Abstract
There is a need for straightforward, novel diagnostic and monitoring technologies to enable the early diagnosis of COPD and its differentiation from other respiratory diseases, to establish the cause of acute exacerbations and to monitor disease progression. We sought to establish whether technologies already in development could potentially address these needs. A systematic horizon scanning review was undertaken to identify technologies in development from a wide range of commercial and non-commercial sources. Technologies were restricted to those likely to be available within 18 months, and then evaluated for degree of innovation, potential for impact, acceptability to users and likelihood of adoption by clinicians and patients with COPD. Eighty technologies were identified, of which 25 were considered particularly promising. Biomarker tests, particularly those using sputum or saliva samples and/or available at the point of care, were positively evaluated, with many offering novel approaches to early diagnosis and to determining the cause for acute exacerbations. Several wrist-worn devices and smartphone-based spirometers offering the facility for self-monitoring and early detection of exacerbations were also considered promising. The most promising identified technologies have the potential to improve COPD care and patient outcomes. Further research and evaluation activities should be focused on these technologies.
Keywords: Chronic obstructive pulmonary disease, diagnosis, monitoring, new technology, horizon scanning
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally and presents a significant burden to patients, carers and health services worldwide.1,2 More than 1.5 million adults are known to be diagnosed with COPD in England and Wales, and a further 3 million adults are estimated to be living with undiagnosed COPD.3,4 Improving the care and outcomes for people with COPD is a priority for the National Health Service (NHS) in England, which aims to reduce premature mortality from respiratory disease, avoid unnecessary hospital admissions and improve the quality of life and support for patients with long-term conditions and their carers.5 However, several current issues remain in the diagnosis and monitoring of COPD, some of which could be resolved by technological developments or novel disease biomarkers.
Several authors have noted limitations in the use of spirometry to diagnose COPD due to differing guidelines as to what constitutes COPD, difficulties in technique encountered by frail or cognitively impaired patients, and a lack of awareness and knowledge in primary care leading to delayed diagnosis, especially in non-smoking subgroups.6–9 In addition, some commentators have proposed a need for improved diagnostic criteria where asthma and COPD coexist, observing that non-specialist clinicians frequently find it difficult to both diagnose and manage this situation,8,10,11 whilst others consider that additional emphasis should be placed on early detection.9,12,13 There is an unmet need for simple, accurate testing approaches that would enable early diagnosis and differentiation from other respiratory diseases.13 As part of the ongoing care of COPD, patient self-monitoring is increasingly being recognized as beneficial, with evidence indicating this may improve health-related quality of life and the recognition and management of acute exacerbations,14,15 and self-monitoring is recommended in guidelines from the National Institute for Health and Care Excellence (NICE).2 However, the most effective approach to self-management in COPD is not known.14 Finally, several commentators identify the need to determine the cause of acute exacerbations as a research need; this would allow treatment to be tailored to the underlying pathology.16,17 Correspondingly the NICE Database of Uncertainties about the Effects of Treatments includes questions about the appropriate use of corticosteroids and antibiotics for the management of acute exacerbations of COPD.18,19 As only around half of exacerbations are associated with a bacterial infection and a only a third of exacerbations demonstrate eosinophilic inflammation, the use of these agents may expose many patients to significant adverse effects and the potential development of antibiotic resistance for no apparent benefit.20,21 There is an unmet need for novel diagnostic or monitoring approaches, particularly those available at the point of care, to establish the cause of acute exacerbations and to monitor the progression of the disease.
Horizon scanning systems, or early awareness and alert systems, aim to identify significant health technologies prior to launch that may require further assessment or planning prior to adoption.22,23 A horizon scanning review uses systematic methods to identify, filter, prioritize and present early information on all new and emerging technologies relevant to the area of interest, however, it does not assess the evidence supporting the identified technologies, validate the claims made about them by developers or comprehensively evaluate the potential impact they may have on clinical care.24,25 We sought to identify new and emerging technologies already in development for the diagnosis or monitoring of COPD that could potentially address the unmet research and clinical practice needs identified above, and then use the views of clinical experts and patients with COPD to establish which of these technologies could be considered the most innovative, acceptable and likely to make an impact on patients and health services in the future. This horizon scanning review forms part of the National Institute for Health Research Horizon Scanning Research and Intelligence Centre work programme. The full report is available on the Centre’s website (http://www.hsric.nihr.ac.uk/news/what-does-the-future-hold-for-copd-diagnostic-and-monitoring-technologies/) and will be used to inform healthcare policymakers, commissioners, researchers, research funders, clinicians and patients about new technologies ‘on the horizon’ for the management of COPD. Ethical approval was obtained from the University of Birmingham’s Internal Ethical Review Committee (reference: IERC2014-5/C1/SF/07).
Methods
Identification and filtration of technologies
Between January and March 2015, potential technologies for the diagnosis and monitoring of COPD were identified by searching relevant online databases and other websites (Table 1) using pre-specified search terms (Table 2) and eliciting suggestions from clinical experts. Initial search findings were filtered to include only those technologies which were new (already licensed/CE marked or launched in the United Kingdom for ≤24 months – in the launch, early post-marketing or early diffusion phase) or emerging (in development and expected to be licensed/CE marked in the next 18 months – in late phase clinical trials, prelaunch or pre-marketing phase), and results were further prioritized to include only those technologies that demonstrated, or claimed to demonstrate, some degree of innovation (either a completely novel technology, with no direct comparators already marketed, or a significant development from existing marketed products).
Table 1.
Search protocol – pre-specified identification sources.
| Source name | Website link |
|---|---|
| Published medical literature | |
| Medline & Medline in Progress, & EMBASE | Accessed via http://www.elibrary.bham.ac.uk/ |
| PubMed.gov | http://www.ncbi.nlm.nih.gov/pubmed |
| The Cochrane Library | http://www.cochranelibrary.com/ |
| ZETOC British Library Database | http://zetoc.mimas.ac.uk/ |
| HTA agencies | |
| AHRQ Healthcare Horizon Scanning System | Status update reports and potential high impact reports via http://www.effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=881 |
| CADTH | http://www.cadth.ca |
| ECRI Institute | http://www.ecri.org |
| EuroScan International Network | http://euroscan.org.uk/ |
| NIHR Horizon Scanning Research & Intelligence Centre database | http://www.hsric.nihr.ac.uk/ |
| Clinical trial registries and research funding databases | |
| ClinicalTrials.gov | http://clinicaltrials.gov/ |
| COPD clinical research network | http://www.copdcrn.org/ |
| Current-controlled trials | http://www.controlled-trials.com/ |
| MRC-funded research | http://www.mrc.ac.uk/research/funded-research/ |
| NIHR Biomedical Research Centres and Units annual dataset (2014) | Not applicable |
| NIHR Evaluation Trials and Studies Project portfolio | http://www.nets.nihr.ac.uk/projects?collection=netscc&meta_P_sand=Project |
| UKCRN portfolio database | http://public.ukcrn.org.uk/search/ |
| World Health Organization International Clinical Trials Registry Platform | http://www.who.int/ictrp/en/ |
| Specialist media and commercial research and development databases | |
| AdvamedSmartbrief | https://www2.smartbrief.com/news/ADVAMED/index.jsp |
| Clinica | http://www.clinica.co.uk/ |
| Fierce Devices | http://www.fiercemedicaldevices.com/ |
| GlobalData Medical | http://globaldata.com/medical/Login.aspx?ReturnUrl=%2fmedical |
| MedGadget | http://www.medgadget.com/ |
| Medical News Today | http://www.medicalnewstoday.com/ |
| Regulatory authorities | |
| USFDA approvals | http://www.fda.gov/newsevents/productsapprovals/default.htm |
| Specialist journals | |
| American Journal of Respiratory and Critical Care Medicine | http://www.atsjournals.org/journal/ajrccm |
| COPD: Journal of Chronic Pulmonary Obstructive Disease | http://informahealthcare.com/loi/cop |
| European Respiratory Journal | http://erj.ersjournals.com/ |
| Expert Reviews of Respiratory Medicine | http://informahealthcare.com/loi/erx |
| International Journal of Chronic Obstructive Pulmonary Disease | http://www.dovepress.com/international-journal-of-chronic-obstructive-pulmonary-disease-journal |
| Thorax | http://thorax.bmj.com/ |
| Professional and patient groups | |
| American Thoracic Society | http://www.thoracic.org/ |
| British Lung Foundation | http://www.blf.org.uk/Home |
| British Thoracic Society | https://www.brit-thoracic.org.uk/ |
| COPD Foundation | http://www.copdfoundation.org/ |
| European Lung foundation | http://www.europeanlung.org/en/ |
| European Respiratory Society | http://www.ersnet.org/ |
| Primary Care Respiratory Society | http://www.pcrs-uk.org/ |
| Royal College of Physicians | https://www.rcplondon.ac.uk/ |
Table 2.
Search protocol – pre-specified search terms.
| Condition and synonyms | Chronic obstructive pulmonary disease |
| COPD | |
| Chronic obstructive airways disease | |
| COAD | |
| Chronic obstructive lung disease | |
| Chronic obstructive respiratory disease | |
| Chronic airflow limitation | |
| Emphysema | |
| Chronic bronchitis | |
| Diagnosis | Diagnosis |
| Recognition | |
| Detection | |
| Identification | |
| Test | |
| Point-of-care test | |
| Monitoring | Monitoring |
| Surveillance | |
| Review | |
| Timeframe | New |
| Emerging |
External input
Clinical experts and patients with COPD were recruited as expert external advisors to the review. Clinical experts were identified from initial scoping and literature searches and the NICE COPD guideline development and review panel. Fifteen clinical experts were contacted via email and asked to act as review advisors, five of whom subsequently agreed, resulting in an expert panel comprising consultant respiratory physicians, a general practitioner with a special interest in respiratory disease and a clinical nurse specialist. Clinical experts were emailed the table of identified technologies and asked to provide their views on each technology’s level of innovation, potential for impact (on patients and the delivery of health services), potential barriers to adoption and any further comments. They were also asked to identify any additional relevant technologies or relevant research they were aware of that had not already been identified.
Patients with COPD were identified from the patient advisory group to the existing Birmingham Lung Improvement StudieS (BLISS) research programme.26 Members of the group were approached using a standard email or letter informing them of the review and inviting them to participate. Three patients subsequently agreed and were sent the table of identified technologies and asked to provide their views on each technology’s level of innovation, potential for impact (on patients and the delivery of health services) and acceptability to users, along with any further comments.
Review output
All identified new and emerging technologies for the diagnosis or monitoring of COPD were included in the final report. Clinical expert and patient views were used to identify those technologies that were considered to demonstrate a high degree of innovation and/or a significant potential for impact and adoption by the NHS in England.
Results
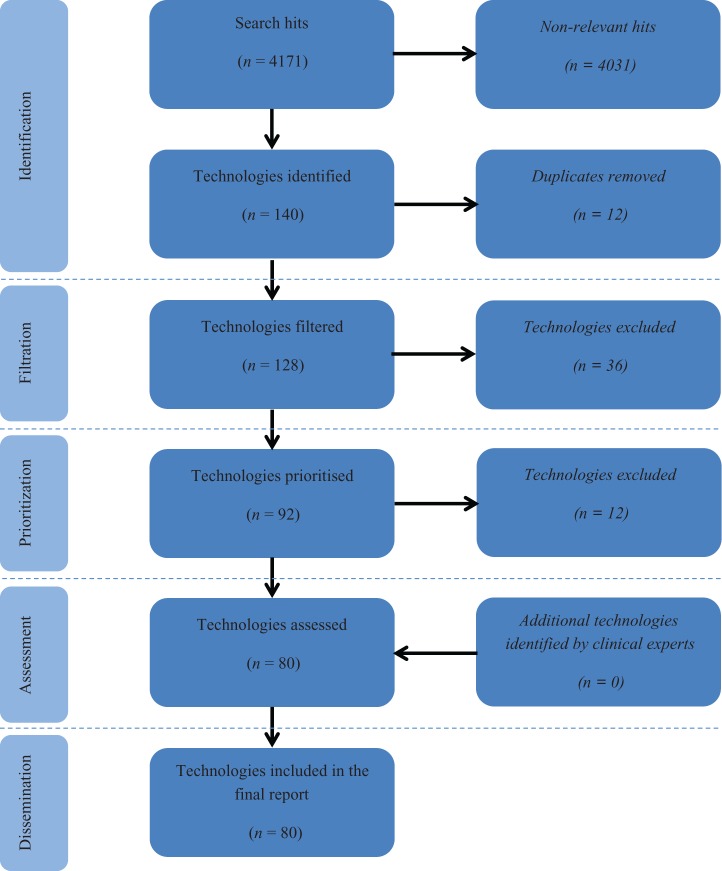
Eighty new and emerging technologies for the diagnosis and monitoring of COPD were identified (Table 3, Figure 1). These included 31 biomarkers, 21 telehealth technologies, 6 wearable technologies, 6 imaging technologies, 4 vital sign monitors, 4 questionnaires, 3 spirometers and 5 additional other technologies. Clinical experts and patients provided comments on all identified technologies, providing useful insights into the innovativeness, potential impact, stage of development and probable timeframe for each technology. Of the technologies identified, 25 (31.3%) were considered particularly promising, demonstrating a high degree of innovation and with a significant potential for impact and adoption by the NHS according to expert and patient comments (Table 4).
Table 3.
Technologies identified by the review.
| Technology type | Technology name | Brief description | Developer | Stage of development |
|---|---|---|---|---|
| Wearable technologies | WHolter | 24 hour ambulatory digital device intended for tracking wheeze and cough. | iSonea Ltd., Israel. | CE marked. FDA approved. |
| Breeze@home | Remote monitoring jacket. | Deep Breeze Ltd., Israel. | Estimated launch May 2015. | |
| Wrist-based pulse oximeter | Wireless, wrist worn, pulse oximeter. | Oxitone Medical Ltd., Israel. | Patent pending. Estimated to reach market by 2015. | |
| VigilCare | Wireless monitoring device detecting step activity and vital signs remotely in real time. | Agali Technologies, Inc., USA. | Estimated launch October 2017. | |
| BuddyWOTCH | Wearable smartwatch intended for home monitoring of walking, oxygenation, temperature, chronicle (image capture of medication, food and liquids) and heartrate. | Aseptika Ltd., UK. | Estimated launch October 2019. The Company intends to deliver its first production unit to NHS clinical partners and beta test volunteers by the end of 2015. | |
| RESpeck | Non-invasive, wireless respiration and movement monitor. | Centre for Speckled Computing, University of Edinburgh, UK. | Pilot trials complete. | |
| Blood biomarkers | Blood eosinophil count | Biomarker to direct corticosteroid therapy during COPD exacerbations. | Glenfield Hospital, UK. | BEAT: COPD study. |
| Aα-Val360 | Biomarker for the identification of patients with early COPD at risk of progression. | Queen Elizabeth Hospital, UK. | In pilot/feasibility trial. | |
| CRP point-of-care test | Rapid point-of-care test to direct antibiotic prescribing in exacerbations of COPD. | Cardiff University, UK. | In trial since July 2014, due to publish trial in February 2018. | |
| Plasma fibrinogen biomarker | Biomarker test to identify individuals at an increased risk of COPD exacerbation. | University of Kentucky, USA. | Qualification Package submitted to the FDA for plasma fibrinogen as a new drug development tool. | |
| Inflammatory biomarker panel | Inflammatory biomarkers to established predictive factors aiming to improve mortality predictions. | GlaxoSmithKline (GSK) plc. | Test of hypothesis trial (ECLIPSE study). | |
| CRP, fibrinogen and leukocyte count inflammatory biomarkers | Inflammatory biomarker panel to monitor for exacerbations of COPD. | Copenhagen University Hospital, Denmark. | Test of hypothesis trial. | |
| PCT | Biomarker test to direct COPD exacerbation treatment. | Holbæk Hospital, Denmark. | Phase IV. | |
| Serum Procalcitonin | Rapid biomarker test for the diagnosis of a COPD exacerbation. | Ramathibodi Hospital, Bangkok. | Proof of concept study. | |
| Heat shock protein 27 biomarker | Biomarker test for the diagnosis of early COPD before it is detectable by lung function tests. | University Department of Surgery at MedUni, Vienna. | Test of hypothesis trial. | |
| Endothelial microparticle biomarkers | Biomarker for the identification of smokers with early emphysema. | Weill Cornell Medical College, USA. | Test of hypothesis trial. | |
| α-2 macroglobulin, haptoglobin, ceruloplasmin and haemopexin biomarkers | Diagnostic biomarker for COPD. | University of Newcastle, UK. | Proof of concept study. Estimated launch January 2018. | |
| ARHGEF1 (Rio guanine nucleotide Exchange factor 1) biomarker | COPD diagnostic and prognostic biomarker. | Lenimen, USA. | Estimated launch May 2016. | |
| Autoantibody Antigen Array – COPD | COPD diagnostic and prognostic biomarker. | University of Colorado, USA. | Estimated launch May 2016. | |
| AlphaKitQuickScreen Device | Point-of-care device intended for the diagnosis of α-1-antitrypsin deficiency. | Grifols, Spain. | CE marked. | |
| Serum uric acid biomarker | Low-cost biomarker for the prediction of an exacerbation of COPD. | University of Athens, Greece. | Test of concept study. | |
| Hydrogen sulphide Biomarker | Biomarker for the diagnosis of a COPD exacerbation. | N.R.S.Medical College, India. | Proof of concept study. | |
| Neutrophil/lymphocyte ratio | A widely available and cost-effective biomarker to determine the cause of an exacerbation of COPD. | Athens Medical School. | Proof of concept study. | |
| Sputum biomarkers | Home use sputum test | Home use test detecting the level of activity of nine respiratory pathogens to provide advance warning of an exacerbation. | Aseptika Ltd., UK. | Phase III trials. |
| Sputum rheology | Low-cost, rapid, easy to access and non-invasive biomarker for COPD diagnosis. | TA Instruments, USA. | Technology is already available but is not currently used for this indication. | |
| Biomarker panel: SP-A, sRAGE, MPO and NGAL | Biomarker panel to diagnose COPD-Asthma overlap. | University of Helsinki, Finland. | Proof of concept study. | |
| FTIR monitoring | Handheld device using infrared spectroscopy to diagnose and monitor COPD. | Glyconics, UK. | Large validation trial. | |
| Saliva biomarkers | COPDSPOC | Point-of-care biosensor for COPD diagnosis. | University Hospital of North Staffordshire, UK. | Pilot/feasibility trial. |
| Saliva CRP and PCT biomarkers | Monitoring biomarker for the detection of an exacerbation of COPD. | University Hospital of North Staffordshire, UK. | Test of concept. | |
| Breath biomarkers | VOC Diagnostic Assay | Immunodiagnostic assay intended for the diagnosis of COPD. | XAir Diagnostics B.V. | Estimated launch April 2016. |
| Exhaled VOCs | Breath test for the diagnosis and monitoring of COPD. | Markes International Ltd., UK. | Currently in trial for application of the existing technology for use in COPD. | |
| 1 3C-Methacetin Breath Test | Breath test for the diagnosis and monitoring of COPD. | Hadassah Medical Centre, Israel. | Phase II/III trial. | |
| SpirometrixFenom™ Point-of-care test | Highly sensitive point-of-care test for the diagnosis of COPD and prediction of an acute exacerbation of COPD. | Spirometrix Inc. | Technology is still under development. | |
| Breath PulmoHealth ‘Check’ COPD detector | Diagnostic COPD breath test. | Akers Biosciences, Inc., USA. | Estimated CE mark September 2016. Estimated Europe launch December 2016. Estimated USA launch March 2017. | |
| Other biomarkers | Metabolomic Biomarker Assay – COPD | Body fluid or tissue biomarker–based assay intended for diagnosis of COPD. | Biocrates Life Sciences AG, Austria. | Estimated launch May 2017. |
| l-Lactate dehydrogenase biomarker | Bronchial aspirate biomarker for the diagnosis of COPD exacerbations. | University Hospital of Split, Croatia. | Proof of concept study. | |
| Hyaluronic acid and heparin sulphate biomarkers | Bronchial aspirate biomarker for the diagnosis of COPD exacerbations. | University Hospital Basel, Switzerland. | Proof of concept study. | |
| Telehealth technologies | Care Innovations Guide | Online interface for the monitoring and patient education. | Intel-GE Care Innovations LLC., USA. | Estimated launch date May 2011. |
| Intel® Health Guide PHS6000 | A touch screen, in-home patient device and online management interface for monitoring and patient–doctor communication. | Intel Corporation. | In phase III trial. | |
| AlereHomeLink | Touch screen device and interface for the monitoring of symptom severity. | Alere Connect, USA. | FDA approved Jan 2014 and CE marked. | |
| Commander Flex | Interactive home telehealth wireless tablet device intended for patient monitoring. | Cardiocom, LLC., USA. | FDA approved. | |
| MedVizer T400 Home Health Monitor | Touch screen, tablet device for the monitoring of symptoms, vital signs and for patient education. | Visual Telecommunication Network, Inc., USA. | Phase II trials | |
| CHROMED monitoring system | Home monitoring system for vital signs and symptoms. | University of Lincoln, UK. | Pilot/feasibility trial. | |
| eHealth Diary | A digital pen enabling for symptom monitoring. | Phoniro systems, Sweden. | Pilot/feasibility trial. | |
| i-DSMP (Internet-based Dyspnoea Self-management Program) | An interface enabling the monitoring of symptoms and exercise. | University of California, USA. | Phase II trial. | |
| Telephone-linked computer–COPD | A computer-based telecommunications system that can monitor, educate and counsel patients. | VA Boston Health Care System, USA. | Phase IV. | |
| InterSpace: Web-based supported self-management programme | COPD self-management website offering doctor/nurse–patient videoconferences. | University hospitals of Leicester, UK. | Feasibility study. | |
| ADAPT | Post-discharge-staged telemonitoring programme. | Prince Phillip Hospital, Llanelli, UK. | Pilot trial. | |
| SmartScope System | A remotely used software application system intended for remote patient monitoring. | Futura Mobile Health, LLC., USA. | Pilot study. Estimated launch May 2016. | |
| GaitTrack App | A smartphone app used in conjunction with a pulse oximeter to monitor gait, heart rate and blood oxygenation. | University of Illinois, USA. | Pilot study. | |
| Respiratory virtual clinics | Multidisciplinary virtual clinics between primary and secondary care to monitor patients with COPD. | King’s Health Partners Integrated Respiratory Team, UK | Feasibility study completed in 2014. | |
| mACEWS (mobile Acute Care Early Warning System) | A mobile monitoring solution to detect the early signs of an exacerbation of COPD. | Airstrip Technologies Inc., USA. | No trials found. | |
| Smart inhaler | Inhaler sensor providing remote monitoring of drug use and therefore prediction of exacerbation. | Propeller Health, USA. | FDA approved. Estimated going to market early 2015. | |
| Health-e-Connect System | Internet-based patient monitoring system intended for remote nebulizer compressor monitoring. | ALR Technologies Inc., USA. | Estimated launch March 2016. | |
| CTC-Actiwise | An algorithmic system intended for the early detection of potentially upcoming exacerbations in patients with COPD. | CareTelCom AB, Sweden. | Estimated launch May 2017. | |
| Semi-automated cough classifier | Daily cough monitoring using ambient sound recording system and a novel semi-automated cough classifier as a marker for exacerbations. | Hull York Medical School, UK. | Pilot study. | |
| Microsoft@ Kinect™-based telemedicine programme | A Microsoft Kinect-based telemedicine system used in order to monitor patients with COPD. | HUA Txagorritxu, Spain. | Pilot study. | |
| Imaging technologies | PRM™ (Parametric response map) COPD | Software capable of diagnosing COPD types on CT scan. | Imbio, USA. | FDA 510(K) clearance in 2014. |
| Mobile SPECTImaging | Imaging sensitive to early changes in COPD and has the possibility of identifying comorbid disease. | Professor Brian Hutton, UK. | Prototype to be installed early 2016. | |
| CT perfusion scan | Imaging capable of the diagnosis of early COPD and those at risk of disease progression. | University of Edinburgh, UK. | Pilot/feasibility trial. | |
| Quantitative CT scans | Imaging able to monitor for the likelihood of a COPD exacerbation. | National Jewish Health, USA. | Technology widely available but not widely used for this indication. | |
| Transthoracic Parametric Doppler/Pulsed Doppler Ultrasound System | A non-invasive and non-imaging ultrasound Doppler and signal processing technology capable of extracting parametric information for the diagnosis of COPD. | EchoSense Ltd., Israel. | Proof of concept study | |
| Human Lung Regional Ventilation Defect Severity Measured by Fluorine-19 Gas MRI | MRI assessment for the monitoring of COPD disease progression. | Duke University Medical Center, USA. | Phase II trial. | |
| Vital sign monitoring technologies | EverOn™ heart and respiration monitor | Disposable sensor placed under a mattress for the detection of respiratory and heart rate, for the detection of COPD exacerbations. | EarlySense Ltd., USA. | FDA approved and CE marked. |
| Respiratory Holter – COPD | A device to detect respiratory rate through QT interval monitoring. | Nicrem S.r.l., Italy. | Preliminary validation stages. Estimated launch February 2018. | |
| Nonin Bluetooth® Smart Model 3230 Finger Pulse Oximeter | A finger pulse oximeter with Bluetooth Smart wireless technology. | Nonin Medical, Inc., USA. | FDA approved. Launched in 2013. | |
| Capno-Pulse | A continuous, non-invasive respiratory monitoring device intended for the monitoring of COPD. | Neetour Medical Ltd., Israel. | Estimated launch March 2018. | |
| Spirometry technologies | MySpiroo | Handheld portable spirometer which monitors lung function via a smartphone app. | MySpiroo, Poland. | Expected launch 2015. |
| MIR Smart One® | Smartphone-based spirometer, connecting wirelessly to an app. | MIR (Medical International Research), Italy. | Launched November 2014. | |
| Smartphone spirometer | Smartphone-based spirometer, utilizing global positioning system location and positioning to monitor COPD. | resp.io, USA. | No trials found. | |
| Questionnaire-based technologies | DECAF scoring system | A simple yet effective predictor of mortality in patients hospitalized with an exacerbation of COPD. | North Tyneside General Hospital, UK. | Validation trial. |
| Asthma-COPD Overlap Syndrome Questionnaire | Questionnaire for the diagnosis of COPD where Asthma co-exists. | US GSK Clinical Trials Call Centre, USA. | Phase II trial. | |
| COPD screening questionnaire | Smoking status and symptom questionnaire for the identification of patients in need for COPD investigations. | Bispebjerg University Hospital, Denmark. | Effectiveness evaluation study. | |
| DOSE index | Multicomponent index with the potential to predict future outcomes in patients with COPD. | Radboud University Nijmegen, Netherlands. | Validation study. | |
| Other technologies | SPPB | Short walking test for the monitoring of COPD. | Royal Brompton & Harefield NHS trust, UK. | Effectiveness study. |
| SonicSense | An ultrasonic sensor for use of COPD monitoring. | The Technology Partnership (TTP), UK. | Prototype stage. | |
| ResPOC point-of-care testing for respiratory viruses | Point-of-care test to detect viral causes of COPD exacerbations. | Ateknea Solutions, Hungary. | Expected commercial launch 2016. | |
| HIRA-TAN semi-quantitative PCR | A rapid, accurate, easily performed PCR test to identify causative bacteria in COPD exacerbations. | Saitama Medical University, Japan. | Validation study. | |
| mPCR | A sensitive, rapid mPCR test for the detection of respiratory viruses from tracheal aspirate and nasal pharyngeal aspirate samples. | All India Institute of Medical Sciences, India. | Proof of concept study. |
BEAT: COPD -: Biomarkers to Target Antibiotic and Systemic Corticosteroid Therapy in COPD; USFDA: US Food and Drug Administration; COPD: chronic obstructive pulmonary disease; NHS: National Health Service; CRP: C-reactive protein; PCT: procalcitonin; FTIR: Fourier transform infrared; SPOC: saliva point of care; VOC: volatile organic compounds; MRI: magnetic resonance imaging; DOSE: dyspnoea, obstruction, smoking, exacerbation; mPCR: multiplex polymerase chain reaction; SPPB: short physical performance battery; CT: computed tomography; ResPOC: Respiratory Virus Point-Of-Care; DECAF: Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation.
Figure 1.
Identification, filtration and prioritization of technologies included in the horizon scanning review. Adapted from the PRISMA flow diagram for systematic reviews.30
Table 4.
Promising technologies – those considered to demonstrate a high degree of innovation, potential for impact, and potential for adoption by the NHS.
| Technology type | Promising technologies |
|---|---|
| Wearable technologies | Wrist-based pulse oximeter |
| BuddyWOTCH | |
| Biomarker technologies | |
| Diagnostic | Aα-Val 360 biomarker |
| Biomarker panel: SP-A, sRAGE, MPO and NGAL for asthma-COPD overlap syndrome | |
| FTIR monitoring | |
| COPD-SPOC sensor | |
| Monitoring | Home use sputum test |
| To determine the cause of an acute exacerbation | Blood eosinophil biomarker |
| CRP point-of-care test | |
| PCT | |
| Telehealth technologies | Virtual Medical Assistant Version 2.0 |
| Commander Flex | |
| MedVizer T400 Home Health Monitor | |
| ADAPT | |
| SmartScope System | |
| Respiratory virtual clinics | |
| Spirometry technologies | MySpi-roo |
| MIR Smart One® | |
| Smartphone spirometer | |
| Questionnaire technologies | DECAF scoring system |
| DOSE index | |
| Other technologies | SPPB |
| ResPOC point-of-care testing for respiratory viruses | |
| HIRA-TAN semi-quantitative PCR | |
| mPCR | |
FTIR: Fourier transform infrared; SPOC: saliva point of care; COPD: chronic obstructive pulmonary disease; DOSE: dyspnoea, obstruction, smoking, exacerbation; mPCR: multiplex polymerase chain reaction; ADAPT: After DischArge Pulmonary Telehealth; CRP: CRP: C-reactive protein; ResPOC: Respiratory Virus Point-Of-Care; DECAF: Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation.
Wearable technologies
All identified wearable technologies were intended to monitor stable COPD through devices worn either across the chest or on the wrist. Whilst devices monitoring respiratory rate, wheeze, blood oxygenation and/or temperature are already available, several new technologies enabled self-monitoring at home with remote access by clinicians, though the feasibility of multiple clinicians interpreting large volumes of recorded data was questioned. Patients commented that users may be unwilling to wear a ‘cumbersome’ looking device and were concerned about ‘having the restriction of a band around [their] chest’. The wrist-based pulse oximeter and BuddyWOTCH devices could offer alternatives to existing fingertip devices, providing continuous monitoring without limiting movement of the user’s hands. In addition, BuddyWOTCH captures physical activity, temperature and heart rate. Both technologies were considered promising, with patients keen to try a more compact, watch style device. Experts considered them ‘potentially useful…for longer term monitoring…in vulnerable groups…[and] for pulmonary rehabilitation’. Concerns were expressed regarding the reliability of wrist-based monitoring; however, a potentially large impact could be expected if these devices demonstrated acceptable performance.
Biomarker technologies
Thirty-one biomarker tests were identified and included in the report, the majority based on blood samples. Alternative samples included sputum, bronchial aspirate and innovatively, saliva and volatile organic compounds from breath samples. Most biomarkers were intended for the diagnosis of COPD, but experts dismissed the majority of blood-based diagnostic biomarkers as being too early in development or ‘possibly as a research tool’ with no demonstrated clinical value, despite some being considered highly innovative. Aα-Val360 was the most promising biomarker in this category, having been proposed as a method to identify patients with early COPD at risk of progression. Experts commented that it was ‘highly innovative…targeting high risk individuals’. Fourier transform infrared spectroscopic monitoring used sputum to rapidly differentiate COPD from other respiratory conditions and determine the risk of an imminent exacerbation; experts commented that this was ‘interesting and if effective then impact and adoption [are] possibly significant’. A further sputum-based biomarker panel to diagnose asthma-COPD overlap was thought to be ‘of interest in secondary care clinics’, whilst experts considered COPD-saliva point of care (SPOC), a novel saliva-based point-of-care test for the diagnosis of COPD incorporating three biomarkers, to have the ‘potential to change practice’.
Several inflammatory blood biomarkers were proposed for the monitoring of COPD for risk of an acute exacerbation. However, fibrinogen and C-reactive protein (CRP) were noted to be ‘non-specific indicators of inflammation’ and therefore ‘unlikely to be much impact or …[high adoption]’. In contrast, procalcitonin was thought to be a ‘more specific marker of infection’. Researchers claimed it was able to differentiate between a bacterial and nonbacterial cause of acute exacerbations, prompting an expert to state ‘…a strong chance of high impact (more appropriate exposure to antibiotics) and adoption’. The Home Use Sputum Test may provide advance warning of an acute exacerbation in the community and experts were very positive, commenting ‘this would be useful and may help identify pathogens early so treatment can be commenced’, and highlighting the potential to ‘reduce antibiotic use’, though also noting ‘identifying a bacterium does not always mean that the cause of symptoms is identified’. Patients commented on its potential to ‘reassure patients’. Other technologies aimed to identify the cause of acute exacerbations in order to better direct specific management. Experts commented that the use of peripheral blood eosinophil count as a biomarker to direct corticosteroid therapy was ‘worth exploring more’, though impact was judged to be ‘unclear, as most exacerbations are in the community and [this biomarker] will need a blood test’. The CRP POC test was considered innovative, providing an accessible fingerprick test in community settings. One expert remarked ‘yes to innovation, yes to potential impact and adoption is a strong possibility if shown to help decision making’.
Telehealth technologies
Telehealth is a popular area of development in technologies for chronic diseases and represented a large proportion of all technologies identified. Many devices were in a late stage of development from established commercial developers and had already obtained a CE mark, making them more likely to come to market within the specified timeframe. However, telehealth was generally viewed negatively by the clinical experts, with comments aimed at the category as a whole rather than in-depth review of each technology. Experts cited a UK multicentre trial of touchscreen telemonitoring for COPD27 and a systematic review and meta-analysis of telehealth for COPD,28 commenting on the current high estimates for cost-effectiveness of similar technologies and the lack of evidence that they improve quality of life or reduce mortality. In addition, experts expressed concern about the burden on clinicians, saying ‘the problem with this kind of technology is that you need a healthcare professional to look at the data’.
Imaging technologies
The majority of imaging technologies were considered highly innovative, but clinical experts believed their use would be restricted to research or specialist centres, so that uptake was likely to be very limited, and these technologies had little potential for impact across the whole of the NHS.
Vital sign monitoring technologies
Four devices offered novel methods for monitoring COPD. Both EverOn and the Respiratory Holter technologies monitored respiratory rate, with one expert noting ‘the accurate long-term measurement of respiratory rate in hospital/home may be useful’. However, neither were particularly novel and experts were unsure whether they would be ‘useful in the real world’. Similarly, Capno-Pulse received mixed comments from experts, with some identifying a role for improved non-invasive arterial blood carbon dioxide monitoring and others unclear whether it had potential for impact. However, patients were generally more positive, saying they would be willing to try these devices.
Spirometry technologies
These devices use a smartphone mounted, handheld device for home monitoring of COPD. Most clinicians are aware that monitoring current spirometric parameters does not reliably detect deterioration or exacerbation in COPD, though changes may be evident in asthma and potentially asthma-COPD overlap.2 However, comments were mostly encouraging, with good potential for adoption when used in conjunction with training and appropriate follow-up. Patients considered these technologies ‘could have [a] considerable impact’ and were ‘definitely’ acceptable to users. Two devices incorporate Bluetooth technology, however, the additional impact of this connectivity was thought to be limited due to the requirement for an interpreting clinician and patients’ ability to respond to the results.
Questionnaire-based technologies
Questionnaires were considered acceptable by patients providing they were not too long as ‘patients do get tired of filling in forms’. Two questionnaires aimed to monitor the severity of exacerbations in order to inform clinical decisions regarding care. The Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation (DECAF) score was considered a ‘useful innovation to predict safe early discharge’, whilst experts disagreed about the Dyspnoea, Obstruction, Smoking, Exacerbation (DOSE) index, with one noting that it is ‘well validated…difficult to see why not used more’ and another commenting ‘not clear to me that it is any advance over existing risk assessment tools’. Two questionnaires were aimed at COPD diagnosis. The COPD screening questionnaire includes smoking history and symptoms and aims to facilitate early diagnosis. Again, experts were divided in their opinions. One commented it was ‘a useful way to identify and encourage people to get spirometry’ whereas another said it was ‘not novel, tools already exist’.
Other technologies
Three technologies aimed to diagnose the cause of acute exacerbations in order to direct treatment. The developer of Respiratory Virus Point-Of-Care (ResPOC), a point-of-care test, claims it is capable of identifying respiratory viruses within 1 hour, with an equivalent accuracy to that of standard laboratory tests. Multiplex-polymerase chain reaction uses similar technology to diagnose viral infections using samples of tracheal or nasal aspirate, whilst HIRA-TAN may provide rapid and sensitive identification of bacterial pathogens. All of these three similar technologies received positive comments from experts, noting they were ‘innovative’ with high ‘potential for impact and adoption’.
Discussion
We identified a large number of new and emerging technologies for the diagnosis and monitoring of COPD, almost a third of which were considered to be innovative with significant potential for impact on patients and health services and be appropriate for adoption by the NHS. The technologies included in this horizon scanning review broadly addressed the issues identified as key research and unmet practice needs. Novel biomarkers offered a potential future alternative approach to early diagnosis and the differentiation from other respiratory diseases, including several based on sputum or saliva samples with results available at the point of care. Experts were less favourable about non-COPD-specific biomarkers of inflammation, though plasma fibrinogen has since become the first COPD biomarker to receive Food and Drug Administration approval for use in interventional clinical trials.29 Many technologies offered the potential to facilitate patient self-monitoring, including vital sign monitors (including wearable technologies), telehealth systems, smartphone-based spirometers and biomarkers, with a home-use sputum test potentially allowing advance warning of an acute exacerbation. Patients emphasized their preference for compact, simple, easy to use and portable monitoring devices that did not interfere with daily life, so that wrist-worn devices were preferred over vest-like wearable devices. Importantly, six innovative technologies were identified with the potential to establish the cause of an acute exacerbation, two of which offered this as at the point of care. These could enable treatment of acute exacerbations to be tailored to the underlying pathology, potentially avoiding the unnecessary use of antibiotics and corticosteroids.
The review used an established horizon scanning review methodology to systematically identify technologies.22,24 The approach aims to ensure that all technologies appropriate to the review are identified, and this is facilitated by the use of multiple online sources, including specialist media, health technology assessment organizations, regulatory authorities, research funders, subscription-only commercial research and development databases, clinical trial registries and professional and patient groups, in addition to bibliographic databases of published medical literature and specialist journals. However, it is not possible to claim that all possible technologies have been identified as this is dependent on commercial, media and academic reporting, though reassuringly clinical experts did not know of any relevant technologies in development that had not already been identified. A particular limitation of horizon scanning reviews is the restricted information on each technology upon which to base inclusion/exclusion, filtration/prioritization and assessment decisions. It is common for there to be limited publicly accessible information and scientific data available for technologies in development or the early launch stage, and we made no attempt to validate developers claims for their products, all of which require further clinical validation using patient-relevant outcomes and information on costs prior to adoption.
The views of clinical experts and patients with COPD provided essential insights into the place of identified technologies in COPD management and the acceptability of individual technologies to users. For some technologies, there was disagreement as to their potential impact, reflecting the individual’s particular perspective, experience of similar developments and local pathways of care. This was important, as we sought consensus before labelling a technology as particularly promising. Telehealth was a particular source of disagreement in this review, with technologies receiving generally negative comments from clinical experts, citing impracticality, the existing evidence base and cost as a barrier to use, whereas patients were broadly in favour of devices that enabled them to self-monitor and communicate with clinicians. Experts also noted the lack of evidence that variables such as cough frequency or inhaler use were associated with the risk of an acute exacerbation, and that clinical trials of such systems typically measured success in terms such as patient satisfaction, as opposed to conventional clinical parameters, admissions or healthcare utilization. In a field becoming increasingly saturated with similar products, yet experiencing little adoption by the NHS, it seems unlikely that telehealth will become part of routine COPD care until it is shown to improve patient outcomes and a feasible cost-benefit balance is achieved.
Conclusion
This review identified several innovative technologies with the potential for impact in a disease with a significant burden in the United Kingdom and worldwide. The most promising technologies have the potential to improve the early diagnosis of COPD, facilitate patient self-monitoring and establish the cause of acute exacerbations, potentially at the point of care. These technologies have the potential to contribute to addressing key priorities in the NHS Outcomes Framework, namely reducing premature deaths from respiratory disease, enhancing the health-related quality of life of patients with COPD and the quality of life of their carers, ensuring people feel supported to manage their condition and reducing unplanned admissions and time in hospital.5 In addition, this review identified many technologies in the fields of telehealth and wearable technologies that were considered to be less promising, impractical or poorly directed. This review enables the focus of future research funding and NHS health technology assessment activities to be appropriately targeted, thereby facilitating the adoption of technologies with the potential to make a significant impact on NHS COPD care and the quality of patients’ lives.
Acknowledgements
The authors would like to thank the clinical experts and members of the BLISS Patient Advisory Group (including their co-ordinator, Dr Alexandra Enocson) who contributed to this review.
Footnotes
Author Contribution: DJW conceived the original study idea, LCD and JS contributed to the development of the study design and methods. LCD collected the data and carried out the analysis with assistance from DJW and JS. All authors (LCD, DJW, JS, SH and RM) were involved in the interpretation of the results. LCD and DJW produced the initial draft of the article, which was then circulated repeatedly to all authors for critical revision, before all authors read and approved the final version. All authors had full access to all of the study data and can take responsibility for the integrity of the data and the accuracy of the analysis. DJW is the guarantor.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute for Health Research (NIHR), who fund the NIHR Horizon Scanning Research & Intelligence Centre. In addition, RM is supported by the NIHR Cambridge Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institute for Health and Care Excellence. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (partial update). NICE guidelines CG101, 2010. National Institute for Health and Care Excellence (NICE). [Google Scholar]
- 3. East of England Public Health Observatory. Modelled estimates of prevalence of COPD, www.apho.org.uk/resource/item.aspx?RID=111122 (2002, accessed 18 October 2014).
- 4. Stang P, Lydick E, Silberman C, et al. The prevalence of COPD: using smoking rates to estimate disease frequency in the general population. Chest 2000; 117: 354S–359S. [DOI] [PubMed] [Google Scholar]
- 5. Department of Health. The NHS Outcomes Framework 2015-16. London: Department of Health, https://www.gov.uk/government/publications/nhs-outcomes-framework-2015-to-2016 (2014, accessed 27 August 2015). [Google Scholar]
- 6. Lam DC, Hui CK, Ip MS. Issues in pulmonary function testing for the screening and diagnosis of chronic obstructive pulmonary disease. Curr Opin Pulm Med 2012; 18: 104–111. [DOI] [PubMed] [Google Scholar]
- 7. Pistelli R, Ferrara L, Misuraca C, et al. Practical management problems of stable chronic obstructive pulmonary disease in the eldery. Curr Opin Pulm Med 2011; 17: S43–S48. [DOI] [PubMed] [Google Scholar]
- 8. Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med 2011; 4: 729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller MR, Levy ML. Chronic obstructive pulmonary disease: missed diagnosis versus misdiagnosis. BMJ 2015; 351: h3021. [DOI] [PubMed] [Google Scholar]
- 10. Barnes PJ. Addressing unmet medical need in COPD management. Future Med 2012: 2–5. DOI: 10.2217/ebo.11.134 [Google Scholar]
- 11. Bateman ED, Reddel HK, van Zyl-Smit RN, et al. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways disease? Lancet Respir Med 2015; 3(9): 719–728. [DOI] [PubMed] [Google Scholar]
- 12. Roche N, Huchon GJ. Current issues in the management of chronic obstructive pulmonary disease. Respirology 1997; 2: 215–229. [DOI] [PubMed] [Google Scholar]
- 13. Casaburi R, Duvall K. Improving early-stage diagnosis and management of chronic obstructive pulmonary disease. Postgrad Med 2014; 126: 141–154. [DOI] [PubMed] [Google Scholar]
- 14. Zwerink M, Brusse KM, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 19(3): CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bischoff EWMA, Akkermans R, Bourbeau J, et al. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ 2012; 345: e7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miravitlles M. Five questions in chronic obstructive pulmonary disease. Expert Rev Respir Med 2013; 7: 1–2. [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Campos JL, Agusti A. Heterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposal. Lancet Respir Med 2015; 3(9): 729–734. [DOI] [PubMed] [Google Scholar]
- 18. UK Database of Uncertainties about the Effects of Treatments (DUETs). Antibiotics for Exacerbations of Chronic Obstructive Pulmonary Disease. NICE, http://www.library.nhs.uk/duets/ViewResource.aspx?resID=419189&tabID=297&summaries=true&resultsPerPage=10&sort=PUBLICATION_DATE&catID=15581 (2012, accessed 08 May 2015). [Google Scholar]
- 19. UK Database of Uncertainties about the Effects of Treatments (DUETs). Systemic Corticosteroids for Acute Exacerbations of Chronic Obstructive Pulmonary Disease. NICE, http://www.library.nhs.uk/duets/ViewResource.aspx?resID=419189&tabID=297&summaries=true&resultsPerPage=10&sort=PUBLICATION_DATE&catID=15581 (2014, accessed 08 May 2015).
- 20. Tichter AM. Are routine antibiotics beneficial for exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med 2013; 62: 592–593. [DOI] [PubMed] [Google Scholar]
- 21. Sethi S. Personalised medicine in exacerbations of COPD: the beginnings. Eur Respir J 2012; 40: 1318–1319. [DOI] [PubMed] [Google Scholar]
- 22. Gutierrez-Ibarluzea I, Simpson S, Benguria-Arrate G. Early awareness and alert systems: an overview of EuroScan methods. Int J Technol Assess Health Care 2012; 28: 301–307. [DOI] [PubMed] [Google Scholar]
- 23. Wild C, Langer T. Emerging health technologies: informing and supporting health policy early. Health Policy 2008; 87: 160–171. [DOI] [PubMed] [Google Scholar]
- 24. EuroScan International Network. A toolkit for the identification and assessment of new and emerging health technologies, http://euroscan.org.uk/methods/ (2014, accessed 08 May 2015).
- 25. Smith J, Ward D, Michaelides M, et al. New and emerging technologies for the treatment of inherited retinal diseases: a horizon scanning review. Eye 2015; 29(9): 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. University of Birmingham. Birmingham Lung Improvement StudieS (BLISS), http://www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/BLISS/index.aspx (2015, accessed 10 May 2015).
- 27. Pinnock H, Hanley J, McCloughan L, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013; 347: f6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLean S, Nurmatov U, Liu JL, et al. Telehealthcare for chronic obstructive pulmonary disease: Cochrane review and meta-analysis. Br J Gen Pract 2012; 62: e739–e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. US Food and Drug Administration. Biomarker Qualification Program. FDA, http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284076.htm (2015, accessed 07/07/2015). [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]