Abstract
Objective
Growth hormone (GH) administration reduces abdominal, but not lower body, fat mass. To gain insight into the underlying mechanisms, this study examined the expression of the GH receptor (GHR) and some of its targets in abdominal and gluteal adipose tissue.
Methods
GHR and GH targets in the lipolytic pathway were assessed (quantitative PCR/Western blotting) in adipose aspirates from premenopausal women [n = 15, age 26.9 ± 6.1 years, body mass index (BMI) 28.0 ± 6.8 kg/m2] and men (n = 28, age 29.2 ± 7.0 years, BMI 26.9 ± 3.7 kg/m2).
Results
GHR mRNA expression was lower in the gluteal depot when compared with the abdominal depot (P = 0.01). Abdominal GHR correlated negatively with age and BMI, whereas gluteal GHR was associated with lower waist-to-hip ratio (WHR), that is, pear shape. In both sites, GHR mRNA correlated strongly with genes important for the regulation of lipolysis: adipose tissue triglyceride lipase (ATGL), hormone-sensitive lipase, perilipin, and CIDEA (all P < 0.001), independently of BMI, WHR, age, and sex. GHR protein was lower in the gluteal fat when compared with the abdominal fat (P = 0.03) and correlated with ATGL protein in the gluteal depot (P < 0.001).
Conclusions
GHR levels correlate with levels of lipases and lipid droplet-associated proteins crucial for lipolysis. Thus, higher GHR expression in the abdominal depot when compared with the gluteal depot may underlie the in vivo effect of GH to specifically reduce abdominal adipose tissue mass.
Introduction
Obesity is associated with increased risk for the development of type 2 diabetes, cardiovascular disease, and premature death (1). The distribution of body fat has a greater impact on cardiometabolic risk than excess total adiposity (2). Abdominal adiposity is a major underlying risk factor for the development of metabolic syndrome and is associated with insulin resistance, dyslipidemia, and both a proinflammatory and prothrombotic state (2,3). In contrast, lower body obesity (subcutaneous adipose tissue stored in the gluteofemoral region, as reflected in hip circumference) is associated with a lower risk of metabolic complications (4,5) and is inversely associated with fasting insulin levels and HbA1c (6-8).
It is well established that growth hormone (GH) influences fat distribution. GH replacement therapy administered to hypopituitary men and women decreases abdominal fat, with suggestions of a preferential effect on visceral than on subcutaneous abdominal depots (9,10). We have shown that in otherwise healthy young men and women with obesity, GH administration results in the reduction of abdominal fat without a decrease in thigh fat or an increase in ectopic fat deposition in liver or muscle (11,12). A similar pattern of fat redistribution with GH administration has been demonstrated in older men (13) and postmenopausal women (14) with obesity. Of note, the effects of GH on visceral adiposity in men and women with central obesity disappeared shortly after GH withdrawal (15,16).
GH is well established to affect adipocyte metabolism by regulating fatty acid (FA) storage and release (17), and limited data suggest depot-dependent effects. Richelsen et al. (18) found that GH decreased lipoprotein lipase activity, the enzyme which regulates the uptake of circulating triglycerides (TGs), comparably in both abdominal and gluteal adipose tissues of women with obesity. Within the adipocyte, TG storage and release are regulated by tightly controlled mechanisms that include variations in the expression of lipases [hormone-sensitive lipase (HSL) and adipose tissue TG lipase (ATGL)] and lipid droplet (LD)-associated proteins, such as perilipin (PLIN1) and CIDEC which protect the LD from lipolytic enzymes in the basal state (19). GH increases adipocyte lipolysis and lipid turnover (20,21) in part by increasing the activity of HSL, which mainly acts as a diglyceride lipase (22). An analysis of GH-regulated pathways in thigh subcutaneous adipose tissue after GH administration in hypopituitary men (23) did not detect changes in the expression of key genes of lipolysis but found increased expression of genes that modulate TG synthesis [lipin 1 (LPIN1) and acyl-coA synthetase short-chain family member 2 (ACSS2)]. Microarray data from patients with acromegaly showed upregulation of the TCA cycle, FA metabolism, and biosynthesis of unsaturated FA pathways (24). Finally, studies in genetically engineered mice suggest that the GH effects on adipose tissue may also be due to changes in adipogenesis and the immune populations within the tissue microenvironment (25,26).
The mechanisms underlying the depot-specific role of GH in human adipose tissue distribution and function remain poorly understood. In children with short stature who were not GH deficient, GH decreased abdominal adipocyte size, but had no effect on gluteal adipocyte size (27). A previous study reported higher GH receptor (GHR) expression in omental versus abdominal subcutaneous fat in lean but not obese women (28); however, differences between abdominal and gluteal adipose tissue depots have not been investigated.
We recently examined the transcriptome of subcutaneous abdominal and gluteal adipose tissues of healthy young volunteers and reported on depot differences in developmental genes (29). Further analysis of these data shows that the GHR signaling pathway (Reactome) was elevated in abdominal adipose tissue when compared with gluteal adipose tissue of women (Gene set enrichment analysis FDR q-value = 0.23. The leading edge subset of genes contributing to the enrichment included GHR, STAT1, STAT5A, STAT5B, CISH, SOCS1, JAK2, IRS1, and IRS2). Thus, in the current study, we used quantita tive PCR (qPCR) and Western blotting to verify depot differences in the expression of GHR. In an effort to understand GH effects on fat distribution, we focused on the association of GHR with the expression of key genes in the lipolytic pathway in abdominal and gluteal adipose tissue using samples from healthy young subjects with a range of body mass index (BMI) in that cohort (29).
Methods
Adipose tissue samples
We used samples from our previously published study (29) of abdominal and gluteal adipose tissue with the addition of seven subjects who were recruited by advertisement at Boston Medical Center, after approval by the BMC Institutional Review Board. Inclusion criteria were as follows: age 18-40 years and percent body fat of 20-50%. Subjects with significant medical illness, glucocorticoid use, smoking, or substance/alcohol abuse, and irregular menstrual cycles (for women) were excluded from the study. The subject characteristics are shown in Table 1.
TABLE 1.
Subject characteristics
| Variable | Total | Males | Females |
|---|---|---|---|
| N | 43 | 28 | 15 |
| Age (years) | 28 ± 6.8 | 29.2 ± 7.0 | 26.9 ± 6.1 |
| Ethnicity (C/AA/Other) | 34/8/1 | 23/4/1 | 11/4/0 |
| BMI (kg/m2) | 27.3 ± 5.0 | 26.9 ± 3.7 | 28.0 ± 6.8 |
| WHR | 0.86 ± 0.07 | 0.88 ± 0.06 | 0.79 ± 0.06 |
Data are shown as mean ± standard deviation.
AA, African American; BMI, body mass index; C, Caucasian; WHR, waist-to-hip ratio.
Fat aspirates
Aspirates of abdominal and gluteal subcutaneous fat were obtained from the mid-abdomen lateral to the umbilicus and the upper outer quadrant of the gluteal area, as previously described (29). Aliquots of adipose tissue (300-500 mg) were immediately frozen in liquid nitrogen and used for measurement of gene and protein expression levels by RT-qPCR and Western blotting.
Gene expression
Total RNA was isolated with Qiazol reagent (Qiagen) and quantified using NanoDrop. Total RNA was reverse-transcribed using a Transcriptor First-Strand cDNA synthesis kit (Roche), and qPCR was performed on a LightCycler 480 (Roche) using the following parameters: one cycle of 95°C for 10 min followed by 40 cycles at 95°C for 10 sec and 608C for 1 min. For each gene, a standard curve was generated with cDNA prepared from pooled RNA samples. Cyclophilin A (PPIA) was used as the reference gene, as in our previous study (29); it did not differ between depots and sexes. Commercially available primers from ThermoFisher Scientific were used (see Supporting Information Table S1 for details). Relative expression levels were calculated using LightCycler 480 relative quantification software (Roche).
Western blotting
Adipose tissue lysates were prepared in 2× cell lysis buffer (#9803, Cell Signaling, Danvers, MA). Approximately 50-80 μg of total protein was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes followed by immunoblot analysis with rabbit anti-human GHR antibody (SAB2100914, Sigma-Aldrich, St. Louis, MO) and rabbit anti-human p44/42 MAPK (Erk1/2) antibody (#9102, Cell Signaling, Danvers, MA) as a loading control. The anti-human ATGL antibody is a gift from Drs. D.W. Gong and C. Sztalryd (University of Maryland, Baltimore, Maryland; ref. 30).
Statistical analysis
JMP Pro version 9 (SAS Institute Inc., Cary, NC) and GraphPad Prism version 5.0 for Windows (GraphPad Software Inc., San Diego, CA) were used for statistical analyses. Variables were tested for normality of distribution using the Wilk-Shapiro test. Variables that were not normally distributed were log transformed. GHR expression in abdominal and gluteal fat aspirates was compared using paired t-test after logarithmic transformation. Linear regression analysis was performed to examine associations between genes, BMI, sex, and age, and Pearson's correlation coefficients are presented. Multivariate standard least-squares regression modeling was performed to control for BMI, waist circumference, hip circumference, age, and sex. Variance inflation factors were less than seven for all variables entered into the models. Values of P < 0.05 were considered statistically significant.
Results
Depot differences
GHR mRNA expression (by qPCR) was 75% higher in the abdominal fat depot than in the gluteal depot in the group as a whole (P = 0.01, n = 43) and within the male group (by 83%, P = 0.04, n = 28). A similar trend toward higher GHR expression in the abdominal fat depot when compared with the gluteal fat depot was noted in the female group (by 61%, P = 0.07, n = 15; Figure 1).
Figure 1.
GHR mRNA expression was significantly higher in the abdominal (ABD) fat depot than in the gluteal (GLU) depot in the group as a whole and within the male group when analyzed separately. There was a trend toward higher GHR expression in the abdominal fat depot when compared with the gluteal fat depot in the female group when analyzed separately. *P < 0.05.
ATGL mRNA levels were significantly higher in the abdominal than the gluteal depot in the group as a whole (by 107%, P < 0.01, n = 43) and within the male group when analyzed separately (P < 0.001, n = 28), with a similar trend in the female group (P = 0.08, n = 15; Figure 2, Panel A). In contrast, there was no significant difference in HSL mRNA levels between fat depots (Figure 2, Panel B). Levels of mRNA for genes that encode the major LD proteins, PLIN1 and CIDEC, did not differ in abdominal and gluteal adipose tissue (Figure 2, Panels C and E). CIDEA mRNA expression was significantly higher in the abdominal depot versus the gluteal depot in the group as a whole (P < 0.001) and in males (P < 0.001) and females (P < 0.05) when analyzed separately (Figure 2, Panel D).
Figure 2.
(A) ATGL mRNA fat depot expression. (B) HSL mRNA fat depot expression. (C) PLIN mRNA fat depot expression. (D) CIDEA mRNA fat depot expression. (E) CIDEC mRNA fat depot expression. *P < 0.05 for comparison between fat depots. †P < 0.05 for comparison between sexes.
GHR protein levels were higher by 62% in abdominal fat when compared with gluteal fat (P = 0.03). On the contrary, no difference was found in ATGL protein levels between the two depots (Figure 3, Panels A and B).
Figure 3.
(A) GHR, ATGL, and total ERK (a loading control) protein expression in abdominal (A) and gluteal (G) fat samples from five representative subjects. (B) Quantification of GHR and ATGL expression and (C) their correlation in abdominal (ABD) and gluteal (GLU) fat. *P < 0.05.
Sex differences
GHR mRNA levels were comparable between sexes in both depots (Figure 1). PLIN1 and ATGL mRNA expression was higher in the female than in the male gluteal fat depot (Figure 2, Panels A and C). No other sex differences in the expression of genes examined were noted.
In a smaller number of subjects (n = 3 males and n = 3 females), protein levels of GHR were higher in males than in females in both the abdominal depot (by 4.1-fold, P = 0.06) and the gluteal depot (by 3.9-fold, P = 0.005).
GHR mRNA expression associations with demographic parameters
Abdominal subcutaneous GHR mRNA expression correlated negatively with BMI and age (r = −0.38, P = 0.03 and r = −0.57, P = 0.0006 respectively), but not with waist-to-hip ratio (WHR; r = −0.001, P = 0.96). In contrast, GHR expression in the gluteal depot was unrelated to BMI and age, but showed a significant inverse correlation with WHR (r = −0.36, P = 0.03). There was no correlation between the GHR mRNA levels in the two depots.
In multiple linear regression analysis, with BMI, sex, and age as independent predictors of abdominal GHR mRNA expression, GHR expression was associated with age, independent of BMI and sex (log GHR = −0.03 Age + 1.21, total model R2 = 0.34, P = 0.007). When WHR, sex, and age were included in the model, the model explained 50% of the variability in abdominal GHR expression, and both age and WHR were statistically significant determinants (log GHR = −0.05 Age + 3.28 WHR − 1.67). When waist and hip were entered in the model separately, higher abdominal GHR mRNA was associated with higher waist circumference (P = 0.002) and lower hip circumference (P = 0.004); no interaction was noted. Models based on BMI, WHR, sex, and age as a function of gluteal GHR expression were not statistically significant.
To explore the determinants of the difference in GHR mRNA levels between the abdominal and gluteal depot, the variable [abdominal log GHR – gluteal log GHR] was entered as the dependent variable, and WHR, age, and sex were entered as independent variables. This model explained 48% of the variability of the GHR depot difference (R2 = 0.48, P = 0.002), with increasing WHR (P = 0.0006) and age (P = 0.0037), but not sex, as important determinants, that is, central fat distribution and age were associated with a larger depot difference (abdominal > gluteal).
Association between GHR mRNA levels and expression of lipolytic genes
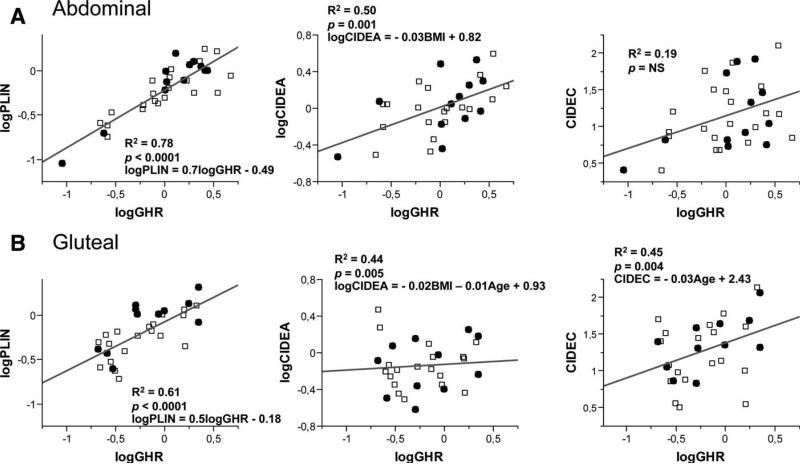
In both abdominal and gluteal depots, GHR mRNA levels correlated highly with ATGL (abdominal: r = 0.88, P < 0.0001; gluteal: r = 0.75, P < 0.0001; Figure 4), HSL (abdominal: r = 0.82, P < 0.0001; gluteal: r = 0.62, P = 0.01; Figure 4), PLIN1 (abdominal: r = 0.88, P < 0.0001; gluteal: r = 0.78, P < 0.0001; Figure 5), and CIDEC expression (abdominal: r = 0.71, P = 0.001; gluteal: r = 0.66, P = 0.005; Figure 5). In the gluteal, but not in the abdominal depot, GHR also correlated with mRNA levels of CIDEA (r = 0.67, P = 0.004; Figure 5). As a control, we examined the association between GHR and the α2-adrenergic receptor, and no correlation was noted in either the abdominal depot or the gluteal depot (not shown).
Figure 4.
Correlations between GHR mRNA and lipolytic enzyme mRNA levels in (A) abdominal and (B) gluteal adipose tissue. □, male subjects; •, female subjects.
Figure 5.
Correlations between GHR mRNA and lipid droplet-associated proteins (mRNA levels) in (A) abdominal and (B) gluteal adipose tissue depots. □, male subjects; •, female subjects.
GHR and ATGL protein levels were also correlated in the gluteal depot (Figure 3C). Multiple regression analysis models were constructed to control for possible confounders. GHR expression remained significantly associated with ATGL, HSL, and PLIN1 expression in both depots after controlling for BMI, age, and sex. In contrast, GHR expression was not associated with CIDEA and CIDEC expression after accounting for these factors.
Discussion
Our study supports the hypothesis that depot differences in the expression of GHR contribute to depot differences in GH effects. GHR expression was higher in abdominal subcutaneous fat when compared with gluteal subcutaneous fat of young healthy adult males and females and correlated significantly with the expression of critical regulators of lipolysis, including ATGL, HSL, PLIN1, and CIDEA in both abdominal and gluteal subcutaneous fat. These cross-sectional observations suggest that depot differences in GHR expression and action may be an important mechanism underlying the depot differences in TG storage and the in vivo effect of GH administration to preferentially reduce abdominal fat. This hypothesis must be verified by analyses of the impact of these variations in GHR expression on GH signaling and on downstream TG synthesis and turnover in vitro and in vivo.
Consistent with the current results, Gravholt et al. (31) found that basal glycerol concentrations (an index of lipolysis) as measured by microdialysis were higher in abdominal than femoral adipose tissue, and a pulse of GH produced a greater increment in lipolysis in abdominal adipose tissue when compared with femoral adipose tissue. The synergistic effect of GH and cortisol to induce lipolysis is also higher in the abdominal depot when compared with the femoral depot (32). We know of no data that assessed GH effects on gluteal adipose tissue lipolysis in vivo. However, in children of short stature who were not GH deficient, GH administration increased lipolysis in vitro in tissue fragments to a similar extent in both abdominal and gluteal adipose tissue, but it enhanced the insulin-stimulated esterification of exogenously added FA preferentially in the gluteal depot (27). These data are consistent with the observed decrease in abdominal subcutaneous adipocyte size, whereas the gluteal adipocyte size did not change. Zhao et al. (23) only focused on the femoral depot, which has many characteristics in common with the gluteal adipose tissue (e.g., increased adipocyte size and higher α2-receptor activity; ref. 33), and noted increased expression of genes related to esterification (LPIN1 and ACCS2), but not to lipolysis, in hypopituitary men treated with GH. We did not detect correlations between GHR and LPIN1 and ACSS2 in our microarray data, and it is possible that GH exerts different effects in healthy volunteers than in GH-deficient hypopituitary subjects.
Conclusion
Our data support the hypothesis that the GH effect to preferentially decrease central obesity is in part mediated by greater sensitivity to GH action at the level of the GHR. With regard to the possibility that the higher GHR mRNA levels have functional significance, we noted that the expression of key transducers of GH signaling, STAT5A (~40%) and STAT5B (~14%), was also higher in abdominal than gluteal adipose tissues (P < 0.005 and P < 0.01, respectively, microarray data). These results underline the need for studies on the mechanisms regulating GHR expression in different subcutaneous depots and how variations in GH action contribute to depot-dependent effects on net TG storage in human adipocytes.
Supplementary Material
Acknowledgments
Funding agencies: NIH P30DK46200, R24DK087669, R01 HL077674, K24 HL092902, and K23 RR23090.
Footnotes
Disclosure: The authors declared no conflict of interest.
Author contributions: K.K., S.K.F., and K.K.M. designed the study. K.K. performed qPCR measurements and analyzed the data. M.J.L. performed Western blotting. K.K., M.A.B., S.K.F., and K.K.M. wrote the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
References
- 1.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105:2696–2698. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- 4.Snijder MB, Henry RM, Visser M, et al. Regional body composition as a determinant of arterial stiffness in the elderly: the Hoorn Study. J Hypertens. 2004;22:2339–2347. doi: 10.1097/00004872-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 6.Rocha PM, Barata JT, Teixeira PJ, Ross R, Sardinha LB. Independent and opposite associations of hip and waist circumference with metabolic syndrome components and with inflammatory and atherothrombotic risk factors in overweight and obese women. Metabolism. 2008;57:1315–1322. doi: 10.1016/j.metabol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 8.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab. 2002;282:E1023–E1028. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- 9.Beauregard C, Utz AL, Schaub AE, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2063–2071. doi: 10.1210/jc.2007-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengtsson BA, Eden S, Lonn L, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 11.Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98:3864–3872. doi: 10.1210/jc.2013-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166:601–611. doi: 10.1530/EJE-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–734. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 14.Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 15.Lin E, Bredella MA, Gerweck AV, et al. Effects of growth hormone withdrawal in obese premenopausal women. Clin Endocrinol (Oxf) 2013;78:914–919. doi: 10.1111/cen.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasarica M, Zachwieja JJ, Dejonge L, Redman S, Smith SR. Effect of growth hormone on body composition and visceral adiposity in middle-aged men with visceral obesity. J Clin Endocrinol Metab. 2007;92:4265–4270. doi: 10.1210/jc.2007-0786. [DOI] [PubMed] [Google Scholar]
- 17.Richelsen B. Action of growth hormone in adipose tissue. Horm Res. 1997;48(Suppl 5):105–110. doi: 10.1159/000191338. [DOI] [PubMed] [Google Scholar]
- 18.Richelsen B, Pedersen SB, Borglum JD, Moller-Pedersen T, Jorgensen J, Jorgensen JO. Growth hormone treatment of obese women for 5 wk: effect on body composition and adipose tissue LPL activity. Am J Physiol. 1994;266:E211–E216. doi: 10.1152/ajpendo.1994.266.2.E211. [DOI] [PubMed] [Google Scholar]
- 19.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson BA, Brummer RJ, Eden S, Rosen T, Sjostrom L. Effects of growth hormone on fat mass and fat distribution. Acta Paediatr Suppl. 1992;383:62–65. discussion 6. [PubMed] [Google Scholar]
- 21.Richelsen B, Pedersen SB, Kristensen K, et al. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism. 2000;49:906–911. doi: 10.1053/meta.2000.6738. [DOI] [PubMed] [Google Scholar]
- 22.Dietz J, Schwartz J. Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism. 1991;40:800–806. doi: 10.1016/0026-0495(91)90006-i. [DOI] [PubMed] [Google Scholar]
- 23.Zhao JT, Cowley MJ, Lee P, Birzniece V, Kaplan W, Ho KK. Identification of novel GH-regulated pathway of lipid metabolism in adipose tissue: a gene expression study in hypopituitary men. J Clin Endocrinol Metab. 2011;96:E1188–E1196. doi: 10.1210/jc.2010-2679. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg I, Tran QT, Barkan AL, Saltiel AR, Chandler WF, Bridges D. Gene expression signature in adipose tissue of acromegaly patients. PLoS One. 2015;10:e0129359. doi: 10.1371/journal.pone.0129359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benencia F, Harshman S, Duran-Ortiz S, et al. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology. 2015;156:1794–1803. doi: 10.1210/en.2014-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olarescu NC, Berryman DE, Householder LA, et al. GH action influences adipogenesis of mouse adipose tissue-derived mesenchymal stem cells. J Endocrinol. 2015;226:13–23. doi: 10.1530/JOE-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum M, Gertner JM, Gidfar N, Hirsch J, Leibel RL. Effects of systemic growth hormone (GH) administration on regional adipose tissue in children with non-GH-deficient short stature. J Clin Endocrinol Metab. 1992;75:151–156. doi: 10.1210/jcem.75.1.1619004. [DOI] [PubMed] [Google Scholar]
- 28.Erman A, Veilleux A, Tchernof A, Goodyer CG. Human growth hormone receptor (GHR) expression in obesity. I. GHR mRNA expression in omental and subcutaneous adipose tissues of obese women. Int J Obes (Lond) 2011;35:1511–1519. doi: 10.1038/ijo.2011.23. [DOI] [PubMed] [Google Scholar]
- 29.Karastergiou K, Fried SK, Xie H, et al. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98:362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell M, Wang H, Chen H, et al. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravholt CH, Schmitz O, Simonsen L, Bulow J, Christiansen JS, Moller N. Effects of a physiological GH pulse on interstitial glycerol in abdominal and femoral adipose tissue. Am J Physiol. 1999;277:E848–E854. doi: 10.1152/ajpendo.1999.277.5.E848. [DOI] [PubMed] [Google Scholar]
- 32.Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Moller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab. 2004;286:E488–E494. doi: 10.1152/ajpendo.00199.2003. [DOI] [PubMed] [Google Scholar]
- 33.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26–29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.