Abstract
Perfluorocarbons (PFCs) are used in biomaterial formulations to increase oxygen (O2) tension and create a homogeneous O2 environment in 3-dimensional tissue constructs. It is unclear how PFCs affect mechanical and transport properties of the scaffold, which are critical for robustness, intracellular signaling, protein transport, and overall device efficacy. In this study, we investigate composite alginate hydrogels containing a perfluorooctyl bromide (PFOB) emulsion stabilized with Pluronic® F68 (F68). We demonstrate that PFC addition significantly affects biomaterial properties and performance. Solution and hydrogel mechanical properties and transport of representative hydrophilic (riboflavin), hydrophobic (methyl and ethyl paraben), and protein (bovine serum albumin, BSA) solutes were compared in alginate/F68 composite hydrogels with or without PFOB. Our results indicate that mechanical properties of the alginate/F68/PFOB hydrogels are not significantly affected under small strains, but a significant decrease fracture stress is observed. The effective diffusivity Deff of hydrophobic small molecules decreases with PFOB emulsion addition, yet the Deff of hydrophilic small molecules remained unaffected. For BSA, the Deff increased and the loading capacity decreased with PFOB emulsion addition. Thus, a trade-off between the desired increased O2 supply provided by PFCs and the mechanical weakening and change in transport of cellular signals must be carefully considered in the design of biomaterials containing PFCs.
Keywords: Perfluorocarbon, alginate, rheology, synthetic oxygen carrier, protein transport
INTRODUCTION
Hypoxia within biomaterial devices has limited the success and advancement of 3-dimensional (3D) tissue engineered constructs.1 Research has focused on increasing oxygen (O2) supply within macroscopic constructs through the addition of biologic or synthetic molecules to enhance oxygenation, including growth factors or signaling molecules to promote angiogenesis2,3 and synthetic O2 vectors, such as perfluorocarbons (PFCs), to increase O2 solubility within the material.4–6
PFCs have been investigated for their O2 delivery capabilities in many biological applications, most notably in the creation of artificial blood substitutes (e.g., Oxygent™ (Alliance Pharmaceutical Corporation), Fluosol (Green Cross Corporation)). Recently, research has focused on methods to incorporate these emulsions into cell-based tissue engineering applications. For example, a PFC-surfactant emulsion added to differentiation media promoted in vitro O2-mediated cell differentiation of the murine myogenic cell line C2Cl2, resulting in cells with greater ability to generate active force, a function of striated muscle.7 Alternatively, a PFC-silicone rubber membrane was used to enhance O2 diffusion to pancreatic bud explants cultured on 2-dimensional (2D) surfaces, resulting in greater endocrine and β-cell differentiation over exocrine and α-cell differentiation, respectively. Oxygent™, a perfluorooctyl bromide (PFOB)/lecithin emulsion, when added to cell culture media, was shown to enhance the partial tissue O2 tension and improve epithelial metabolism within tissue-engineered tracheal constructs formed on commercially available polyesteuruethane foam scaffolds.8 PFC emulsions also enhance O2 delivery and promote the growth of hepatocytes in the extra capillary space of hepatic hollow fiber bioreactors.8,9 For pancreatic transplantation, preservation of islet functionality is crucial to the success of the transplantation and PFC solutions have been used to maintain tissue oxygenation prior to transplantation.10–14 Our group and others have explored the addition of PFC emulsions to alginate or alginate-composite microcapsules to enhance encapsulated cell viability and functionality.4,6,15,16
PFCs, such as perfluorooctyl bromide (PFOB), are highly hydrophobic (KOW,PFOB = 6.87),17 necessitating the use of a surfactant, such as Pluronic® F68 (F68), for biomaterial formulations. Many of the PFC formulations used in the aforementioned investigations use a surfactant to solubilize their PFCs, but to date, no studies exist which discuss the specific implications of surfactant use in the PFC emulsion in terms of its effect on mechanical properties and transport within these devices. This work aims to address these issues in order to better guide the design of tissue engineered constructs containing PFCs.
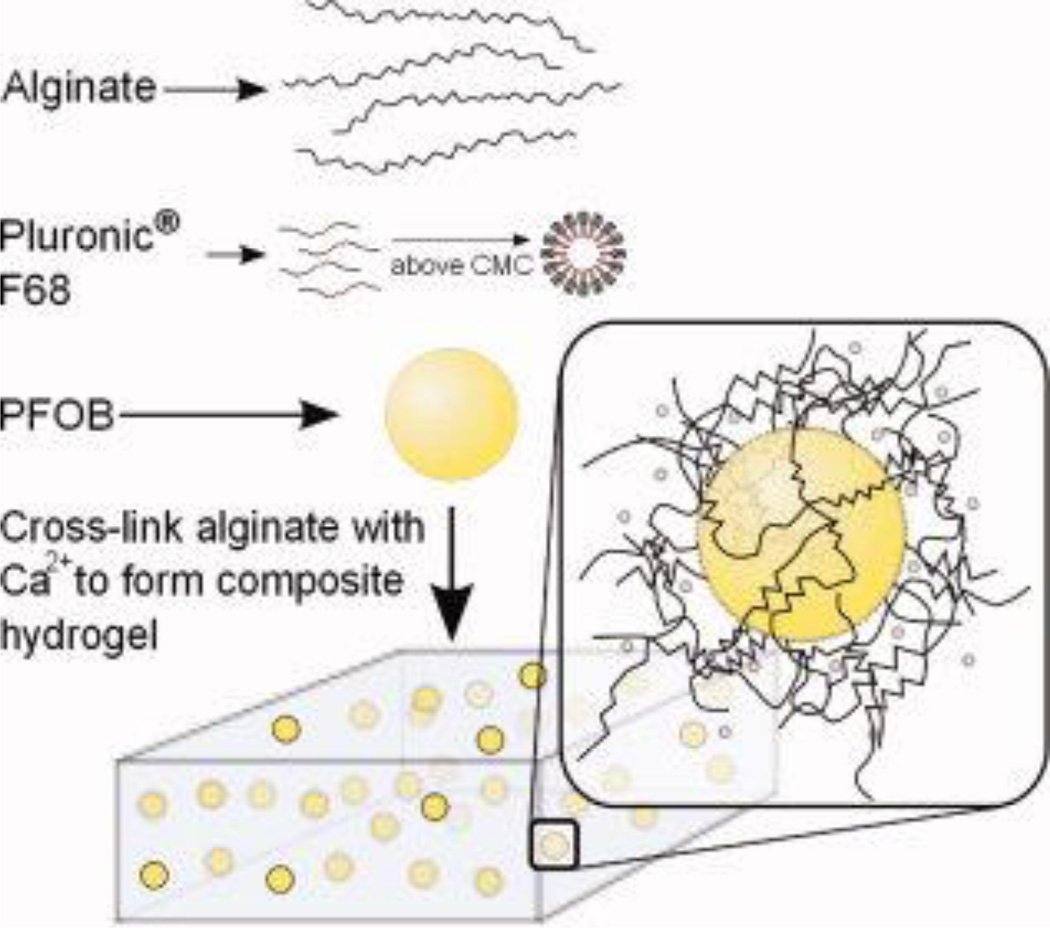
Previously, our laboratory has investigated the use of F68/PFOB emulsions in cell-seeded alginate hydrogels for increased O2 transport and enhanced cell viability within biomedical devices, including cell immobilization and encapsulation matrices4,15 as well as investigated the mechanical and transport implications for F68 addition to alginate hydrogels. F68 is a tri-block copolymer consisting of polyethylene oxide and polypropylene oxide units and is used at a concentration above its critical micelle concentration (CMC; 0.04 mM or 0.034 wt%) to stabilize the PFOB, forming micelles which can be entrapped within the alginate network during cross-linking (schematic shown in Figure 1). Our laboratory has previously determined that use of 1–2 wt% F68 in biomaterial design does not significantly affect mechanical properties, yet affects protein transport, which alters the final function of the biomaterial.18 However, results indicate that use of 5 wt% F68 significantly affects mechanical properties of an alginate hydrogel18 and therefore, this study focuses solely on the use of 1 or 2 wt% F68 in combination with PFOB in alginate hydrogels.
Figure 1.
The hydrogel system investigated in this work is comprised of 1% w/v alginate solution which contains a perfluorocarbon (perfluorooctyl bromide, PFOB) and a non-ionic surfactant (Pluronic® F68) at varying concentrations. Hydrogels are formed by internal crosslinking with 50 mM CaEDTA, which is hydrolyzed by 50 mM GDL. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
© IF THIS IMAGE HAS BEEN PROVIDED BY OR IS OWNED BY A THIRD PARTY, AS INDICATED IN THE CAPTION LINE, THEN FURTHER PERMISSION MAY BE NEEDED BEFORE ANY FURTHER USE. PLEASE CONTACT WILEY'S PERMISSIONS DEPARTMENT ON PERMISSIONSWILEY.COM OR USE THE RIGHTSLINK SERVICE BY CLICKING ON THE 'REQUEST PERMISSION' LINK ACCOMPANYING THIS ARTICLE. WILEY OR AUTHOR OWNED IMAGES MAY BE USED FOR NON-COMMERCIAL PURPOSES, SUBJECT TO PROPER CITATION OF THE ARTICLE, AUTHOR, AND PUBLISHER.
Specifically, this work investigates the addition of F68/PFOB emulsions to alginate hydrogels using bulk rheology, small and large strain mechanical testing, and loading and release of proteins and pharmaceuticals. While current research has shown encouraging evidence for O2 transport enhancement through the incorporation of synthetic O2 carriers within tissue engineering constructs,5,15 it is vital to understand their effect, not just on O2 transport within the bulk material, but also on the mechanical integrity and transport phenomena of biological and pharmaceutical molecules for the design of truly superior devices.
MATERIALS AND METHODS
Materials
Cell culture-grade sodium alginate (lot#1353824, %G ≥ 60, Mv~240 kDa, determined from intrinsic viscosity in 100 mM sodium chloride19) was obtained from Sigma Aldrich (St. Louis, MO). Polymer solutions were made with water purified using a Thermo Scientific Barnstead NANOPure® Infinity system (nanopure water) purified to 18 MΩ-cm. PFOB and methyl and ethyl paraben (MP and EP) were obtained from Acros Organics, a division of Thermo Fisher Scientific. Cell culture-grade Pluronic® F68, bovine serum albumin (BSA), HEPES buffer, D(+)-glucose, and sodium citrate were obtained from Sigma Aldrich. BCA Protein Assay kits were obtained from Pierce (New York, NY). All additional materials were obtained from Thermo Fisher Scientific (Waltham, MA) unless otherwise noted. All experiments were run at room temperature (25°C) and concentrations of Pluronic® F68 are low enough that they do not form thermoreversible gels under the conditions utilized.
Sample preparation
Sodium alginate solutions were prepared in nanopure water with final concentrations of glucose and HEPES buffer of 0.1% w/v and 10 mM, respectively, and stirred for 24 hours. F68, PFOB and nanopure water were emulsified for 30–60 minutes using a Thermo Fisher Aquasonic® 75HT sonicator (Waltham, MA) until no visible phase separation was observed. The emulsion was added to a concentrated alginate solution to create a final alginate concentration of 1% w/v. Solutions with 1% F68 and 0–7% PFOB or 2% F68 and 0–10% PFOB were stable for more than 30 minutes. Solutions containing 1% F68 and 10% PFOB began to phase separate within 30 minutes, so solution properties for this formulation are not reported.
Alginate hydrogels were prepared using in situ release of calcium ions, using a modified method to that previously reported.18,20 Briefly, CaEDTA was added to an alginate or alginate/F68/PFOB solution at a final concentration of 50 mM CaEDTA. Glucono-δ-lactone (GDL) was added initiate calcium release (50 mM). Solutions reached their gel point within 30 minutes and were stored in a humidified chamber (25°C, 90% humidity). PFOB-containing composite hydrogels are white and opaque, indicating a two-phase system. All hydrogel formulations were soaked in 100 mM CaCl2 containing the equivalent F68 concentration (24–48 hours). There was minimal PFOB loss and gels remained opaque. Care was required to prevent fracture of the alginate/F68/PFOB hydrogels. Hydrogels containing 1% F68/10% PFOB were successfully made due to the formation of the alginate network, preventing phase separation. The final alginate concentration for all solution formulations in this study is 1% w/v, though swelling studies indicate that during gelation more water is lost from formulations containing F68.18
Rheological and structural characterization
Solution viscosities were measured on a TA Instruments AR-G2 stress-controlled rheometer (New Castle, DE) using a 40 mm steel parallel plate geometry at 25 ± 0.1 °C and shear rates from 0.1 to 100 s−1. Alginate hydrogel mechanical properties were measured on a TA Instruments AR2000 stress-controlled rheometer (New Castle, DE) with a solvent trap using a 60 mm acrylic parallel plate geometry at 25 ± 0.1 °C using oscillation frequency sweeps from 0.01 to 100 Hz within the linear viscoelastic regime. All samples were cast in 100 mm petri dishes (n=4) and each sample was run once. Uniaxial compression tests were performed on a TA Instruments AR-G2 stress-controlled rheometer using a 40 mm steel parallel plate geometry at 25 ± 0.1 °C and 10% strain/minute until fracture was observed. The compression modulus was determined from the slope of the stress-strain curve for strains from 0 to 0.1. The fracture stress and strain were determined from the point at which the stress sharply decreased following the first fracture event. Gels for compression tests were formed and soaked in 24-well plates (3.5 mL alginate solution, n=6) as previously described.
Solute loading, release, and quantification
Protein and small molecules can be loaded into hydrogels using two methods: in situ loading and imbibing. In situ loading of BSA could not be performed due to the electrostatic interaction between alginate and BSA in solution21 and therefore this study only explored the loading and release of imbibed molecules. For riboflavin, fully formed hydrogels were soaked in a solution of 75 µg riboflavin per mL of nanopure water for 48 hours in 6-well non-tissue culture plates in a humidified incubator (≥90% humidity) at 25°C and hydrogels were flipped and stirred every 6 hours. For the paraben studies, the same procedure was followed to imbibe the hydrogels except a 0.05 wt% paraben (methyl or ethyl) in a 1% or 2% F68 solution prepared in nanopure water due to the hydrophobicity of the paraben molecules. Also, for this reason, transport of parabens within 1% alginate hydrogels without surfactant could not be performed. For experiments with BSA, the same procedure was utilized to imbibe the hydrogels, using a 25 mg/mL BSA solution.
Protein release studies were based on a protocol previously developed in our laboratory.18 Briefly, hydrogels formed in 12-well non-tissue culture plates were soaked in a 100 mM CaCl2 solutions containing BSA, riboflavin, or paraben, then moved from one well of a new 12-well plate to the next, each containing fresh nanopure water. Gels remained in each well for a predetermined amount of time (2, 4, 6, 8, 12, 15, 20, 40, 60, 80, 100, and 120 minutes), chosen based on trial runs which evaluated the maintenance of sink conditions. After 120 minutes, hydrogels dissolved in 5 mL of 100 mM sodium citrate to complete the mass balance analysis (used to calculate the total loading capacity). Six replicates were run for each condition and averages are reported. BSA concentration was quantified using a BCA Protein Assay kit by following the provided protocol and measured at 562 nm on a μQuant spectrophotometer (BioTek Instruments, Inc., Richmond, VA). Riboflavin concentration was measured on a μQuant spectrophotometer at 370 nm using a standard riboflavin curve. Similarly, paraben concentration was measured on a μQuant spectrophotometer at 280 nm using a standard curve of either methyl or ethyl paraben dissolved in 1% or 2% F68. All samples measured on the spectrophotometer were quantified in triplicate for each of the 6 hydrogels analyzed. Transport of a solute is modeled as solute release from a bulk hydrogel. No significant change in F68 or PFOB concentration was expected or observed during the experiment and no increase in hydrogel swelling was found. An effective diffusion coefficient (Deff) was calculated to capture overall transport dynamics in one parameter. The Deff was determined using experimental data and a 3D diffusion model19,22,23 shown in Equation 1, as previously described.18 Briefly, the mass of solute released while maintaining sink conditions throughout the 120 minute time course was quantified and fit to the model.
| (1) |
In Equation 1, h is half of the hydrogel thickness, r is the radius, t is time, Mt is the amount of protein released at time t, M∞ is the amount of protein released at infinite time, and j and k are integers. αj is defined as the roots of the zero-order Bessel function (Equation 2), and βk is defined by Equation 3. Summations in Equation 1 were evaluated for j=1,2,…,15 and k=0,1,…,150. The Deff was determined by the minimization of an objective function shown in Equation 4, where Mt* is the theoretical release fraction at time t, and the Me* is the experimental release fraction at the corresponding time t.
| (2) |
| (3) |
| (4) |
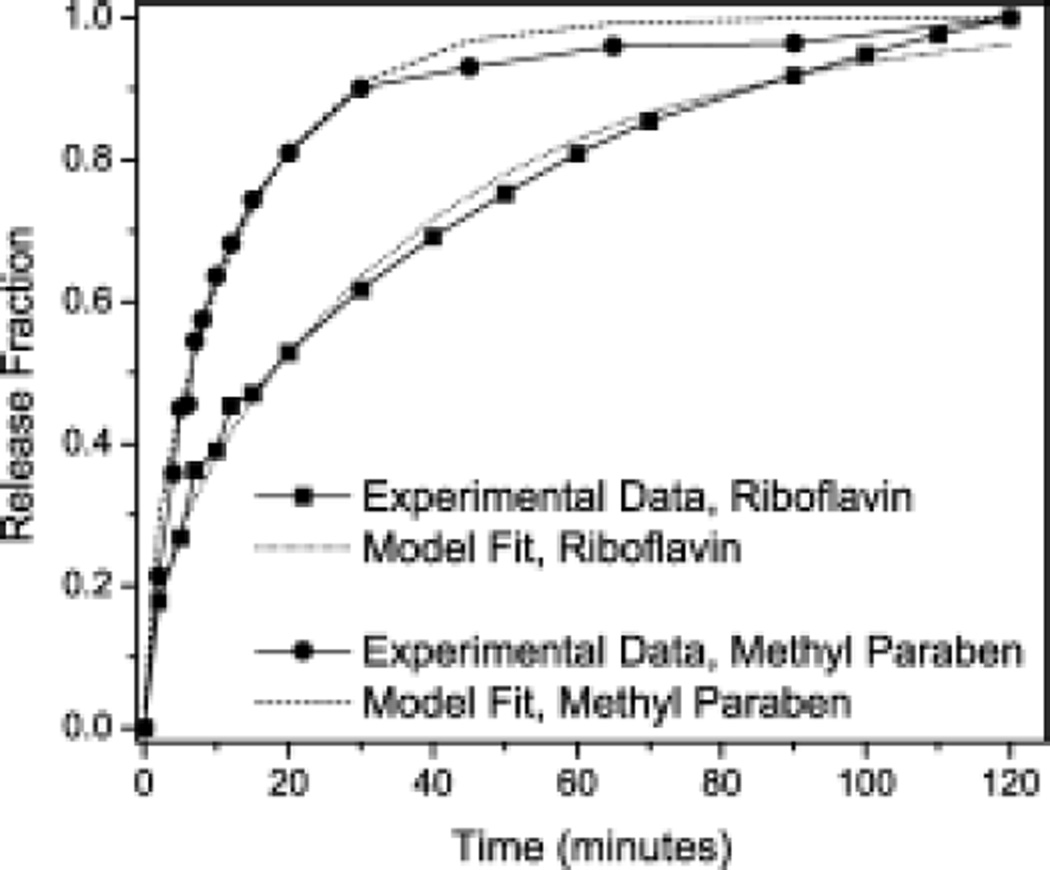
The Deff in porous media is a function of the diffusion coefficient, D, the porosity of the material, ε, the constrictivity, δ, and the tortuosity, τ as defined by Deff = (Dεδ)/τ (Equation 5).24,25 Therefore, a change in any of these variables will lead to a change in effective diffusivity. From release results (a representative release plot is shown in Figure 2), Deff was calculated.18
Figure 2.
Representative experimental release data for riboflavin (●) and methyl paraben (▪) and corresponding model fits from 1% alginate/2% F68/10% PFOB composite hydrogels. All % values are in w/v.
© IF THIS IMAGE HAS BEEN PROVIDED BY OR IS OWNED BY A THIRD PARTY, AS INDICATED IN THE CAPTION LINE, THEN FURTHER PERMISSION MAY BE NEEDED BEFORE ANY FURTHER USE. PLEASE CONTACT WILEY'S PERMISSIONS DEPARTMENT ON PERMISSIONSWILEY.COM OR USE THE RIGHTSLINK SERVICE BY CLICKING ON THE 'REQUEST PERMISSION' LINK ACCOMPANYING THIS ARTICLE. WILEY OR AUTHOR OWNED IMAGES MAY BE USED FOR NON-COMMERCIAL PURPOSES, SUBJECT TO PROPER CITATION OF THE ARTICLE, AUTHOR, AND PUBLISHER.
Particle size analysis
Dynamic light scattering (DLS) was performed using a Brookhaven Instrument Corporation ZetaPlus Particle Size Analyzer (Holtsville, NY) to determine the effect of increasing F68 concentration on the effective diameter of a PFOB emulsion. F68 was dissolved in 10 mM sodium chloride, PFOB was added, and the solution was sonicated for 30 minutes. A 90° angle, temperature of 25°C, and wavelength of 660 nm were used. Size distributions were analyzed using the Brookhaven Instrument Corporation Particle Analyzing software. Samples exhibited a bimodal size distribution, showing a small population of F68 micelles (diameter of 1–2 nm) and a larger population of F68/PFOB emulsion droplets (diameter >> 10 nm). Effective diameter and the corresponding standard deviations of the analysis are reported for the large population of F68/PFOB droplets only. Formulations of 1% F68 and 10% PFOB were not investigated due to phase separation.
Scanning Electron Microscopy (SEM)
Hydrogels were prepared as described above, fixed in ethanol, critical-point freeze dried, sputtered with a thin layer of gold, and imaged using a Magellan™ 400 SEM (FEI Inc., Hillsboro, OR).
Statistical analysis
All samples were run in triplicate unless otherwise noted. Rheological properties at each independent variable and protein loading and release were analyzed for significance using a Student’s t-test. All reported values are mean ± standard deviation. Deff was analyzed using a Student’s t-test comparison of samples one standard deviation above and below the average release rate for each sample type. Differences were considered statistically significant for p≤0.05.
RESULTS AND DISCUSSION
Mechanical properties
PFCs have been investigated as O2 carriers for biomedical applications to increase the overall O2 solubility in a 3D device or to create a homogeneous O2 environment.4,5,7,10,15,26 Creating a more homogeneous O2 concentration profile within a construct may enhance device efficacy by reducing hypoxia-related cell death due to non-uniform O2 distribution.4,15 Consequently, it is equally important to monitor how PFC incorporation affects other device properties, such as mechanical integrity and solute transport.
Solution viscosity
The viscosity of alginate/F68/PFOB emulsions was measured over shear rates from 0.1–100 s−1 and the viscosity profile resembled Newtonian-like behavior for shear rates greater than 1 s−1 (data not shown). Results at 10 s−1 were analyzed for statistical significance because this was in the middle of the Newtonian regime. At 10 s−1, all solutions containing a PFOB emulsion were statistically similar to each other, with values between 0.051–0.052 Pa s (p>0.05), and statistically greater than the 1% alginate solution, with a viscosity of 0.043 Pa s (p<0.05). However, the solutions containing PFOB were also statistically similar to 1% alginate with 1% or 2% F68, indicating that the change in the viscosity is mainly due to surfactant addition.18 This is in agreement with our previous work on alginate/F68 solutions.18 Increasing trends in viscosity should be monitored further for greater PFC incorporation or use of a PFC with higher molecular weight (>10%) since we expect significant increases in the viscosity to affect processing parameters in biomedical applications. For example, in cell encapsulation processes a dramatic increase in polymer viscosity affects capsule size, morphology, and immobilized cell viability, due to an increase in stress exerted on the cell membrane as the cell solution is extruded through a needle.27
Particle sizing in solution
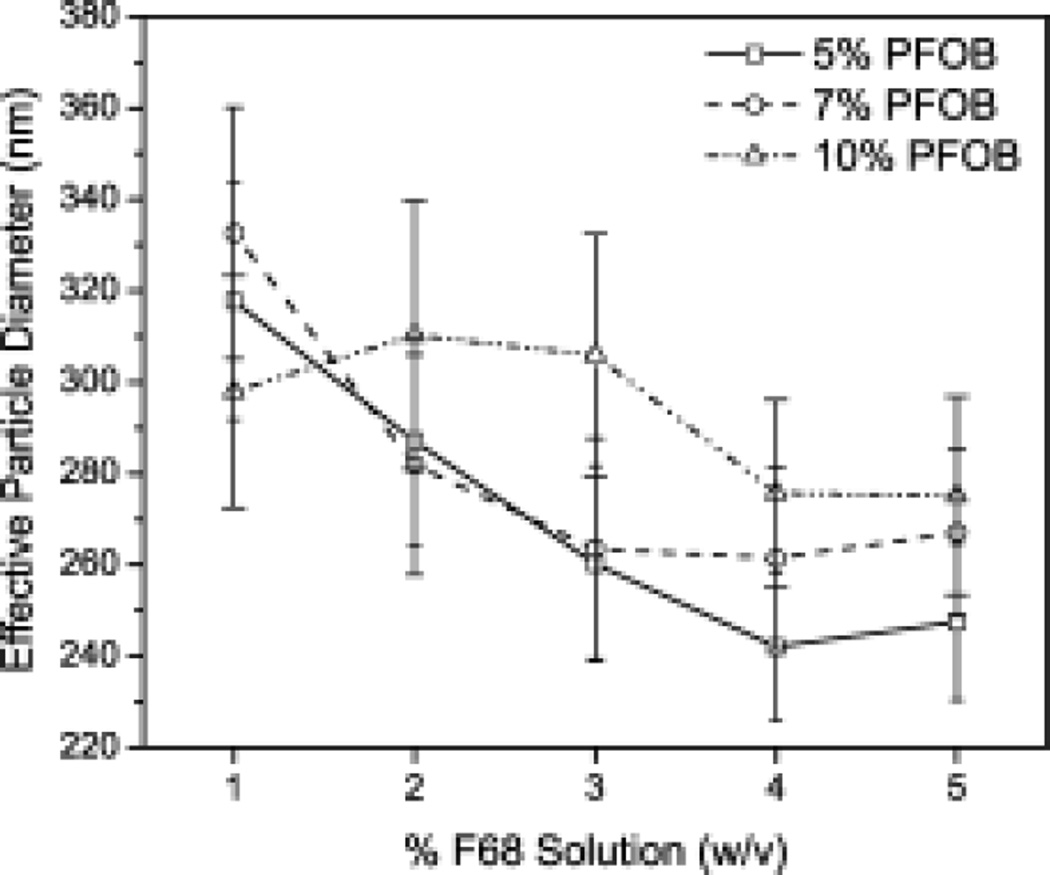
Distribution of F68/PFOB emulsion size within the alginate solution may affect solution properties28 or have a major impact on bulk properties of a material (e.g., Deff).29 F68/PFOB particle size in F68 solutions ranged from 240–330 nm, decreasing in particle size with increasing F68 concentration (Figure 3). Results show that smaller emulsion droplets were achieved with higher F68 concentrations, but final size depends on both F68 and PFOB concentration which suggests that the emulsion particle size in a hydrogel matrix depends on F68 and PFOB concentration as well as homogenization technique (e.g., sonication, high-shear mixing). Specifically, in the results depicted in Figure 3, for a specific PFOB concentration, there is no significant difference in particle size across 1–5% F68 (p<0.05). However, the standard deviations get smaller, suggesting a more uniform particle size is achieved at high PFOB concentrations. Secondly, for 3%, 4%, and 5% F68 solutions, the average particle size for 5% and 10% PFOB are statistically different (p<0.05), also due to the narrowing of the range of particle sizes in solution. Overall, these changes in particle size have implications in the oxygen diffusion of the hydrogel because differences in PFC particle size can lead to differences in O2 transport (site Stabler, JBMR, 2012). Therefore, formulation design and optimization may be required for a particular oxygen demand.
Figure 3.
Average particle size of F68/PFOB emulsions is concentration dependent. Average diameter of F68/PFOB emulsions decrease in increasing F68, but increase with increasing PFOB. 10% PFOB emulsions (∆) show the largest particle size, followed by 7% PFOB (○) and 5% PFOB (□). All % values are in w/v. Error is reported as the standard deviation across all samples taken.
© IF THIS IMAGE HAS BEEN PROVIDED BY OR IS OWNED BY A THIRD PARTY, AS INDICATED IN THE CAPTION LINE, THEN FURTHER PERMISSION MAY BE NEEDED BEFORE ANY FURTHER USE. PLEASE CONTACT WILEY'S PERMISSIONS DEPARTMENT ON PERMISSIONSWILEY.COM OR USE THE RIGHTSLINK SERVICE BY CLICKING ON THE 'REQUEST PERMISSION' LINK ACCOMPANYING THIS ARTICLE. WILEY OR AUTHOR OWNED IMAGES MAY BE USED FOR NON-COMMERCIAL PURPOSES, SUBJECT TO PROPER CITATION OF THE ARTICLE, AUTHOR, AND PUBLISHER.
Hydrogel mechanical properties
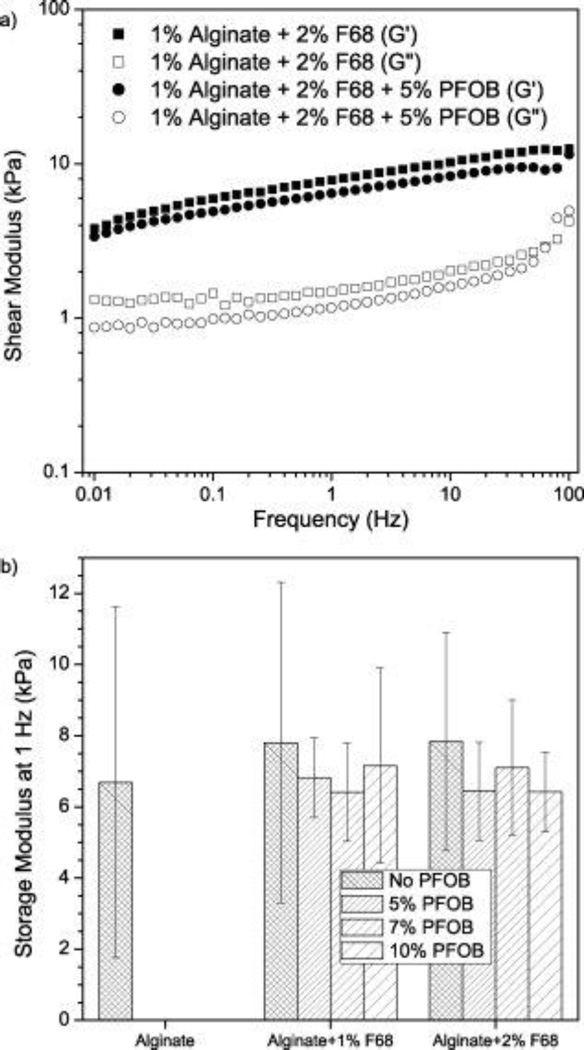
Bulk mechanical properties of alginate/F68/PFOB hydrogels were evaluated to monitor the effect of PFOB incorporation on the storage modulus (G’, solid-like behavior) and loss modulus (G”, liquid-like behavior) of the composite hydrogels. G’ and G” were measured using small oscillatory shear rheometry. Representative data are shown in Figure 4, and the spectra are characteristic of elastic gels18,30 with a weak dependence of G’ on frequency and a minimum in G” with frequency.
Figure 4.
Shear modulus of alginate/F68/PFOB composite matrices. (a) Representative plot of storage (G′, filled symbols) and loss (G″, open symbols) moduli as a function of oscillation frequency. Both G′ and G″ show a weak frequency dependence. Alginate/2% F68 (▪, □) and alginate/2% F68/5% PFOB (●, ○) have similar profiles (n = 4). (b) Storage modulus at a frequency of 1 Hz. Comparison is made to show statistical similarity amongst alginate hydrogels containing F68/PFOB emulsions (p > 0.05, n = 4). All % values are in w/v.
Figure 4b shows G’ at 1 Hz for all formulations to highlight the statistical similarity. The addition of F68/PFOB emulsions to alginate hydrogels did not significantly change the magnitude of G’ at any frequency measured for all formulations investigated (p>0.05). G’ maintains a weak frequency dependence with addition of F68/PFOB emulsions, where the values of G’ measured at 0.01 Hz are ~4 kPa, and increase to ~10 kPa at 100 Hz (data not shown). Similarly, uniaxial compression was performed under small strain and the Young’s modulus (E) was not significantly affected by PFOB addition (~9.5 kPa, p>0.05), as shown in Table 1. Therefore, for the formulations investigated in this study, there were no significant changes in the small strain rheological behavior following the incorporation of F68/PFOB emulsions. A notable outcome of these data is that the stiffness of alginate hydrogels containing F68/PFOB emulsions is similar to the alginate control (~7 kPa), suggesting that the overall gaseous exchange of the composite material can be improved without sacrificing macroscale mechanical durability under small strains.
Table 1.
Mechanical properties from compression of alginate/F68/PFOB composite hydrogels.
| E (kPa) | Fracture Strain | |
|---|---|---|
| 1% F68 | ||
| 0% PFOB | 9 ± 1.4 | 0.83 ± 0.02 |
| 7% PFOB | 10 ± 1.2* | 0.85 ± 0.01* |
| 10% PFOB | 10 ± 1.2* | 0.82 ± 0.04* |
| 2% F68 | ||
| 0% PFOB | 9 ± 0.7 | 0.85 ± 0.01 |
| 7% PFOB | 10 ± 0.6* | 0.85 ± 0.02* |
| 10% PFOB | 9 ± 0.4* | 0.78 ± 0.04* |
Statistical similarity to 0% PFOB (p ≥ 0.05, n = 4).
Standard deviations are reported.
However, physical observation during experimentation demonstrated that alginate/F68/PFOB composite hydrogels behaved differently and were more prone to fracture during experimentation. Fracture properties were investigated using uniaxial compression to explore the response to high strain. High strain mechanical testing showed a significant decrease in fracture stress for formulations containing 10% PFOB (Figure 5, p<0.05). Fracture stress for 10% PFOB formulations was 50% less than the formulations containing only F68. There was no significant difference in the measured fracture strain (~80% strain, Table 1) suggesting that the F68/PFOB emulsion does not contribute to changes in alginate chain conformation. Therefore, the decrease in fracture stress may be attributed to local concentrations of stress around the F68/PFOB inclusions.
Figure 5.
Fracture stress from uniaxial compression, compressed at a strain rate of 10%/min. The 10% PFOB formulations show lower fracture stress than alginate/F68 composite hydrogels. (*) denotes statistical difference from the appropriate F68 control sample (p ≤ 0.05, n ≥ 4). All % values are in w/v.
© IF THIS IMAGE HAS BEEN PROVIDED BY OR IS OWNED BY A THIRD PARTY, AS INDICATED IN THE CAPTION LINE, THEN FURTHER PERMISSION MAY BE NEEDED BEFORE ANY FURTHER USE. PLEASE CONTACT WILEY'S PERMISSIONS DEPARTMENT ON PERMISSIONSWILEY.COM OR USE THE RIGHTSLINK SERVICE BY CLICKING ON THE 'REQUEST PERMISSION' LINK ACCOMPANYING THIS ARTICLE. WILEY OR AUTHOR OWNED IMAGES MAY BE USED FOR NON-COMMERCIAL PURPOSES, SUBJECT TO PROPER CITATION OF THE ARTICLE, AUTHOR, AND PUBLISHER.
We expect that the F68/PFOB micelles locally deform the network possibly causing localized stress along the chains during gelation, leading to the observed early fracture. The exact size of the F68/PFOB micelles within the alginate hydrogel is unknown due to the variation among particles sizes in solution prior to gelation (see Figure 3) as well as changes in particle size over time due to micelle aggregation.
Results presented here suggest that use of 3D scaffolds containing PFC emulsions may not be suitable for applications where high compressive strength is desired, such as cartilage tissue engineering.31 While some composite formulations offer improved hydrogel mechanical strength (e.g., multi-network scaffolds), the incorporation of PFCs to enhance O2 supply can potentially negate the added mechanical benefits. Consequently, the application of PFCs in 3D biomaterials should be limited to situations where high compressive stress is not required or PFCs should be incorporated in the polymer itself through chemical modifications.
Transport of small molecules and proteins
Recent focus on the use of PFCs to enhance O2 transport within a biomaterial has led to promising results and better device performance.5,7,15,26 Addition of a surfactant leads to changes in transport of small molecules and proteins within a hydrogel.18,32 Therefore, investigation of F68/PFOB emulsions within alginate hydrogels is necessary to understand the limitations of using PFCs in biomaterial formulations.
To investigate these effects, the release of two hydrophobic small molecules (methyl and ethyl paraben), a small, water-soluble metabolite (riboflavin), and a large, multi-domain protein (BSA) were measured over a two hour period. During the release period, it is expected that some free F68 and F68/PFOB micelles are able to leave the hydrogels. However, due to the lack of observed hydrogel swelling during the release period and the relatively short time for release, it was determined that the loss does not significantly affect the overall release of solute.18 Nonetheless, the following calculated diffusion coefficients are considered to represent effective diffusivity (Deff) from the composite hydrogels. Any facilitation of solute release due to F68 loss is captured by Deff. Overall, results indicate that the size and hydrophobicity of the molecule play crucial roles in determining the effect of PFOB on molecular transport within the hydrogel.
Parabens
MP and EP are commonly used as anti-fungals in shampoos, lotions, and other over-the-counter cosmetics. In our study, MP and EP represent hydrophobic small molecules which have increasing octanol-water (KOW)33 and micelle (Pluronic® F127)-water (KMW)34 partition coefficients with increasing chain length (see Table 2). It was expected that EP should prefer the PFOB phase more than MP, decreasing the Deff. The partition coefficient affects a solute’s diffusion coefficient in a given material, and therefore an increase in the KOW and KMW will lead to a decrease in the diffusion coefficient, D, an important parameter in Deff.24,25 Our results support this phenomenon by showing that the Deff of MP is larger than the Deff of EP for all composite hydrogel formulations investigated (Table 2). PFOB emulsion inclusion decreased the Deff of both MP and EP in alginate/F68/PFOB hydrogels (up to a 32% decrease) and kept the MP and EP from fully diffusing out of the hydrogel after 2 hours (only 88%), demonstrating an interaction of MP and EP with F68/PFOB emulsion, preventing complete release even though the size of the paraben molecules is smaller than the hydrogel mesh size.
Table 2.
Transport properties of riboflavin, methyl paraben, and ethyl paraben in alginate/F68/PFOB composite hydrogels.
| Loading Capacity g /g dry polymer |
% Release (2 hrs) |
Deff (× 10−10) m2/s |
Obj. Fxn |
|
|---|---|---|---|---|
| Methyl Paraben Kow=2.029 Kmw (F127:water)=77 ± 230 | ||||
| 1% F68 | 3.3 ± 0.2 | 89 ± 1.8 | 8.5 ± 0.6 | 0.0 |
| 1% F68 + 10% PFOB | 4.0 ± 0.3 | 90 ± 1.7 | 6.6 ± 0.5* | 0.0 |
| 2% F68 | 3.3 ± 0.4 | 90 ± 2.3 | 8.0 ± 0.8 | 0.0 |
| 2% F68 + 10% PFOB | 3.0 ± 0.3 | 89 ± 2.3 | 6.0 ± 0.2* | 0.0 |
| Ethyl Paraben Kow=2.9829 Kmw (F127:water)=560 ± 930 | ||||
| 1% F68 | 3.0 ± 0.5 | 89 ± 1.3 | 7.2 ± 0.6 | 0.0 |
| 1% F68 + 10% PFOB | 4.0 ± 0.4 | 91 ± 1.2 | 5.2 ± 0.2* | 0.0 |
| 2% F68 | 2.7 ± 0.3 | 91 ± 1.1 | 7.3 ± 0.7 | 0.0 |
| 2% F68 + 10% PFOB | 2.7 ± 0.1 | 87 ± 1.8* | 5.0 ± 0.2* | 0.1 |
| Riboflavin Kow=0.35 | ||||
| 1% Alginate Only18 | 50 ± 3 | 99 ± 1.3 | 1.7 ± 0.1 | 0.1 |
| 1% F68 | 45 ± 4 | 99 ± 0.7 | 1.9 ± 0.2 | 0.2 |
| 1% F68 + 10% PFOB | 48 ± 6 | 88 ± 2.2* | 2.0 ± 0.3 | 0.1 |
| 2% F68 | 52 ± 3 | 97 ± 0.8 | 1.9 ± 0.2 | 0.1 |
| 2% F68 + 10% PFOB | 49 ± 3 | 92 ± 0.4* | 2.2 ± 0.2 | 0.2 |
Alginate Only (Riboflavin) is provided as a comparison.18
Statistical difference from sample with F68 but without PFOB (p ≤ 0.05, n = 6).
Standard deviations are reported.
Results imply potential difficulties in using a PFC-containing hydrogel as a delivery vehicle for hydrophobic small molecules. O2 delivery is not relevant for all drug delivery systems and is often overlooked in favor of tunable release kinetics. However, a combination therapy utilizing cell-seeded scaffolds containing drug delivery vehicles may be desired, thus providing a sufficient O2 supply to the seeded cells would enhance construct efficacy. Our results demonstrate that PFC addition interferes with release kinetics for hydrophobic small molecules.
Riboflavin
Diffusion and transport of a small, water soluble metabolite, riboflavin (vitamin B2), was investigated to show the importance of size and molecular characteristics in diffusion and transport within alginate/F68/PFOB composite hydrogels. Unlike MP and EP, the octanol-water partition coefficient is below 1, indicating preference for the water phase of the composite hydrogel. The Deff of riboflavin in alginate/F68/PFOB hydrogels was not significantly affected by the addition of 10% PFOB (Deff ≈2×10−10 m2/s for all formulations). However, the percent release of riboflavin significantly decreased (12% decrease for 1% F68/10% PFOB and 5% decrease for 2% F68/10% PFOB). For a water-soluble vitamin small molecule, transport within the hydrogel is largely unaffected by F6818 and PFOB emulsion addition.
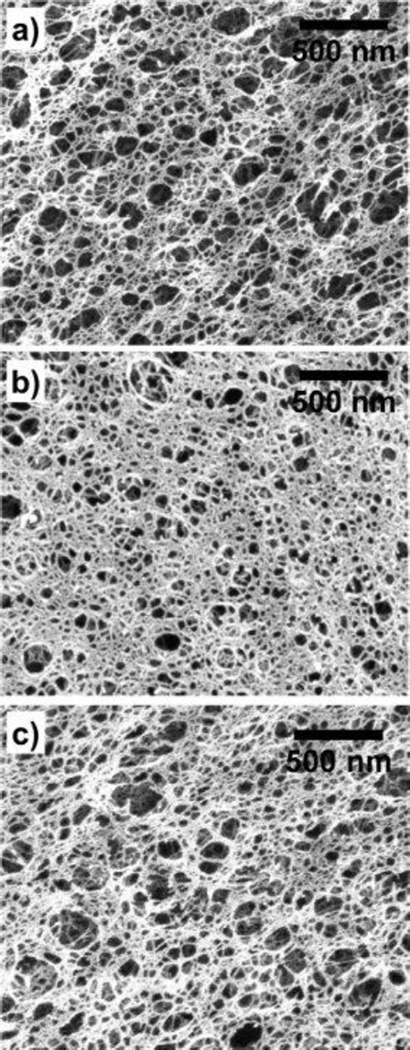
The lack of statistically significant changes in the transport of riboflavin in the presence of PFOB indicates that the structure and hydrophobicity of the small molecule play critical roles in the transport within alginate/F68/PFOB hydrogels. Compared to MP and EP, riboflavin does not interact with the F68/PFOB emulsion (KOW = 0.35), yet it is similar in size to MP and EP (~0.1 nm).35 As shown in the literature, the porosity shown by SEM can indicate large differences in pore structure of a given material.36,37 Literature reports the average mesh size for the alginate/F68 hydrogels to be on the order of 10 nm through imaging and swelling analysis18 and SEM micrographs shown in Figure 6 did not indicate a dramatic changes in mesh size with PFOB addition. Therefore, diffusion limitations due to size exclusion are not expected for MP, EP, or riboflavin.
Figure 6.
Scanning electron micrographs (SEM) of alginate/F68/PFOB composite matrices shows no significant change in mesh size with the addition of PFOB. (a) 1% alginate/1% F68. (b) 1% alginate/1% F68/5% PFOB. (c) 1% alginate/1% F68/10% PFOB. All % values are in w/v.
© IF THIS IMAGE HAS BEEN PROVIDED BY OR IS OWNED BY A THIRD PARTY, AS INDICATED IN THE CAPTION LINE, THEN FURTHER PERMISSION MAY BE NEEDED BEFORE ANY FURTHER USE. PLEASE CONTACT WILEY'S PERMISSIONS DEPARTMENT ON PERMISSIONSWILEY.COM OR USE THE RIGHTSLINK SERVICE BY CLICKING ON THE 'REQUEST PERMISSION' LINK ACCOMPANYING THIS ARTICLE. WILEY OR AUTHOR OWNED IMAGES MAY BE USED FOR NON-COMMERCIAL PURPOSES, SUBJECT TO PROPER CITATION OF THE ARTICLE, AUTHOR, AND PUBLISHER.
Bovine serum albumin
BSA was investigated due to the multiple structural domains present within the molecule to demonstrate the complexity involved in understanding the effect of PFOB on protein transport. The transport of BSA was significantly affected by the addition of PFOB to composite hydrogels. Table 3 shows a significant decrease in BSA loading capacity as well as in the percent released after two hours with the addition of PFOB. For example, there was a 22% decrease in loading capacity and a 13% decrease in percent released for composite hydrogels containing 2% F68/10% PFOB. A decrease in release percentage may be due to increased BSA particle size due to interaction of BSA with F68 alone (as previously reported)18 or with both F68 and PFOB. Consequently, a significant increase in Deff was found (29% for 2% F68/10% PFOB). Therefore, aside from any changes to matrix mechanical properties, such as pore size or modulus, the increase in Deff can be partially explained by an increase in constrictivity (δ), the ratio of particle diameter to pore diameter.24,25 An increase in particle size would lead to an increase in Deff through an increase in δ (see Equation 5).
Table 3.
Transport properties of BSA in alginate/F68/PFOB composite hydrogels.
| Loading Capacity g /g dry polymer |
% Release (2 hrs) |
Deff (× 10−10) m2/s |
Obj. Fxn |
|
|---|---|---|---|---|
| BSA | ||||
| 1% Alginate Only18 | 51 ± 4 | 95 ± 3 | 0.5 ± 0.10 | 0.2 |
| 1% F68 | 35 ± 4 | 88 ± 6 | 0.6 ± 0.08 | 0.0 |
| 1% F68 + 10% PFOB | 27 ± 2* | 76 ± 4* | 0.8 ± 0.04* | 0.1 |
| 2% F68 | 29 ± 1 | 82 ± 4 | 0.7 ± 0.05 | 0.0 |
| 2% F68 + 10% PFOB | 23 ± 2* | 71 ± 4* | 0.9 ± 0.04* | 0.1 |
Alginate only was added as a comparison.18
Statistical difference from sample without PFOB (p ≤ 0.05, n = 6).
Standard deviations are reported.
Structurally complex molecules, such as serum albumin or insulin, have important functions in vivo. Successful transport of such proteins within engineered scaffolds is crucial for efficacious device design and function. While the addition of a PFC emulsion will enhance O2 transport within a 3D tissue engineering construct,4,15 we expect that PFCs will limit the release of cell-secreted proteins, signaling molecules, or other metabolic agents. For example, addition of a PFC emulsion to encapsulated islets or β-cells16 may alter the release profile of secreted insulin from the encapsulation device, negatively affecting device efficacy. Insulin has been shown to aggregate over time38 and our results suggest that interaction between the insulin hexamer and PFC droplets will increase the aggregation potential.
PFCs have also been shown to direct stem cell differentiation through enhanced O2 delivery,5,39 leading to promising results in the in vivo differentiation of mesenchymal stem cells into osteocytes.5 The results obtained by Kimelman-Bleich and colleagues were not consistent across all of the in vivo models examined,5 indicating that the mechanism of action of the PFC addition is not well understood. Therefore, the changes in transport and mechanical integrity demonstrated in this work indicate that PFC emulsion addition may impede overall 3D tissue engineering device efficacy, even though PFC addition enhances the viability of cells within a 3D biomaterial.5,16,26 Therefore, the use of PFCs in 2D constructs, such as that demonstrated by Fraker, et al.,26 where PFCs were incorporated into a silicone membrane used for the differentiation of murine embryonic pancreatic bud explants26 is recommended. In this design, protein transport limitations are not a factor in device design and instead, the 2D construct provides an ideal way to study the role of varying O2 tension on stem cell differentiation.
CONCLUSION
Results indicate that while addition of PFOB does not significantly affect bulk mechanical properties and hydrogel microstructure under conditions of small strain or the transport of small hydrophilic molecules, there is a significant change in transport of small hydrophobic molecules and large proteins as well as the mechanical behavior at high strains. This may lead to a possible mechanical destabilization or decrease in cell viability under certain in vivo conditions. Thus, although PFCs may provide unique benefits in terms of O2 supply, this must be carefully weighed against potential problems in transport of biomolecules within a cell-based device and mechanical robustness at large strains.
Acknowledgments
This work was supported by the National Science Foundation (NSF)-sponsored Center for Hierarchical Manufacturing (CMMI-0531171) and the University of Massachusetts Commercial Ventures and Intellectual Property Office. We acknowledge use of the NSF-funded Materials Research Science and Engineering Center (MRSEC) on Polymers (DMR-0213695) central facilities. J.C.W. acknowledges support from the NSF-sponsored Institute for Cellular Engineering IGERT program (DGE-0654128). W.L.S. acknowledges support from a National Research Service Award T32 GM08515 from the National Institutes of Health.
Contributor Information
Joseph C. White, Email: jwhite@ecs.umass.edu.
Whitney L. Stoppel, Email: wstoppel@ecs.umass.edu.
Susan C. Roberts, Email: sroberts@ecs.umass.edu.
Surita R. Bhatia, Email: sbhatia@ecs.umass.edu.
REFERENCES
- 1.Stoppel WL, Roberts SC. Oxygen Supply for Tissue Engineering. In: Bhatia SK, editor. Engineering Biomaterials for Regenerative Medicine. Springer New York: 2012. pp. 41–86. [Google Scholar]
- 2.Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials. 2011;32(25):5782–5789. doi: 10.1016/j.biomaterials.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 3.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. Journal of Thrombosis and Haemostasis. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 4.Chin K, Khattak SF, Bhatia SR, Roberts SC. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnology Progress. 2008;24(2):358–366. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- 5.Kimelman-Bleich N, Pelled G, Sheyn D, Kallai I, Zilberman Y, Mizrahi O, Tal Y, Tawackoli W, Gazit Z, Gazit D. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009;30(27):4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Goh F, Gross JD, Simpson NE, Sambanis A. Limited beneficial effects of perfluorocarbon emulsions on encapsulated cells in culture Experimental and modeling studies. Journal of Biotechnology. 2010;150(2):232–239. doi: 10.1016/j.jbiotec.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita H, Shimizu K, Morioka Y, Nagamori E. Enhancement of C2C12 differentiation by perfluorocarbon-mediated oxygen delivery. Journal of Bioscience and Bioengineering. 2010;110(3):359–362. doi: 10.1016/j.jbiosc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Tan Q, El-Badry AM, Contaldo C, Steiner R, Hillinger S, Welti M, Hilbe M, Spahn DR, Jaussi R, Higuera G, et al. The Effect of Perfluorocarbon-Based Artificial Oxygen Carriers on Tissue-Engineered Trachea. Tissue Engineering Part A. 2009;15(9):2471–2480. doi: 10.1089/ten.tea.2008.0461. [DOI] [PubMed] [Google Scholar]
- 9.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288(3):H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 10.Maillard E, Sanchez-Dominguez M, Kleiss C, Langlois A, Sencier MC, Vodouhe C, Beitigier W, Krafft MP, Pinget M, Belcourt A, et al. Perfluorocarbons: New Tool for Islets Preservation In Vitro. Transplantation Proceedings. 2008;40(2):372–374. doi: 10.1016/j.transproceed.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Qualley SA, Rigley TH, Marsh CL, Stevens RB. Prolonged preservation of the human pancreas prior to islet isolation using the two-layer (University of Wisconsin solution [UW]/perfluorocarbon) method. Transplantation. 2000;69(8):384. [Google Scholar]
- 12.Ricordi C, Fraker C, Szust J, Al-Abdullah I, Poggioli R, Kirlew T, Khan A, Alejandro R. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75(9):1524–1527. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 13.Tanioka Y, Tanaka T, Gotoh T, Kakinoki K, Li S, Yoshikawa T, Sakai T, Fujino Y, Suzuki Y, Kuroda Y. Vienna, AUSTRIA: 2004. Sep 05–10, Augmentation of tissue ATP level during digestion using preoxygenated perfluorocarbon results in high islet yield - New strategy for islet isolation; pp. 220–222. [DOI] [PubMed] [Google Scholar]
- 14.Kin T, Mirbolooki M, Salehi P, Tsukada M, O'Gorman D, Imes S, Ryan EA, Shapiro AMJ, Lakey JRT. Islet isolation and transplantation outcomes of pancreas preserved with University of Wisconsin solution versus two-layer method using preoxygenated perfluorocarbon. Transplantation. 2006;82(10):1286–1290. doi: 10.1097/01.tp.0000244347.61060.af. [DOI] [PubMed] [Google Scholar]
- 15.Khattak SF, Chin KS, Bhatia SR, Roberts SC. Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnology and Bioengineering. 2007;96(1):156–166. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AS, Fisher RJ, Weir GC, Colton CK. Oxygen consumption and diffusion in assemblages of respiring spheres: Performance enhancement of a bioartificial pancreas. Chemical Engineering Science. 2009;64(22):4470–4487. [Google Scholar]
- 17.Riess JG. Oxygen carriers ("blood substitutes") - Raison d'Etre, chemistry, and some physiology. Chemical Reviews. 2001;101(9):2797–2919. doi: 10.1021/cr970143c. [DOI] [PubMed] [Google Scholar]
- 18.Stoppel WL, White JC, Horava SD, Bhatia SR, Roberts SC. Transport of biological molecules in surfactant–alginate composite hydrogels. Acta Biomaterialia. 2011;7(11):3988–3998. doi: 10.1016/j.actbio.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnology and Bioengineering. 1999;65(5):605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Draget KI, Ostgaard K, Smidsrod O. Homogeneous alginate gels – A technical approach. Carbohydrate Polymers. 1990;14(2):159–178. [Google Scholar]
- 21.Zhao Y, Li F, Carvajal MT, Harris MT. Interactions between bovine serum albumin and alginate: An evaluation of alginate as protein carrier. Journal of Colloid and Interface Science. 2009;332(2):345–353. doi: 10.1016/j.jcis.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 22.Fu JC, Hagemeir C, Moyer DL, Ng EW. Unified Mathematical-Model for Diffusion from Drug-Polymer Composite Tablets. Journal of Biomedical Materials Research. 1976;10(5):743–758. doi: 10.1002/jbm.820100507. [DOI] [PubMed] [Google Scholar]
- 23.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Advanced Drug Delivery Reviews. 2001;48(2–3):139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 24.Crank J. The mathematics of diffusion. Oxford, Eng: Clarendon Press; 1975. p. viii.p. 414. [Google Scholar]
- 25.Johnson AT. Biological process engineering: an analogical approach to fluid flow, heat transfer, and mass transfer applied to biological systems. Wiley; 1999. [Google Scholar]
- 26.Fraker CA, Alvarez S, Papadopoulos P, Giraldo J, Gu WY, Ricordi C, Inverardi L, Dominguez-Bendala J. Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem Cells. 2007;25(12):3155–3164. doi: 10.1634/stemcells.2007-0445. [DOI] [PubMed] [Google Scholar]
- 27.Pozrikidis C. Modeling and simulation of capsules and biological cells. Boca Raton, Fla. London: CRC; 2003. p. x.p. 333. [Google Scholar]
- 28.Luckham PF, Ukeje MA. Effect of particle size distribution on the rheology of dispersed systems. Journal of Colloid and Interface Science. 1999;220(2):347–356. doi: 10.1006/jcis.1999.6515. [DOI] [PubMed] [Google Scholar]
- 29.Deleris I, Zouid I, Souchon I, Trelea IC. Calculation of apparent diffusion coefficients of aroma compounds in dairy emulsions based on fat content and physicochemical properties in each phase. Journal of Food Engineering. 2009;94(3–4):205–214. [Google Scholar]
- 30.Stokke BT, Draget KI, Smidsrod O, Yuguchi Y, Urakawa H, Kajiwara K. Small-angle X-ray scattering and rheological characterization of alginate gels. 1. Ca-alginate gels. Macromolecules. 2000;33(5):1853–1863. doi: 10.1021/bm034105g. [DOI] [PubMed] [Google Scholar]
- 31.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Advanced Materials. 2003;15(14):1155–1158. [Google Scholar]
- 32.Shao ZZ, Li YP, Krishnamoorthy R, Chermak T, Mitra AK. Differential-effects of anionic, cationic, nonionic, and physiological surfactants on the dissociation, alpha-chymotryptic degradation, and enteral absorption of insulin hexamers. Pharmaceutical Research. 1993;10(2):243–251. doi: 10.1023/a:1018990928259. [DOI] [PubMed] [Google Scholar]
- 33.Yalkowsky SH, Valvani SC, Roseman TJ. Solubility and partitioning VI: Octanol solubility and octanol-water partition-coefficients. Journal of Pharmaceutical Sciences. 1983;72(8):866–870. doi: 10.1002/jps.2600720808. [DOI] [PubMed] [Google Scholar]
- 34.Sharma PK, Reilly MJ, Jones DN, Robinson PM, Bhatia SR. The effect of pharmaceuticals on the nanoscale structure of PEO-PPO-PEO micelles. Colloids and Surfaces B-Biointerfaces. 2008;61(1):53–60. doi: 10.1016/j.colsurfb.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Shin HS, Kim SY, Lee YM, Lee KH, Kim SJ, Rogers CE. Permeation of solutes through interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and poly(acrylic acid) Journal of Applied Polymer Science. 1998;69(3):479–486. [Google Scholar]
- 36.Calvino-Casilda V, Lopez-Peinado AJ, Vaganova E, Yitzchaik S, Pacios IE, Pierola IF. Porosity Inherent to Chemically Crosslinked Polymers. Poly(N-vinylimidazole) Hydrogels. The Journal of Physical Chemistry B. 2008;112(10):2809–2817. doi: 10.1021/jp7106473. [DOI] [PubMed] [Google Scholar]
- 37.Henry JA, Simonet M, Pandit A, Neuenschwander P. Characterization of a slowly degrading biodegradable polyesterurethane for tissue engineering scaffolds. Journal of Biomedical Materials Research Part A. 2007;82A(3):669–679. doi: 10.1002/jbm.a.31094. [DOI] [PubMed] [Google Scholar]
- 38.Hovgaard L, Jacobs H, Mazer NA, Kim SW. Stabilization of insulin by alkylmaltosides. A. Spectroscopic evaluation. International Journal of Pharmaceutics. 1996;132(1–2):107–113. [Google Scholar]
- 39.Radisic M, Park H, Gerecht S, Cannizzaro C, Langer R, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362(1484):1357–1368. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]