Summary
Immunological reagents for wild, non-model species are limited or often non-existent for many species.
In this study, we compare the reactivity of a new anti-passerine IgY secondary antibody with existing secondary antibodies developed for use with birds. Samples from 41 species from the following six avian orders were analysed: Anseriformes (1 family, 1 species), Columbiformes (1 family, 2 species), Galliformes (1 family, 1 species), Passeriformes (16 families, 34 species), Piciformes (1 family, 2 species) and Suliformes (1 family, 1 species). Direct ELISAs were performed to detect total IgY using goat anti-passerine IgY, goat anti-chicken IgY or goat anti-bird IgY secondary antibodies.
The anti-passerine antibody exhibited significantly higher IgY reactivity compared to the anti-chicken and/or anti-bird antibodies in 80% of the passerine families tested. Birds in the order Piciformes (woodpeckers) and order Suliformes (cormorants) were poorly detected by all three secondary antibodies. A comparison of serum and plasma IgY levels was made within the same individuals for two passerine species (house finch and white-crowned sparrow), and serum exhibited significantly more IgY than the plasma for all three secondary antibodies. This result indicates that serum may be preferred to plasma when measuring total antibody levels in blood.
This study indicates that the anti-passerine IgY secondary antibody can effectively be used in immunological assays to detect passerine IgY for species in most passerine families and is preferred over anti-chicken and anti-bird secondary antibodies for the majority of passerine species. This anti-passerine antibody will allow for more accurate detection and quantification of IgY in more wild bird species than was possible with previously available secondary antibodies.
Keywords: bird, Ecoimmunology, ELISA, IgY, non-model organisms, passerine
Introduction
Studies of the antibody-mediated immune response in non-model avian species have been a challenge due to the lack of species-specific secondary antibodies. Although species-specific antibodies are available for multiple domestic poultry species, species-specific antibodies are not commercially available for wild birds. Anti-chicken secondary antibodies have been used to detect antibodies in some wild birds (e.g. Martínez et al. 2003; Cray & Villar 2008), but the efficacy of using a chicken-specific antibody to detect antibodies across all avian species is unknown and likely highly variable. A secondary antibody that recognizes multiple avian species was first reported by Chiles & Reisen (1998), in which an ‘anti-bird’ antibody was produced in goats using sera from white-crowned sparrows (Zonotrichia leucophrys), ringed turtle doves (Streptopelia risoria), domestic chickens (Gallus gallus domesticus) and Muscovy ducks (Cairina moschata). This secondary antibody has been used in indirect enzyme-linked immunosorbent assays (ELISAs) for the detection of flaviviruses (Ebel et al. 2002; Hofmeister et al. 2016), alpha-viruses (Fassbinder-Orth, Barak & Brown 2013) and poxviruses (Ha et al. 2013; Ellison et al. 2014). While this anti-bird antibody is commercially available and provides breadth in an avian coverage, reliable detection of antibodies in a specific group of birds by broad-spectrum antibodies such as this is unknown, and no other wild bird-specific secondary antibodies are commercially available. Furthermore, no studies have been performed that have compared the relative effectiveness of available secondary antibodies to recognize primary antibodies in plasma or serum samples across multiple wild bird species. Additionally, comparisons of secondary antibody effectiveness in a non-model species are essential when one is relying on cross-reactive capability of antibodies for use in their species. The use of poor cross-reactive secondary antibodies may potentially produce unreliable results.
Antibody measurements can be broadly classified into functional assays and detection-based assays. Functional assays measure the binding activity of antibodies present in samples through immunoprecipitation-based tests such as agglutination or neutralization tests. Functional antibody tests have been widely used in wild birds. For example, agglutination tests have been used to detect anti-Mycoplasma antibodies in house finches (Haemorhous mexicanus) and black-capped chickadees (Poecile atricapillus; Dhondt, Dhondt & Hochachka 2015), anti-Toxoplasma antibodies in black-headed gulls (Chroicocephalus ridibundus; Miao et al. 2014) and anti-avian influenza antibodies (through a hemagglutination inhibition assay) in various species of ducks (Killian 2008; Wibawa et al. 2012). The plaque reduction neutralization test (PRNT) is a gold standard assay used to detect neutralizing antibodies against various arthropod-borne viruses (arboviruses) and has been used in hundreds of wild bird species (Beaty, Calisher & Shope 1989; Reisen et al. 2010). However, the PRNT assay requires culturing of the sample and may be limited to a higher level Biosafety Laboratory depending on the type of samples.
Detection-based assays for antibody quantification include immunocytochemistry, immunoblotting or immunosorbent assays (e.g. ELISAs). These assays rely on the use of secondary antibodies raised against the target antibody, where the secondary antibody has been conjugated with various enzymes [e.g. alkaline phosphatase or horseradish peroxidase (HRP)] or fluorescent markers [e.g. fluorescein isothiocyanate(FITC)]. The majority of detection-based studies that have been reported for measuring antibody levels in birds have been ELISAs. Competitive ELISAs and indirect ELISAs are the most common ELISAs that have been used in wild bird studies. Competitive ELISAs (also called blocking ELISAs) involve the competition of test samples with antigen-specific anti-sera from model species (e.g. mouse, rabbit) and a secondary antibody for that model species. Therefore, blocking ELISAs do not require secondary antibodies for non-model species and have been used in wild bird species to detect flaviviruses (Hobson-Peters 2012) and avian influenza (Curran et al. 2014). While competitive ELISAs have been developed as useful detection tools for pathogen exposure, their use for more general antibody quantification (e.g. total immunoglobulin concentration in sera) is more limited due to the target antigen-specific nature of the assay.
Immunological studies of non-model avian species often involve the collection of samples (typically serum or plasma) from birds in the wild using various methods of collection and storage conditions. However, very few studies have compared antibody titres between simultaneously collected plasma and serum samples, and those studies that have been performed have been focused on human clinical immunoassays (e.g. Siev et al. 2011). Therefore, further comparisons of serum and plasma samples for antibody titres are warranted, especially in studies of non-model, wild organisms, where sample storage conditions (and therefore plasma protein stability) are more variable. In this study, we compared the levels of IgY (the avian equivalent to mammalian IgG) from serum and plasma samples collected in parallel from the same individuals.
As part of a collaborative project with the National Science Foundation Research Coordination Network in Ecoimmunology, a goat anti-passerine IgY antibody was produced by Bethyl Laboratories, Inc., using serum and yolk-derived IgY from 15 passerine species from seven different families. In this study, we compare the specificity of this new antibody to previously available antibodies from Bethyl, a goat anti-chicken IgY-HRP antibody and a goat anti-bird IgY-HRP using serum and plasma samples from 41 species across six avian orders.
Materials and methods
SECONDARY ANTIBODIES
Polyclonal goat anti-chicken IgY, conjugated with HRP (Bethyl A30-106P) and polyclonal goat anti-bird IgY-HRP (Bethyl A140-110P) were purchased from Bethyl Laboratories, Inc (Montgomery, TX, USA). To make the anti-passerine antibody, sera and yolk from 15 species in nine different families in the Passeriformes order were used to produce the polyclonal goat anti-passerine IgY antibody at Bethyl Laboratories, Inc. (Bethyl A140-129A, Table 1). Each of the nine families represented in the IgY antigen pool accounted for an average of 11% of the total pool, with a low of 5% (Troglodytidae) and a high of 16% (Hirundinidae). All secondary antibodies were made against both heavy (H) and light (L) chains of IgY isolates from serum and/or yolk from selected avian species. Heavy chains define the immunoglobulin isotype (IgY, IgM, IgA, etc.), but in birds, one type of light chain (lambda) is shared across all antibody isotypes (Benčina et al. 2014). Therefore, it is possible that some of the total Ig detected by the secondary antibodies also included IgA and IgM due to light chain similarities among isotypes.
Table 1.
Species origin of samples used to produce the goat anti-passerine IgY secondary antibody
| Infraorder | Family | Species |
|---|---|---|
| Corvida | Corvidae | Florida scrub jay (Aphelocoma coerulescens) |
| Passerida | Estrilididae | Zebra finch (Taeniopygia guttata) |
| Fringillidae | House finch (Haemorhous mexicanus) | |
| Thick-billed euphonia (Euphonia laniirostris) | ||
| Hirundinidae | Cliff swallow (Petrochelidon pyrrhonota) | |
| Violet-green swallow (Tachycineta thalassina) | ||
| Passeridae | House sparrow (Passer domesticus) | |
| Turdidae | Clay-coloured robin (Turdus grayi) | |
| Western bluebird (Sialia mexicana) | ||
| Thraupidae | Blue-grey tanager (Thraupis episcopus) | |
| Flame-rumped tanager (Ramphocelus flammigerus) | ||
| Yellow-bellied seedeater (Sporophila nigricollis) | ||
| Troglodytidae | House wren (Troglodytes aedon) | |
| Tyrannidae | Ash-throated flycatcher (Myiarchus cinerascens) | |
| Piratic flycatcher (Legatus leucophaius) |
SAMPLE COLLECTION
Serum and/or plasma samples used to test the secondary antibodies were collected through jugular or brachial venipuncture of 221 birds from 41 species across 21 families (Table 2). For the comparison of IgY content between serum and plasma in the same individuals, serum and plasma were collected within 5 min of each other from the same individuals of white-crowned sparrows (Z. leucophrys) and house finches (H. mexicanus; n = 6 per species). All procedures involving animals were approved by the following institutional animal care and use committees: Creighton University IACUC #0915; Oklahoma State University IACUC #AS095 and #AS107; Virginia Tech Institutional IACUC #BIOL-12-124; Indiana University Bloomington IACUC #12-050; Los Alamos National Laboratory IACUC #186; USGS National Wildlife Health Center IACUC # EP040528, EP030909-A1 and EP040713; Millikin University IACUC # 2013-5; Michigan State University IACUC# AUF 02-09-016-00; and Cornell IACUC# 2010-0015. Appropriate Federal and State permits were also obtained: USGS Federal bird banding permit numbers: 20948, 23593, 20261, 23694, 23629, 07732 and 22796; USFWS permit numbers: MB158404-0, MB04762-3, MB19427-0, MB-050243-0 and TE824723-7; State permits: Illinois State permit number: NH13.5412; Nebraska Game and Parks Scientific permit number: 254; New Mexico Game and Fish Scientific Permit number: 3187; Oklahoma State permit number: 5578; Virginia Department of Game and Inland Fisheries permit number: 044569; and Florida Fish and Wildlife Conservation Commission LSSC-10-00205.
Table 2.
Avian species analysed for total IgY content in serum or plasma samples
| Order | Family | Species | n | Serum/Plasma |
|---|---|---|---|---|
| Anseriformes | Anatidae | Mallard (Anas platyrhynchos) | 1 | Serum |
| Columbiformes | Columbidae | Rock dove (Columba livia) | 7 | Serum |
| Mourning dove (Zenaida macroura) | 5 | Plasma | ||
| Galliformes | Phasianidae | Domestic chicken (Gallus gallus) | 4 | Serum |
| Passeriformes | Cardinalidae | Indigo bunting (Passerina cyanea) | 5 | Plasma |
| Northern cardinal (Cardinalis cardinalis) | 5 | Plasma | ||
| Rose-breasted grosbeak (Pheucticus ludovicianus) | 5 | Plasma | ||
| Corvidae | American crow (Corvus brachyrhynchos) | 6 | Serum | |
| Blue jay (Cyanocitta cristata) | 5 | Plasma | ||
| Florida scrub jay (Aphelocoma coerulescens) | 10 | Plasma | ||
| Emberizidae | Dark-eyed junco (Junco hyemalis) | 5 | Plasma | |
| Eastern towhee (Pipilo erythrophthalmus) | 5 | Plasma | ||
| Field sparrow (Spizella pusilla) | 5 | Plasma | ||
| White-crowned sparrow (Zonotrichia leucophrys) | 6 | Serum, Plasma | ||
| Estrilididae | Java sparrow (Lonchura oryzivora) | 6 | Serum | |
| Zebra finch (Taeniopygia guttata) | 11 | Plasma | ||
| Fringillidae | Hawai‘i amakihi (Hemignathus virens) | 6 | Serum | |
| American goldfinch (Spinus tristis) | 5 | Plasma | ||
| House finch (Haemorhous mexicanus) | 11 | Serum, Plasma | ||
| Hirundinidae | Cliff swallow (Petrochelidon pyrrhonota) | 5 | Serum | |
| Icteridae | Brown-headed cowbird (Molothrus ater) | 5 | Plasma | |
| Common grackle (Quiscalus quiscula) | 5 | Plasma | ||
| Red-winged blackbird (Agelaius phoeniceus) | 6 | Plasma | ||
| Mimidae | Brown thrasher (Toxostoma rufum) | 5 | Plasma | |
| Grey catbird (Dumetella carolinensis) | 5 | Plasma | ||
| Paridae | Black-capped chickadee (Poecile atricapillus) | 5 | Plasma | |
| Tufted titmouse (Baeolophus bicolor) | 5 | Plasma | ||
| Parulidae | Ovenbird (Seiurus aurocapilla) | 1 | Plasma | |
| Passeridae | House sparrow (Passer domesticus) | 5 | Serum | |
| Sittidae | Red-breasted nuthatch (Sitta canadensis) | 4 | Plasma | |
| White-breasted nuthatch (Sitta carolinensis) | 5 | Plasma | ||
| Thraupidae | Red-crested cardinal (Paroaria coronata) | 6 | Serum | |
| Troglodytidae | Carolina wren (Thryothorus ludovicianus) | 5 | Plasma | |
| Turdidae | American robin (Turdus migratorius) | 3 | Serum | |
| Eastern bluebird (Sialia sialis) | 4 | Plasma | ||
| Hermit thrush (Catharus guttatus) | 1 | Plasma | ||
| Swainson’s thrush (Catharus ustulatus) | 2 | Plasma | ||
| Veery (Catharus fuscescens) | 1 | Plasma | ||
| Wood thrush (Hylocichla mustelina) | 1 | Plasma | ||
| Zosteropidae | Japanese white-eye (Zosterops japonicus) | 6 | Serum | |
| Piciformes | Picidae | Downy woodpecker (Dryobates pubescens) | 5 | Plasma |
| Red-bellied woodpecker (Melanerpes carolinus) | 5 | Plasma | ||
| Suliformes | Phalacrocoracidae | Double-crested cormorant (Phalacrocorax auritus) | 1 | Serum |
ENZYME-LINKED IMMUNOSORBENT ASSAYS
Total IgY ELISAs were performed according to Fassbinder-Orth, Barak & Brown (2013), with modifications. Briefly, serum or plasma was diluted 1 : 15 000 with coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3, pH 9.6), and 75 μL was added in triplicate to a flat-bottomed 96-well plate (Nunc, Roskilde, Denmark) and incubated overnight at 4 °C. A maximum of 24 samples were on each plate with pooled domestic chicken and cow sera (n = 5 individuals/species/pool) serving as positive and negative controls for standardization among plates. After the overnight incubation, we removed the coating solution and blocked plates with 200 μL/well of blocking buffer [PBS (10 mM phosphate buffer, 150 mM NaCl, pH 7.4) with 5% non-fat dry milk, 0.05% Tween] and incubated for 30 min at room temperature. Plates were washed four times with wash buffer (PBS with 0.05% Tween) using a BioTek ELx50 Automated Strip Washer (BioTek Instruments, Winooski, VT, USA). One hundred microlitres of goat anti-passerine IgY-HRP antibody, goat anti-chicken IgY-HRP antibody or goat anti-bird IgY-HRP antibody diluted 1 : 10 000 with blocking buffer was added per well, incubated for 1 h at 37 °C and washed. One hundred microlitres of tetramethylbenzidine (TMB)-peroxidase substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD, USA), made 35 min prior to completion of the 1 h incubation, was added to each well and incubated at room temperature in the dark for 8 min, and the reaction was stopped with 100 μL of 1 M H2SO4. Plates were read at 450 nm immediately using a Synergy HT microplate reader (BioTek). Values are reported as positive sample value/negative control value (P/N). P/N values >2.0 were considered positive.
STATISTICAL ANALYSES
We used 1-sample Kolmogorov–Smirnov tests to test for normal distribution of P/N data. We found that the data were not normally distributed (P < 0.02 in all cases) and were skewed right; therefore, we used log-transformed P/N values for all statistical analyses. Following the data transformation, P/N for each antibody was normally distributed (P > 0.221 in all cases). We used paired t-tests to determine whether the P/N was significantly different between plasma and serum samples.
To test for differences in P/N for the three secondary antibodies within each order, we used a repeated measures ANOVA (RMANOVA) with P/N as the dependent variable, secondary antibody type as the repeated variable and order as between-subjects factor.
To test for differences in P/N for the three secondary antibodies within families of the order Passeriformes, we used a RMANOVA with P/N as the dependent variable, secondary antibody type as the repeated variable and family as the between-subjects factor.
We used α = 0.05 to determine statistical significance. For any RMANOVA that failed Mauchly’s Test of Sphericity, we used the Greenhouse–Geisser adjustment. Following testing of statistically significant between-subjects factors (either order or family) by repeated factor (antibody type) interactions, we ran separate RMANOVAs within each of the between-subjects levels (orders or families). When we found statistically significant results in RMANOVAs within orders or families, we used paired t-tests as post hoc tests to determine pairwise differences among antibodies. All analyses were completed with SPSS 21.0 (IBM Corp., Armonk, NY, USA). Orders and families where less than four samples were available were not included in these analyses. Specifically, insufficient samples were available to perform statistical analyses on the Anseriformes or Suliformes orders.
ASSAY VARIATION
We calculated intra-assay variation, reported as coefficients of variation (CV) by determining the CV for each experimental sample among its three replicates, then taking the average CV across all samples within each assay (anti-chicken, anti-bird or anti-passerine assays). Interassay variation was determined by calculating the CV of cow and chicken sera pools (negative and positive controls, respectively) run on 11 different plates per secondary antibody assay.
Results
ASSAY VARIATION
The mean intra-assay variation for the three secondary antibody assays was 3.39%, and the mean interassay variation was 10.31% (Table 3). The higher levels of interassay variation can be attributed to possible plate-to-plate variations that may have occurred over the course of several months when processing the 221 samples used for each of the three antibody assays.
Table 3.
Intra-assay and interassay variation
| Assay | CV (%) |
|---|---|
| Intra-assay | |
| Anti-chicken | 2.66 |
| Anti-bird | 4.04 |
| Anti-passerine | 3.48 |
| Mean Intra-assay | 3.39 |
| Interassay | |
| Anti-chicken | |
| Cow | 8.22 |
| Chicken | 8.73 |
| Anti-bird | |
| Cow | 3.99 |
| Chicken | 25.5 |
| Anti-passerine | |
| Cow | 4.46 |
| Chicken | 10.96 |
| Mean Interassay | 10.31 |
COMPARISON OF PLASMA AND SERUM SAMPLES
IgY levels were higher in serum than plasma for the three secondary antibody assays in the house finch (t2 = −13.250, P = 0.006) and were also significantly higher in the white-crowned sparrow (t2 = −5.427, P = 0.032). When both species were considered together, serum contained significantly higher levels of IgY than plasma for all three of the secondary antibodies (t5 = −4.749, P = 0.005, Fig. 1).
Fig. 1.
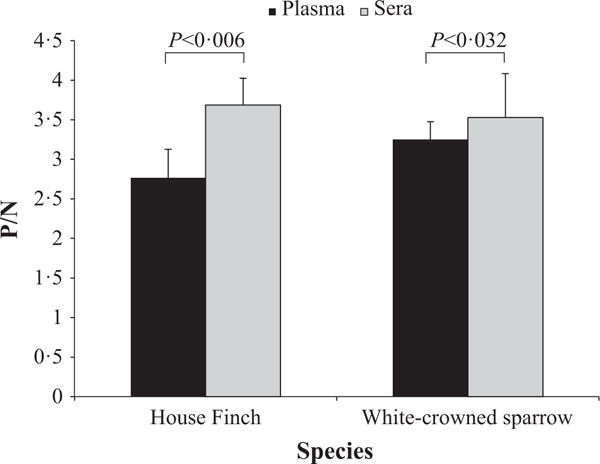
Mean Positive/Negative (P/N) values ± SEM for house finches and white-crowned sparrows (n = 6 per species) detected in plasma and serum from the same individuals. Values shown are mean values for the three secondary antibodies (anti-chicken, anti-bird, anti-passerine).
DIFFERENCES WITHIN ORDERS
There was a significant interaction between secondary antibody type and order (F5.218, 426 = 15.853, P < 0.001), demonstrating that the three types of secondary antibodies produce different results for P/N values across orders. There was not a single secondary antibody that produced the best results across all orders, rather some secondary antibodies performed better in some orders. Differences in total IgY detection among the three secondary antibodies for all orders are shown in Fig. 2.
Fig. 2.
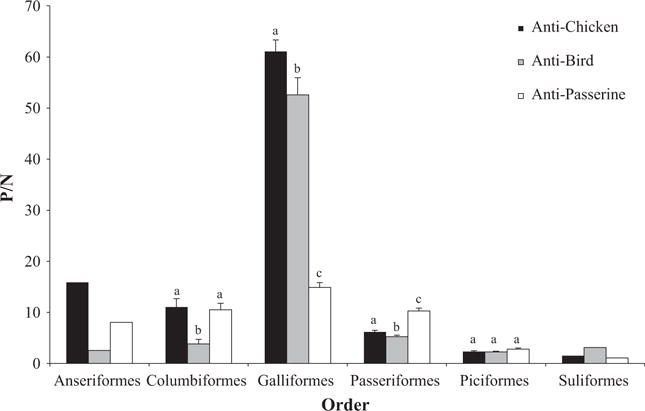
Mean Positive/Negative (P/N) values ± SEM for all avian orders by antibody type. Superscripts above bars signify significant differences (P < 0.05) within orders. Bars not sharing the same superscript are statistically different. Anseriformes and Suliformes values are representative of one individual each, and therefore, no statistical analyses were performed for those orders.
Columbiformes had significantly greater P/N values with the anti-chicken antibody than the anti-bird antibody (t11 = 4.573, P = 0.001) and greater P/N values with the anti-passerine antibody than the anti-bird antibody (t11 = 4.887, P < 0.001), but there was no difference in P/N values between anti-chicken and anti-passerine antibodies (t11 = 0.187, P = 0.855). Galliformes had significantly greater P/N values with the anti-chicken antibody than each the anti-bird (t3 = 4.416, P = 0.022) and the anti-passerine (t3 = 46.643, P < 0.001), and the anti-bird P/N value was significantly greater than the anti-passerine (t3 = 37.202, P < 0.001). Passeriformes had significantly greater P/N values with the anti-passerine antibody than each the anti-bird (t192 = 13.341, P < 0.001) and the anti-chicken (t192 = 14.706, P < 0.001). The P/N value was also greater for the anti-chicken than the anti-bird secondary antibody (t192 = 2.5766, P = 0.011). There were no significant differences in P/N values among secondary antibodies in the Piciformes order (F2,18 = 3.58, P = 0.062).
DIFFERENCES WITHIN FAMILIES OF THE PASSERIFORMES ORDER
There was a significant interaction between secondary antibody type and family within Passeriformes (F26.305, 354 = 6.703, P < 0.001), demonstrating that differences in P/N values among antibodies vary within each family. All orders examined in this study (except Passeriformes) were represented by only one family. Therefore, we only analysed differences in activity among the three secondary antibodies for families within the order Passeriformes (Fig. 3).
Fig. 3.
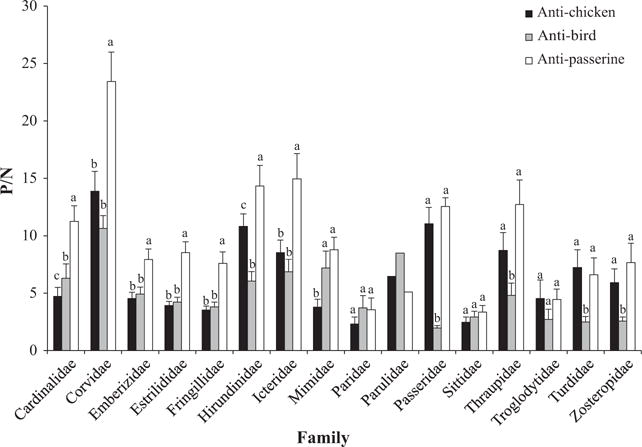
Mean Positive/Negative (P/N) values ± SEM for families within the order Passeriformes by secondary antibody type. Superscripts above bars signify significant differences (P < 0.05) within orders. Bars not sharing the same superscript are statistically different. Parulidae values are representative of one bird, and therefore, no statistical analyses were performed for that family.
The anti-passerine antibody detected significantly more IgY than either anti-chicken or anti-bird for the following families: Cardinalidae (P < 0.001), Corvidae (P < 0.001), Emberizidae (P < 0.002), Estrilidae (P < 0.001), Fringillidae (P < 0.001), Hirundinidae (P < 0.049) and Icteridae (P < 0.021). The anti-passerine antibody detected significantly more IgY than the anti-bird antibody (but not the anti-chicken antibody) for the following families: Passeridae (P < 0.001), Thraupidae (P < 0.002), Turdidae (P < 0.008) and Zosteropidae (P < 0.017). The anti-passerine antibody detected significantly more IgY than the anti-chicken antibody (but not the anti-bird antibody) in Mimidae (P < 0.001) and Paridae (P = 0.05). The anti-passerine antibody detected significantly more IgY than the anti-bird antibody (but not the anti-chicken antibody) in the Troglodytidae (P = 0.013). There were no significant differences among the three secondary antibodies for the Sittidae (F2,16 = 3.311, P = 0.063).
Discussion
This is the first study to compare the effectiveness of multiple secondary antibodies to quantify IgY levels across wild bird species. The results of this study will enhance the ability of avian immunologists specializing in wild birds to devise and analyse antibody-based measurements and assays due to increased available information about relative reactivities of available secondary anti-avian IgY antibodies.
The anti-chicken antibody produced the highest P/N value for any order or family in the chicken (P/N = 61 ± 2.3). This is expected because the secondary antibody was made against only the chicken and no other species. The P/N value for the anti-bird antibody in the chicken was also very high (P/N = 53 ± 3.4), likely indicating that a significant portion of the anti-bird antibody specifically recognizes chicken IgY. The anti-bird antibody was produced against domestic chickens, white-crowned sparrows, ringed turtle doves and Muscovy ducks. However, in this study, the anti-bird antibody detected significantly less IgY than the anti-passerine antibody for Emberizidae (white-crowned sparrows) and Columbidae (ringed turtle doves). More samples are needed from Anatidae (Muscovy ducks) to confirm the similar trend observed in this study (anti-bird< anti-passerine, anti-chicken). These results suggest that the detection capabilities of the anti-bird antibody are strongest in galliforms and may not be ideal in other taxa. Birds from Piciformes and Suliformes exhibited very low P/N ratios for all secondary antibodies (P/N < 3) and did not show any differences among secondary antibodies. The cross-reactivity of antibodies from these orders with the secondary antibodies used in this study was likely minimal.
The anti-passerine IgY antibody detected significantly more IgY than the anti-chicken or anti-bird IgY secondary antibodies for 47% of the passerine families studied (7/15); it detected significantly more IgY than either the anti-bird or anti-chicken antibodies for 33% of passerine families (5/15). The remaining families, Paridae, Sittidae and Troglodytidae, exhibited the lowest P/N ratios of all passerine families (mean P/N for all secondary antibodies = 3.3 ± 0.85, compared to 7.9 ± 1.11 for all other passerine families) and did not show any differences among secondary antibodies. Possible reasons for the low detection in these families could include a lack of cross-recognition of IgY by the secondary antibodies used in this study. No members of the Paridae or Sittidae families were used to make the anti-passerine antibody. Although one species from Troglodytidae (house wren) was used to make the secondary anti-passerine antibody, the goat anti-passerine secondary antibody product that was produced did not recognize members of the Troglodytidae family well. The IgY in these three families may have been significantly different, antigenically, compared to the target antibodies used to make the secondary antibodies, which would reduce cross-reactivity of the secondary antibodies with IgY from these three families. It is also possible that low circulating antibody levels in the individuals sampled for this study could have contributed to the results. Further investigation is required to understand whether this pattern of low antibody detection may be a common phenomenon of these particular species.
Some species exhibited high levels of antibodies, regardless of the secondary antibody used. For example, birds from the passerine family Corvidae exhibited higher levels of IgY for the three secondary antibodies compared to all other passerine families (although it was not significantly different from Hirundinidae for all three secondary antibodies, nor from Mimidae for anti-bird, nor from Passeridae for anti-chicken). Hirundinidae and Passeridae were significantly higher than all other families (except Corvidae) for the anti-chicken antibody only (data not shown). The Corvidae family represented only 8% of the total antigen pool in the formation of the antibody (mean family representation was 11%), and so antigenic exposure of the goat to corvid IgY was not predicted to generate the high levels of antibody detected in this study. The three species sampled from Corvidae (American crow, blue jay and Florida scrub jay) exhibited high levels of antibodies with the three secondary antibodies, which may indicate high levels of circulating antibody levels in this family compared to other families surveyed here.
Serum samples had significantly more IgY than plasma samples collected from the same individuals (white-crowned sparrows and house finches) in this experiment. This result is expected for several reasons. First, decreased protein levels have been found in plasma compared to serum for proteins such as Beta-2-microglobulin (Bjerrum & Birgens 1986) and cytokines IL-1β and TNF-α (Dossus et al. 2009). Additionally, anticoagulants such as heparin, EDTA or sodium citrate, all commonly used in sample collection vessels such as microhematocrit capillary tubes, have been shown to interfere with immunoassays (Haab et al. 2005), likely through the disruption of protein interactions and stability (Lopez & Peng 2003). Lastly, long-term or improper storage of plasma likely causes fibrin polymerization, leading to possible precipitation of plasma proteins, including antibodies. Given these results, when antibody quantification is essential to a study, serum collection may be preferred over plasma collection. However, this study was done on a small number of samples from two species and should be repeated with more species and samples to validate these results.
The results of this study provide comparative antibody detection data for researchers interested in analysing antibody levels in wild birds. The new anti-passerine antibody detected higher levels of IgY than the other secondary antibodies for most passerine birds, and based on these data, we suggest using the newly developed anti-passerine IgY antibody to detect antibodies in birds within the order Passeriformes. However, some avian orders and families did not yield significant antibody detection with any secondary antibody, and caution should be used before designing an antibody-based assay using the secondary antibodies reported here. For example, birds in the Piciformes and Suliformes orders and birds from some families within Passeriformes (Paridae, Sittidae and Troglodytidae) exhibited low antibody detection with all of the secondary antibodies, and the use of these secondary antibodies to detect antibodies in these species is not recommended. Finally, care should be taken when using any broad-spectrum secondary anti-IgY antibody to make quantitative IgY comparisons among different orders or families of birds, given differences in the detection ability of secondary antibodies among different target groups.
Acknowledgments
We thank Bethyl Laboratories for providing the anti-passerine antibody for sample testing. We thank Brianne Addison, Rachel Hanauer, Dana Hawley and Kirk Klasing for contributing samples for the production of Bethyl Laboratories’ anti-passerine antibody. We also thank Ellecia Rainwater, Molly Hiatt and Cara Franey for their technical assistance. This research was funded by Creighton College of Arts and Sciences Research Initiative Grant to CFO and National Institutes of Health grant 1R15HD066378-01 to JLG. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. government.
Footnotes
Data accessibility
Complete ELISA data for all species tested have been uploaded to DataDryad (http://datadryad.org/resource/doi:10.5061/dryad.k3v6h).
References
- Beaty BJ, Calisher CH, Shope RE. Diagnostic procedures for viral, rickettsial and chlamydial infections. In: Schmidt NJ, Emmons RW, editors. Arboviruses. American Public Health Association; Washington, DC: 1989. pp. 797–855. [Google Scholar]
- Benčina M, Cizelj I, Berčič RL, Narat M, Benčina D, Dovč P. Shared epitopes of avian immunoglobulin light chains. Veterinary Immunology and Immunopathology. 2014;158:175–181. doi: 10.1016/j.vetimm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Bjerrum OW, Birgens HS. Measurement of beta-2-micro-globulin in serum and plasma by an enzyme-linked immunosorbent assay (ELISA) Clinica Chimica Acta. 1986;155:69–76. doi: 10.1016/0009-8981(86)90100-2. [DOI] [PubMed] [Google Scholar]
- Chiles RE, Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. Journal of Vector Ecology. 1998;23:123–135. [PubMed] [Google Scholar]
- Cray C, Villar D. Cross-reactivity of anti-chicken IgY antibody with immunoglobulins of exotic avian species. Veterinary Clinical Pathology. 2008;37:328–331. doi: 10.1111/j.1939-165X.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- Curran JM, Robertson ID, Ellis TM, Selleck PW. Evaluation of avian influenza serologic and virologic diagnostic methods in wild Anseriformes and Charadriiformes. Avian Diseases. 2014;1:53–59. doi: 10.1637/10531-031513-Reg.1. [DOI] [PubMed] [Google Scholar]
- Dhondt AA, Dhondt KV, Hochachka WM. Response of black-capped chickadees to house finch Mycoplasma gallisepticum. PLoS One. 2015;10:e0124820. doi: 10.1371/journal.pone.0124820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. Journal of Immunological Methods. 2009;350:125–132. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Dupuis AP, Nicholas D, Young D, Maffei J, Kramer LD. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerging Infectious Diseases. 2002;8:979–982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison KS, Hofmeister EK, Ribic CA, Sample DW. Relatively high prevalence of pox-like lesions in Henslow’s sparrow (Ammodrammus henslowii) among nine species of migratory grassland passerines in Wisconsin, USA. Journal of Wildlife Diseases. 2014;50:810–816. doi: 10.7589/2013-09-252. [DOI] [PubMed] [Google Scholar]
- Fassbinder-Orth C, Barak V, Brown C. Immune responses of a native and an invasive bird to Buggy Creek virus (Togaviridae: Alphavirus) and its arthropod vector, the swallow bug (Oeciacus vicarius) PLoS One. 2013;8:e58045. doi: 10.1371/journal.pone.0058045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HJ, Alley M, Howe L, Gartrell B. Evaluation of the pathogenicity of avipoxvirus strains isolated from wild birds in New Zealand and the efficacy of a fowlpox vaccine in passerines. Veterinary Microbiology. 2013;165:268–274. doi: 10.1016/j.vetmic.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Haab BB, Geierstanger BH, Michailidis G, Vitzthum F, Forrester S, Okon R, et al. Immunoassay and antibody microarray analysis of the HUPO Plasma Proteome Project reference specimens: systematic variation between sample types and calibration of mass spectrometry data. Proteomics. 2005;5:3278–3291. doi: 10.1002/pmic.200401276. [DOI] [PubMed] [Google Scholar]
- Hobson-Peters J. Approaches for the development of rapid serological assays for surveillance and diagnosis of infections caused by zoonotic flaviviruses of the Japanese encephalitis virus serocomplex. Journal of Biomedicine and Biotechnology. 2012;2012:379738–379738. doi: 10.1155/2012/379738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister E, Dusek R, Fassbinder-Orth C, Owen B, Franson JC. Susceptibility and antibody response of vesper sparrows (Pooecetes gramineus) to West Nile virus: a potential amplification host in sage-brush grassland habitat. Journal of Wildlife Diseases. 2016;52:345–353. doi: 10.7589/2015-06-148. [DOI] [PubMed] [Google Scholar]
- Killian ML. Hemagglutination assay for the avian influenza virus. Methods in Molecular Biology. 2008;436:47–52. doi: 10.1007/978-1-59745-279-3_7. [DOI] [PubMed] [Google Scholar]
- Lopez JB, Peng CL. Can fluoride-oxalate and sodium citrate stabilise homocysteine levels after blood collection? Clinical Chemistry and Laboratory Medicine. 2003;41:1369–1372. doi: 10.1515/CCLM.2003.210. [DOI] [PubMed] [Google Scholar]
- Martínez J, Tomás G, Merino S, Arriero E, Moreno J. Detection of serum immunoglobulins in wild birds by direct ELISA: a methodological study to validate the technique in different species using antichicken antibodies. Functional Ecology. 2003;17:700–706. [Google Scholar]
- Miao Q, Han JQ, Xiang X, Yuan FZ, Liu YZ, Duan G, Zhu XQ, Zou FC. Prevalence of antibody to Toxoplasma gondii in black-headed gulls (Chroicocephalus ridibundus), Dianchi Lake, China. Journal of Wildlife Diseases. 2014;50:717–719. doi: 10.7589/2014-01-016. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Wheeler SS, Garcia S, Fang Y. Migratory birds and the dispersal of arboviruses in California. The American Journal of Tropical Medicine and Hygiene. 2010;83:808–815. doi: 10.4269/ajtmh.2010.10-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siev M, Yu X, Prados-Rosales R, Martiniuk FT, Casadevall A, Achkar JM. Correlation between serum and plasma antibody titers to mycobacterial antigens. Clinical and Vaccine Immunology. 2011;18:173–1. doi: 10.1128/CVI.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibawa H, Henning J, Waluyati DE, Usman TB, Lowther S, Bingham J, Junaidi A, Meers J. Comparison of serological assays for detecting antibodies in ducks exposed to H5 subtype avian influenza virus. BMC Veterinary Research. 2012;8:117. doi: 10.1186/1746-6148-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]


