Abstract
The purpose of the current study was to apply a high throughput assay to systematically screen a library of food and drug administration (FDA)-approved drugs as potential ligands for the cannabinoid receptor 2 (CB2). A cell-based, homogenous time resolved fluorescence (HTRF) method for measuring changes in intracellular cAMP levels was validated and found to be suitable for testing ligands that may act on CB2. Among the 640 FDA-approved drugs screened, raloxifene, a drug used to treat/prevent post-menopausal osteoporosis, was identified for the first time to be a novel CB2 inverse agonist. Our results demonstrated that by acting on CB2, raloxifene enhances forskolin-stimulated cAMP accumulation in a concentration-dependant manner. Furthermore, our data showed that raloxifene competes concentration-dependently for specific [3H]CP-55,940 binding to CB2. In addition, raloxifene pretreatment caused a rightward shift of the concentration-response curves of the cannabinoid agonists CP-55,940, HU-210, and WIN55,212-2. Raloxifene antagonism is most likely competitive in nature, as these rightward shifts were parallel and were not associated with any changes in the efficacy of cannabinoid agonists on CB2. Our discovery that raloxfiene is as an inverse agonist for CB2 suggests that it might be possible to repurpose this FDA-approved drug for novel therapeutic indications for which CB2 is a target. Furthermore, identifying raloxifene as a CB2 inverse agonist also provides important novel mechanisms of actions to explain the known therapeutic effects of raloxifene.
Keywords: cannabinoid receptor, inverse agonist, drug repurposing, raloxifene, osteoporosis
1. Introduction
Two cannabinoid receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), have been identified and cloned [1,2]. Both CB1 and CB2 are coupled to Gi/o proteins and the activation of these receptors leads to the inhibition of adenylate cyclase activity [3,4].
CB1 receptors are distributed in the central nervous system as well as several peripheral tissues [3,4]. CB2 receptors are primarily located in immune cells, including neutrophils, monocytes, natural killer cells, T cells, B cells, macrophages, mast cells, and microglia cells [3,4]. This distribution suggests an important role for the CB2 receptor in mediating many of the immumnomodulatory, but not the psychoactive effects produced by cannabinoids, for which CB1 receptor is the prime target.
Because CB2 ligands have a wide range of therapeutic potentials, many novel agonists and antagonists for CB2 receptors have been synthesized by pharmaceutical industry as well as academic laboratories [5,6]. However, it is estimated that pharmaceutical product development requires at least 10 to 15 years and costs between $500 million and $2 billion [7,8].
Virtually all clinically used drugs exhibit effects on biological targets other than those for which they were designed. This property of drugs may result in drug repurposing, which refers to the process of finding new uses of existing drugs outside the scope of the original indication [9,10]. The benefits of drug repurposing include the existing approval by regulatory agencies for human use and the availability of human pharmacokinetics data and safety profiles for the approved drug. As a result, drug repurposing is potentially a time, cost-effective and low risk drug development approach. Therefore, systematically profiling food and drug administration (FDA)-approved drugs against a variety of novel targets will provide mechanistic insights into potentially novel therapeutic effects of the existing drugs for drug repurposing [9,10].
In the current study, first of all we validated high throughput cAMP assay appropriate for testing novel ligands for CB2 receptor. There are many cAMP assays available for screening purposes[11,12]. Homogenous Time Resolved Fluorescence (HTRF) is based on the principle of competition of antibody binding sites between the native cAMP produced by cells and the d2-labeled cAMP [11,13]. One distinct advantage of this assay over the other technologies is HTRF’s ratiometric measurement. This feature is extremely advantageous because it allows the reduction of well-to-well variation and it eliminates the interference of compound autofluorescence. This assay has been successfully miniaturized and still maintains accuracy and reproducibility. It is non- radioactive and does not require separation or washing steps. It is not labor intensive, is cost-effective, and has high sensitivity in the upper femtomolar range. These qualities make the cell-based HTRF cAMP assay the assay of choice for this study [11,13].
In an attempt to rapidly and efficiently identify drugs that may act as agonist or inverse agonist for CB2, in this study we screened a library of compounds consisting 640 FDA-approved drugs using the validated high throughput cAMP assay. All of the compounds in the library have well-characterized bioactivity, bioavailability, and safety profiles which could enhance drug repurposing. Our rational of screening this library of FDA-approved drugs is that if novel cannabinoid ligands are found from this library, this may provide novel therapeutic implications for these marketed drugs. In addition, identifying novel cannabinoid ligands from FDA-approved drugs can provide novel mechanisms of actions for the known therapeutic effects these drugs.
It is well known that raloxifene (Evista, Eli Lilly and Company), a selective estrogen receptor modulator (SERM), works as an agonist at estrogen receptors in the bone and acts as an antagonist at the estrogen receptors in the breast [14,15]. As a result, not only does raloxifene decrease the risk of vertebral fractures, it is also reduces the prevalence of hormone-positive breast cancer [14,15]. In the current study, our screening of FDA-approved drugs against CB2 identified raloxifene as a potential inverse agonist for the CB2 cannabinoid receptor. This initial finding prompted us to further characterize the pharmacological profile of raloxifene. In follow-up experiments, we investigated the pharmacological profiles of raloxifene for CB2 by conducting cell-based cAMP accumulation assays, as well as competitive radioligand binding assays. This study provides first evidence that raloxifene is a novel CB2 inverse agonist. Our discovery that raloxifene is an inverse agonist for CB2 suggests it might be possible to repurpose this FDA-approved drug for novel therapeutic indications for which CB2 is a target. Furthermore, identifying raloxifene as a novel CB2 inverse agonist also provides important novel mechanisms of actions to explain the known therapeutic effects of raloxifene.
2. Materials and Methods
2.1. Materials
Dulbecco’s Modified Eagles’s Medium (DMEM), penicillin/streptomycin, L-glutamine, trypsin, and geneticin were purchased from Mediatech (Manassas, VA). Fetal bovine serum was obtained from Atlanta Biologicals (Lawrenceville, GA). Glass tubes used for cAMP accumulation assays were obtained from Kimble Chase (Vineland, NJ). These tubes were silanized by exposure to dichlorodimethylsilane (Sigma-Aldrich, St. Louis, MO) vapor for 3 h under vacuum. 384-well, round bottom, low volume white plates were purchased from Grenier Bio One (Monroe, NC). The cell-based HTRF cAMP HiRange assay kits were purchased from CisBio International (Bedford, MA). Forskolin was obtained from Sigma (St. Louis, MO). The chemical library containing 640 FDA approved drugs were purchased from Enzo Life Sciences (Farmingdale, NY).
2.2. Cell Transfection and Culture
Human Embryonic Kidney 293 (HEK293) cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere consisting of 5% CO2, at 37°C. Expression plasmids containing the CB2 cannabinoid receptors were stably transfected into HEK293 cells using lipofectamine, according to manufacturer’s instructions. Stably transfected cells were selected in culture medium containing 800 μg/ml geneticin. Having established cell lines stably expressing CB2 receptors, the cells were maintained in growth medium containing 400 μg/ml of geneticin until needed for experiments.
2.3. Cell-based HTRF cAMP Assay
Cellular cAMP levels were measured using reagents supplied by Cisbio International (HTRF cAMP HiRange kit). Cultured cells were washed twice with phosphate-buffered saline (8.1 mM NaH2PO4, 1.5 mM KH2PO4, 138 mM NaCl, and 2.7 mM KCl, pH 7.2), and then dissociated in phosphate-buffered saline containing 1 mM EDTA. Dissociated cells were collected by centrifugation for 5 min at 2000g. The cells were resuspended in cell buffer (DMEM plus 0.2 % fatty acid free bovine serum albumin) and centrifuged a second time at 2000g for 5 min at 4°C. Subsequently, the cells were resuspended in an appropriate final volume of cell buffer plus the phosphodiesterase inhibitor Ro 20-1724 (2 μM). 5000 cells were added at 5 μl per well into 384-well, round bottom, low volume white plates (Grenier Bio One, Monroe, NC). Compounds were diluted in drug buffer (DMEM plus 2.5 % fatty acid free bovine serum albumin) and added to the assay plate at 5 μl per well. Following incubation of cells with the drugs or vehicle for 7 minutes at room temperature, d2-conjugated cAMP and Europium cryptate-conjugated anti-cAMP antibody were added to the assay plate at 5 μl per well. After 2 hour incubation at room temperature, the plate was read on a TECAN GENious Pro microplate reader with excitation at 337 nm and emissions at 665 nm and 620 nm. To assess receptor antagonism, HEK293 cells stably expressing CB2 were pre-incubated for 20 minutes with vehicle or raloxifene at a concentration of 1 or 10 μM before subject to stimulation with cannabinoid agonists.
2.4. Cell Harvesting and Membrane Preparation
Cells were washed twice with cold phosphate-buffered saline (PBS) consisting of 8.1 mM NaH2PO4, 1.5 mM KH2PO4, 138 mM NaCl, 2.7 mM KCl, pH 7.2, and scraped off the tissue culture plates. Subsequently, the cells were homogenized in membrane buffer (50 mM Tris–HCl, 5 mM MgCl2, 2.5 mM EDTA, pH 7.4) with a Polytron homogenizer. After the homogenate was centrifuged at 46 000 × g for 30 min at 4 °C, the pellet was resuspended in membrane buffer and stored at −80 °C. Protein concentrations were determined by Bradford assay using a BioRad protein reagent kit.
2.5. Ligand Binding Assays
Drug dilutions were made in binding buffer (membrane buffer containing 0.5 mg/ml fatty acid free BSA) and then added to the assay tubes. [3H]CP55940 was used as a labeled ligand for competition binding assays for CB2. Binding assays were performed in 0.5 ml of binding buffer containing 0.1 mg/ml BSA for 60 min at 30°C. Membranes (80 μg) were incubated with [3H]CP55940 in siliconized culture tubes, with unlabeled ligands at various concentrations. Free and bound radioligands were separated by rapid filtration through GF/B filters (Whatman International, Florham Park, New Jersey, USA). The filters were washed three times with 3 ml of cold wash buffer (50 mmol/l Tris–HCl, pH 7.4, containing 1 mg/ml of BSA). The bound [3H]CP55940 was determined by liquid scintillation counting in 5 ml of CytoScint liquid scintillation fluid (MP Biomedicals, Solon, Ohio, USA). The assays were performed in duplicate, and the results represent the averaged data from at least three independent experiments.
2.6. Data Analysis
Data analyses for cell-based HTRF cAMP assays were performed based on the ratio of fluorescence intensity of each well at 620 nm and 665 nm. Data are expressed as delta F%, which is defined as [(standard or sample ratio – ratio of the negative control)/ratio of the negative control] x 100. The standard curves were generated by plotting delta F% versus cAMP concentrations using non-linear least squares fit (Prism software, GraphPad, San Diego, CA). Unknowns are determined from the standard curve as nanomolar concentrations of cAMP. After the unknowns are determined, the sigmoidal concentration-response equations were used (via Prism program, GraphPad Software, San Diego, CA) to generate the curves of the tested compounds. Data from ligand binding assays were analyzed, and competition binding curves were generated with the non-linear regression analyses using the Prism program.
3. Results
3.1. Z′ Factor Determination
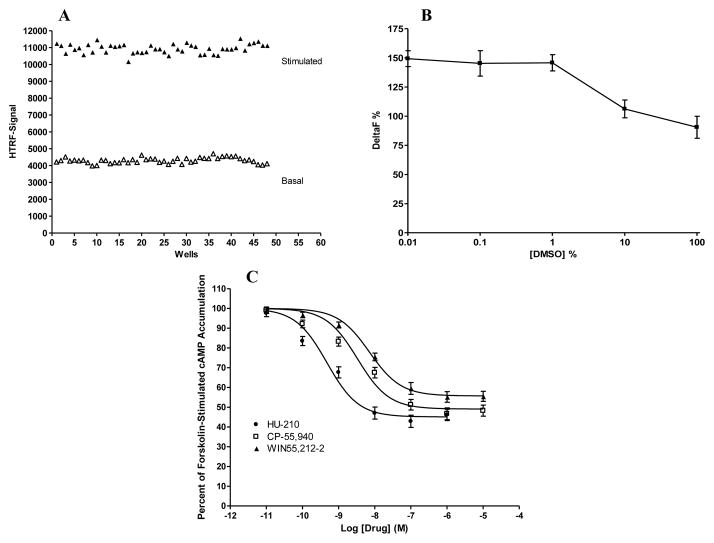
To determine the Z′ value, experiments were performed in 384-well plates using many replicates of the cell-based HTRF cAMP assay with positive and negative controls (Fig. 1A). For positive controls, the HEK293 cells expressing CB2 were treated with the CB2 agonist CP-55,940 at a concentration of 100 nM for 7 minutes at room temperature. For negative controls, the cells were treated with vehicle for 7 minutes. The Z′ value was calculated using the formula: Z′ = 1–3[(standard deviation of negative control) + standard deviation of positive control)]/[(mean of positive control) − (mean of negative control)] [16]. In the current study, the Z factor was determined to be 0.79.
Fig. 1. Validation of the cell-based, HTRF cAMP assay for CB2 cannabinoid receptor.
(A) Z′ factor determination. The solid symbols represent positive controls (cells stimulated with 100 nM CP-55,940), while the open symbols represent negative controls (cells stimulated with vehicle). The Z′ factor was calculated to be 0.79 using 48 positive and 48 negative control points. (B) DMSO tolerance. HEK293 cells stably expressing CB2 were treated with different concentrations of DMSO. Delta F % was calculated using the following formula: Delta F % = [(standard or sample ratio – ratio of the negative control)/ratio of the negative control] x 100. Data shown represent the mean ± S.E.M. of three experiments each performed in duplicate. (C) Pharmacological testing of known cannabinoid agonists. HEK293 cells stably expressing CB2 were treated with different concentrations of cannabinoid agonists HU-210, CP-55,940, and WIN55,212-2 for 7 minutes. Results are expressed as percent forskolin-stimulated cAMP accumulation. Data shown represent the mean ± SEM of five independent experiments.
3.2. Tolerance to Dimethyl Sulfoxide (DMSO)
One important condition to define is the concentration of dimethyl sulfoxide (DMSO) that the HTRF cAMP assay is able to tolerate without any loss in signal. For this purpose, we tested the effect of DMSO at concentrations ranging from 0.01% to 100 %. As shown in Fig. 1B, the cell-based HTRF cAMP assay for CB2 can tolerate DMSO up to 1 % without any loss of signal.
3.3. Pharmacological Testing of Known Cannabinoid Agonists
The ability of cannabinoid agonists to activate CB2 was verified using the HTRF cAMP assay. As shown in Fig. 1C, in HEK293 cells stably expressing CB2, HU-210, CP-55,940, and WIN55,212-2 inhibited forskolin-stimulated cAMP accumulation in a concentration-dependant manner, with a rank order of potency of HU-210 > CP-55,940 > WIN55,212-2. In addition, these three compounds failed to elicit any response in HEK293 cells transfected with an empty vector (data not shown).
3.4. Effects of Raloxifene on Forskolin-stimulated cAMP Accumulation
In an attempt to searching novel ligands for CB2, each compound from a chemical library containing 640 FDA-approved drugs was tested for its ability to activate CB2. The screening of this library resulted in the identification of raloxifene as a potential CB2 inverse agonist.
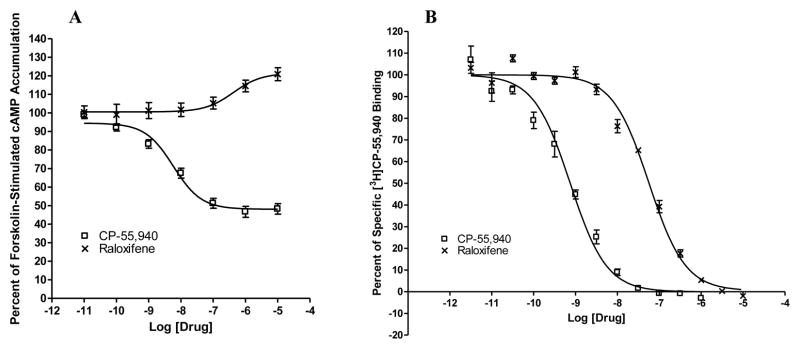
As shown in Fig. 2A, in HEK293 cells stably expressing CB2, the cannabinoid agonist CP-55,940 concentration-dependently inhibited forskolin-stimulated cAMP production. Most importantly, in Fig. 2A we report for the first time that raloxifene behaved as an inverse agonist for CB2 by enhancing forskolin-stimulated cAMP accumulation in a concentration-dependant manner. Furthermore, neither CP-55,940 nor raloxifene had any effects on forskolin-stimulated cAMP accumulation in empty vector- transfected HEK293 cells (data not shown).
Fig. 2. Raloxifene acts on CB2 cannabinoid receptor.
(A) Effects of raloxifene on forskolin-stimulated cAMP accumulation. HEK293 cells stably expressing CB2 were treated with different concentrations of CP-55,940 and raloxifene for 7 minutes. Results are expressed as percent forskolin-stimulated cAMP accumulation. Data shown represent the mean ± SEM of five independent experiments. (B) Competition of [3H]CP-55,940 binding by raloxifene. CP-55,950 and raloxifene were used to compete for specific [3H] CP-55,940 binding to membranes prepared from HEK293 cells stably expressing CB2. Data shown represent the mean ± SEM of three experiments performed in duplicate.
3.5. Competition of [3H]CP-55,940 Binding by Raloxifene
In order to investigate whether raloxifene bind to the CB2 receptor, we performed competition ligand binding experiments using membranes prepared from HEK293 cells stably transfected with CB2. As shown in Fig. 2B, cananbinoid agonist CP-55,940 competed for specific [3H]CP-55,940 binding. Furthermore, Raloxifene was also able to compete for specific [3H]CP-55,940 binding in a concentration-dependent manner. In addition, there was no detectable level of specific [3H]CP-55,940 binding in membranes prepared from HEK293 cells transfected with an empty vector (data not shown).
3.6. Antagonism of Cannabinoid Agonist-induced Inhibition of Forskolin-stimulated cAMP Accumulation by Raloxifene
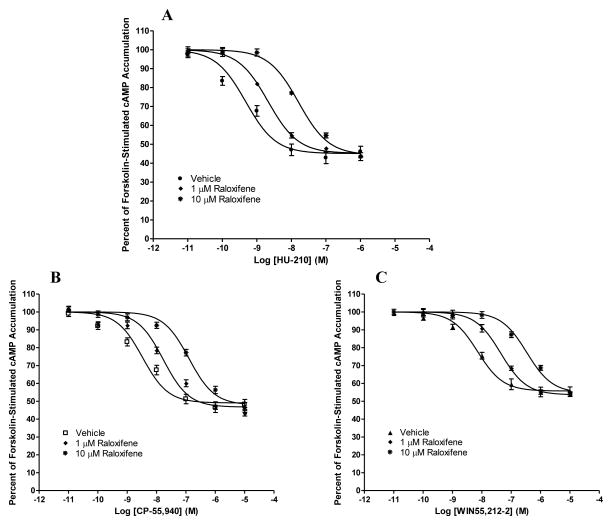
As shown in Fig. 3A–3C, in HEK293 cells stably expressing CB2, the cannabinoid agonists CP-55, 940, HU-210, and WIN55212-2 concentration-dependently inhibited forskolin-stimulated cAMP production. Most importantly, in a concentration-dependent manner, 1 μM and 10 μM raloxifene pretreatments resulted in a rightward, parallel shift of the concentration-response curves for the three cannabinoid agonists (Fig 3A–3C).
Fig. 3. Antagonism of cannabinoid agonist-induced inhibition of forskolin-stimulated cAMP accumulation by raloxifene.
HEK293 cells stably expressing CB2 were pre-incubated for 20 minutes with vehicle or raloxifene at a concentration of 1 or 10 μM before subject to stimulation with cannabinoid agonists (A) HU-210, (B) CP-55,940, and (C) WIN55,212-2 for 7 minutes. Results are expressed as percent forskolin-stimulated cAMP accumulation. Data shown represent the mean ± SEM of five independent experiments.
4. Discussion
Agonist binding to CB2 leads to Gi coupling and inhibition of adenylate cyclase [3,4]. As a result, there is a decrease in intracellular cAMP levels which was measured as an increase in HTRF signal in this study. First of all, in the current study, we validated a cell-based, HTRF cAMP assay for screening novel ligands for CB2.
The Z′ factor is a standard statistical parameter used to evaluate the robustness of a high throughput assay [16]. The Z′ factor can range between 0 and 1, with values approaching 1 indicating excellent assay robustness. In this study the calculated Z′ factor for the assay was 0.79. Since Z′ factor greater than 0.5 indicates a suitable difference between signal and background values with low variability, our results demonstrate that the cell-based, HTRF cAMP assay is robust and suitable for screening ligands that activate CB2.
Since most chemical compound libraries come pre-dissolved in dimethylsulfoxide (DMSO), it is critical to determine the maximum concentration that a compound can be screened before DMSO reaches a concentration that is too high to be tolerated by the assay [12]. Therefore, we determined the effect of DMSO on the cell-based, HTRF cAMP assay. We tested DMSO at a variety of concentrations and the results showed that the assay can tolerate DMSO up to 1 %. These data indicate that the assay is suitable for screening ligands that may act on CB2 at a DMSO concentration of less than 1 %.
To validate further that the cell-based, HTRF cAMP assay is suitable for assaying ligands that may activate CB2, we performed concentration-response studies for three known cannabinoid agonists. The rank order of potency of these agonists in inhibiting cAMP levels in HEK293 cells expressing CB2 was HU-210 > CP-55,940 > WIN55,212-2. These data are consistent with previous reports regarding the potency of these CB2 agonists [3,4]. These results also confirmed the suitability of this cell-based, HTRF cAMP assay for testing ligands for CB2.
In an attempt to discover novel ligands for CB2, each compound from a chemical library containing 640 FDA-approved drugs was tested for its ability to activate CB2 using the validated HTRF cAMP assay. If a compound is an agonist, it will inhibit the forskolin-stimulated cAMP response, which is shown as an increase in HTRF signal. In contrast, if a compound is an inverse agonist, it will further increase the forskolin-stimulated cAMP response, which is characterized as a decrease in HTRF signal. The screening of the 640 FDA-approaved drug library at 1 μM resulted in the identification of raloxifene as a potential inverse agonist for CB2, since it caused a decrease in HTRF signal.
In previous reports, we have demonstrated that CB2 receptors expressed in HEK293 cells exhibit constitutive activity, since the expression of CB2 caused a decrease of cellular cAMP levels compared with vector transfected HEK293 cells [17]. In addition, previously we have shown that SR144528, a known inverse agonist for CB2, is able to enhance forskolin-stimulated cAMP accumulation in HEK293 cells stably expressing CB2 [17]. Similar to SR144528, in the current study raloxifene was able to enhance cAMP accumulation concentration-dependently in HEK293 cells stably expressing human CB2. Since raloxifene did not have any effect on forskolin-stimulated cAMP accumulation in empty vector-transfected HEK293 cells, this suggests that the effect of raloxifene on cAMP accumulation was mediated through CB2 receptor.
Consistent with previous reports[18], in the current study, the cannabinoid agonist CP-55,940 competed concentration-dependently the specific binding of [3H]CP-55,940 to CB2. Similarly, raloxifene was able to compete, in a concentration-dependent manner, for specific [3H]CP-55,940 binding to CB2. These data further demonstrate that raloxifene acted on the same receptor as the cannabinoid agonist CP-55,940.
To further characterize the pharmacological properties of raloxifene, we evaluated its ability to antagonize the effects of the synthetic cannabinoid agonists CP-55,940, HU-210, and WIN55,212-2. Raloxifene concentration-dependently caused rightward shifts of the CP-55,940, HU-210, and WIN55,212-2 concentration-response curves. These data indicate that the raloxifene antagonism is most likely competitive in nature, as these rightward shifts were parallel and were not associated with any change in the efficacy of these agonists.
Raloxifene belongs to the class of selective estrogen receptor modulators (SERMs), which exhibit estrogen agonist activity in some target tissues while exert estrogen antagonist activity in other tissues [14,15]. Raloxifene has been approved for the treatment and prevention of post-menopausal osteoporosis and is currently under study for other therapeutic indications such as breast cancer [14,15].
Estrogen deficiency is the main cause of post-menopausal osteoporosis [14,15]. When estrogen is deficient, bone turnover increases, and bone resorption increases more than bone formation, leading to bone loss. The effects of raloxfiene on bone have been investigated in great detail and are well established. A large clinical trial, the Multiple Outcomes of Raloxifene Evaluation (MORE), was conducted in post-menopausal women with osteoporosis. The results form the MORE trial demonstrated that raloxifene reduced the incidence of new vertebral fractures by 30% and 50% (in women with and without prevalent vertebral fractures, respectively) compared to placebo [14,15].
The biological actions of raloxifene are well known to be mediated through binding to estrogen receptors [14,15]. However, to our knowledge, this report is the first to demonstrate that raloxifene is an inverse agonist for CB2. Recently, there is accumulating evidence to suggest that CB2 inverse agonists is effective for controlling inflammatory cell migration, thus is useful for a variety of inflammatory diseases, such as arthritis and multiple sclerosis [19]. Thus, our identification of raloxifene as a novel CB2 inverse agonist suggests that this FDA-approved drug for post-menopausal osteoporosis has great potential to be repurposed for other therapeutic indications.
Cannabinoids and their receptors play important roles in bone metabolism by regulating bone cell function [20]. It has been found that CB2 inverse agonist such as SR144528 is able to inhibit osteoclast formation and bone resorption, thus reducing bone loss [20]. Therefore, our discovery that raloxifene is a CB2 inverse agonist implicates a possible novel mechanism for the anti-osteoporosis activity of raloxifene--it might be partially mediated through the CB2 cannabinoid receptor in the bone.
In summary, we have validated a cell based, HTRF cAMP assay for testing ligands for CB2, and using this assay, we have screened a library of FDA-approved drugs against CB2. In this study, we report for the first time that raloxifene binds to CB2 and is an inverse agonist for CB2. Our discovery that raloxfiene is an inverse agonist for CB2 suggests that it might be possible to repurpose this FDA-approved drug for novel therapeutic indications for which CB2 is a target. Furthermore, identifying raloxifene as a CB2 inverse agonist also provides important novel mechanisms of actions to explain the known therapeutic effects raloxifene.
Highlights.
A cell-based, high-throughput assay was validated for the CB2 cannabinoid receptor.
Using the validated assay a library of FDA-approved drugs was screened against CB2.
Anti-osteoporosis drug raloxifene was discovered to be a novel CB2 inverse agonist.
Our discovery provides new insights into the possibility of repurposing raloxifene.
Our data suggest novel mechanisms for the known therapeutic effects of raloxifene.
Acknowledgments
This study was supported in part by the National Institutes of Health Grants EY13632 and DA11551.
Abbreviations
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- GPCR
G protein-coupled receptor
- FDA
food and drug administration
- HTRF
homogenous time-resolved fluorescence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 3.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 4.Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 5.Riether D. Selective cannabinoid receptor 2 modulators: a patent review 2009--present. Expert opinion on therapeutic patents. 2012;22:495–510. doi: 10.1517/13543776.2012.682570. [DOI] [PubMed] [Google Scholar]
- 6.Marriott KS, Huffman JW. Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor. Current topics in medicinal chemistry. 2008;8:187–204. doi: 10.2174/156802608783498014. [DOI] [PubMed] [Google Scholar]
- 7.Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health affairs. 2006;25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 8.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. Journal of health economics. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 9.Carley DW. Drug repurposing: identify, develop and commercialize new uses for existing or abandoned drugs. Part II. IDrugs: the investigational drugs journal. 2005;8:310–313. [PubMed] [Google Scholar]
- 10.Carley DW. Drug repurposing: identify, develop and commercialize new uses for existing or abandoned drugs. Part I, IDrugs: the investigational drugs journal. 2005;8:306–309. [PubMed] [Google Scholar]
- 11.Gabriel D, Vernier M, Pfeifer MJ, Dasen B, Tenaillon L, Bouhelal R. High throughput screening technologies for direct cyclic AMP measurement. Assay Drug Dev Technol. 2003;1:291–303. doi: 10.1089/15406580360545107. [DOI] [PubMed] [Google Scholar]
- 12.Williams C. cAMP detection methods in HTS: selecting the best from the rest. Nature reviews Drug discovery. 2004;3:125–135. doi: 10.1038/nrd1306. [DOI] [PubMed] [Google Scholar]
- 13.Degorce F, Card A, Soh S, Trinquet E, Knapik GP, Xie B. HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications. Current chemical genomics. 2009;3:22–32. doi: 10.2174/1875397300903010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muchmore DB. Raloxifene: A selective estrogen receptor modulator (SERM) with multiple target system effects. Oncologist. 2000;5:388–392. doi: 10.1634/theoncologist.5-5-388. [DOI] [PubMed] [Google Scholar]
- 15.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening: the official journal of the Society for Biomolecular Screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 17.Feng W, Song ZH. Effects of D3.49A, R3.50A, and A6.34E mutations on ligand binding and activation of the cannabinoid-2 (CB2) receptor. Biochemical pharmacology. 2003;65:1077–1085. doi: 10.1016/s0006-2952(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 18.Song ZH, Slowey CA, Hurst DP, Reggio PH. The difference between the CB(1) and CB(2) cannabinoid receptors at position 5.46 is crucial for the selectivity of WIN55212-2 for CB(2) Molecular pharmacology. 1999;56:834–840. [PubMed] [Google Scholar]
- 19.Lunn CA, Reich E-P, Fine JS, Lavey B, Kozlowski JA, Hipkin RW, Lundell DJ, Bober L. Biology and therapeutic potential of cannabinoid CB2 receptor inverse agonists. British journal of pharmacology. 2008;153:226–239. doi: 10.1038/sj.bjp.0707480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idris AI, Sophocleous A, Landao-Bassonga E, van’t Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149:5619–5626. doi: 10.1210/en.2008-0150. [DOI] [PubMed] [Google Scholar]