2):S38–S43 [DOI] [PubMed] [Google Scholar]
- 34.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C. and McKeon F. (1999). p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398:714–718 [DOI] [PubMed] [Google Scholar]
- 35.Blanpain C. and Fuchs E. (2007). p63: revving up epithelial stem-cell potential. Nat Cell Biol 9:731–733 [DOI] [PubMed] [Google Scholar]
- 36.Romano RA, Solomon LW. and Sinha S. (2012). Tp63 in oral development, neoplasia, and autoimmunity. J Dent Res 91:125–132 [DOI] [PubMed] [Google Scholar]
- 37.Ou HD, Löhr F, Vogel V, Mäntele W. and Dötsch V. (2007). Structural evolution of C-terminal domains in the p53 family. EMBO J 26:3463–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, Brugge JS. and Ellisen LW. (2006). p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 8:551–561 [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Moro A, Zhang JJ, Cheng W, Qiu W. and Kim PC. (2007). The role of Shh transcription activator Gli2 in chick cloacal development. Dev Biol 303:448–460 [DOI] [PubMed] [Google Scholar]
- 40.Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, et al. (2006). Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev 20:1028–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Singh S, Cherukuri P, Li H, Yuan Z, Ellisen LW, Wang B, Robbins D. and DiRenzo J. (2008). Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells. Stem Cells 26:1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Liu YF, Du YR, Mardaryev AN, Yang W, Chen H, Xu ZM, Xu CQ, Zhang XR, et al. (2013). Cbx4 regulates the proliferation of thymic epithelial cells and thymus function. Development 140:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, Yu M. and Tian W. (2013). An inductive signalling network regulates mammalian tooth morphogenesis with implications for tooth regeneration. Cell Prolif 46:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koyama E, Wu C, Shimo T, Iwamoto M, Ohmori T, Kurisu K, Ookura T, Bashir MM, Abrams WR, et al. (2001). Development of stratum intermedium and its role as a Sonic hedgehog-signaling structure during odontogenesis. Dev Dyn 222:178–191 [DOI] [PubMed] [Google Scholar]
- 45.Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA. and Thesleff I. (1999). Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol 147:105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawano S, Saito M, Handa K, Morotomi T, Toyono T, Seta Y, Nakamura N, Uchida T, Toyoshima K, et al. (2004). Characterization of dental epithelial progenitor cells derived from cervical-loop epithelium in a rat lower incisor. J Dent Res 83:129–133 [DOI] [PubMed] [Google Scholar]
- 47.Wöltgens JH, Lyaruu DM, Bronckers AL, Bervoets TJ. and Van Duin M. (1995). Biomineralization during early stages of the developing tooth in vitro with special reference to secretory stage of amelogenesis. Int J Dev Biol 39:203–212 [PubMed] [Google Scholar]
- 48.Štefková K, Procházková J. and Pacherník J. (2015). Alkaline phosphatase in stem cells. Stem Cells Int 2015:628368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakita M. and Hinrichsen K. (1980). Ultrastructure of the ameloblast-stratum intermedium border during ameloblast differentiation. Acta Anat (Basel) 108:10–29 [DOI] [PubMed] [Google Scholar]
- 50.Barron MJ. and Hinrichsen K. (1980). Ultrastructure of the ameloblast-stratum intermedium border during ameloblast differentiation. Acta Anat (Basel) 108:10–29 [DOI] [PubMed] [Google Scholar]
- 51.Matthiessen ME. and Mollgard K. (1973). Cell junctions of the human enamel organ. Z Zellforsch Mikrosk Anat 146:69–81 [DOI] [PubMed] [Google Scholar]
- 52.Kallenbach E. (1978). Fine structure of the stratum intermedium, stellate reticulum, and outer enamel epithelium in the enamel organ of the kitten. J Anat 126(Pt 2):247–260 [PMC free article] [PubMed] [Google Scholar]
- 53.Thesleff I, Barrach HJ, Foidart JM, Vaheri A, Pratt RM. and Martin GR. (1981). Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev Biol 81:182–192 [DOI] [PubMed] [Google Scholar]
- 54.Thesleff I, Jalkanen M, Vainio S. and Bernfield M. (1988). Cell surface proteoglycan expression correlates with epithelial-mesenchymal interaction during tooth morphogenesis. Dev Biol 129:565–572 [DOI] [PubMed] [Google Scholar]
- 55.Yoshiba K, Yoshiba N, Aberdam D, Meneguzzi G, Perrin-Schmitt F, Stoetzel C, Ruch JV. and Lesot H. (1998). Expression and localization of laminin-5 subunits during mouse tooth development. Dev Dyn 211:164–176 [DOI] [PubMed] [Google Scholar]
- 56.Ida-Yonemuchi H, Ohshiro K, Swelam W, Metwaly H. and Saku T. (2005). Perlecan, a basement membrane-type heparan sulfate proteoglycan, in the enamel organ: its intraepithelial localization in the stellate reticulum. J Histochem Cytochem 53:763–772 [DOI] [PubMed] [Google Scholar]
- 57.Kallenbach E. (1972). Granules in cisternae of the rough endoplasmic reticulum (RER) of preameloblasts and ameloblasts and a possible function of the RER in preameloblasts of rat incisor. J Ultrastruct Res 39:96–105 [DOI] [PubMed] [Google Scholar]
- 58.Skobe Z, Prostak KS. and Stern DN. (1989). Scanning electron microscopy of monkey secretory- and transitional-stage enamel organ cells. J Dent Res 68:1173–1181 [DOI] [PubMed] [Google Scholar]