Abstract
Two infants with retinoblastoma and 13q syndrome with multiorgan system anomalies were treated with targeted intra-arterial chemotherapy (IAC) using one-to-three cycles of melphalan 5 mg to avoid systemic chemotherapeutic side effects. Both patients showed good response, with tumor control and no systemic chemotherapy side effects. Of the treatment modalities currently available, IAC may represent an optimal balance between tumor extermination and adverse drug reactions in this patient population with classically reduced multiorgan reserve.
Keywords: 13q syndrome, intra-arterial chemotherapy, retina, retinoblastoma
Introduction
Nearly, a decade after the first description of an infant with unilateral retinoblastoma (RB), developmental delay, extremity abnormalities, and a long-arm deletion of chromosome 13, a report of 23 patients with RB and various combinations of mild-to-severe psychomotor retardation, craniofacial abnormalities, short webbed necks, neural tube and congenital heart defects, renal abnormalities, and limb and genitourinary deformities named the condition "13q syndrome."[1] A number of 13q genes have since been discovered, the differential loss of which are responsible for the heterogeneous mixture of anomalies above. The RB1 gene (13q14.2) encodes the RB protein, which guards against unregulated cell cycle progression. Congenital loss of one or both copies of this gene confers an elevated risk of developing RB and other tumors. While rare in the general population, 13q syndrome is thought to occur in approximately 5–10% of RB patients.[2]
A variety of treatments options for RB is currently available, including intravenous chemotherapy (IVC), intra-arterial chemotherapy (IAC), external beam radiotherapy, and enucleation. IAC is a targeted option that involves cannulation of the ophthalmic artery and selective delivery of chemotherapy to the eye. Its focused nature, rapid efficacy, and minimal systemic adverse reactions make it a particularly appealing option for 13q syndrome patients. Herein, we describe two infants with RB and 13q syndrome, whose multiorgan involvement led our team to employ minimal exposure (one-to-three course) IAC.
Case Reports
Case 1
An ex-full-term, a 14-month-old boy, was found to have unilateral renal agenesis on prenatal screening. At 2 months of age, his parents noticed an eye abnormality and sought consultation [Figure 1]. Because of nonfocal developmental delay, plagiocephaly, dysmorphic facies, hypotonicity, and a neural tube defect, a karyotype was performed, revealing a de novo 13q14 deletion.
Figure 1.
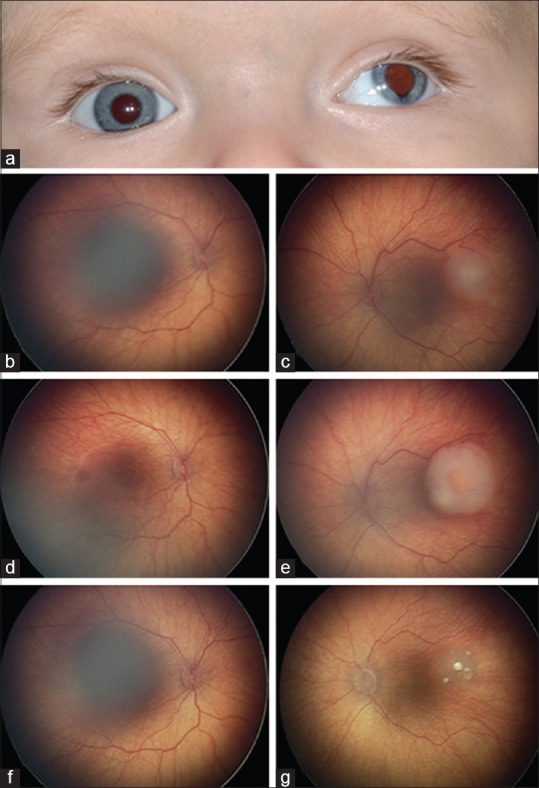
Case 1 - a 14-month-old boy with 13q syndrome who presented with (a) left-sided exotropia and atypical iris thinning, (b) a normal right eye, and (c) a left eye with retinoblastoma 6 mm in diameter and 3 mm in thickness. (d) His right eye remained unchanged following one round of intravenous chemotherapy while (e) the tumor in his left grew, prompting treatment with intra-arterial chemotherapy (5 mg melphalan). (f) The right eye remained normal. (g) The left eye showed complete tumor regression following one intra-arterial chemotherapy cycle though two more were administered due to the history of chemotherapy resistance
On examination, posterior embryotoxon was noted in both eyes. The right eye was otherwise normal. The left eye showed iris thinning inferiorly, a temporal macular tumor measuring 6 mm × 6 mm × 3 mm with overlying subretinal fluid, one millimeter from his intact fovea, and was classified as Group B (international classification of RB). There was no evidence of vitreous or subretinal seeding.
Given the patient's young age and genetic status, systemic IVC was administered to protect against metastatic disease and pinealoblastoma. After one cycle, the main tumor grew larger, suggesting nonresponse, and prompting consideration for IAC. Because of the malignancy's initial chemotherapy resistance, three sessions of melphalan 5 mg IAC were performed. Complete tumor control after the first session was achieved and maintained at 10 months follow-up.
Case 2
At 6 months of age, leukocoria and exotropia were noted in the right eye of a developmentally delayed girl. Three months later, she was diagnosed with a unilateral, macular, 12 mm × 10 mm × 5 mm RB with subretinal seeding and no sign of subretinal fluid, consistent with a Group C classification [Figure 2]. Shortly thereafter, she received a diagnosis of 13q syndrome, manifesting as macrocephaly, dysplastic and hypoplastic corpus callosum, facial dysmorphism (broad nasal bridge, hypertelorism), bifid uvula, and left hip dysplasia. She received a single course of IAC (melphalan 5 mg) to address her malignancy, leading to a femoral artery spasm requiring heparin anticoagulation. The single IAC cycle yielded complete tumor control. Brain magnetic resonance imaging showed no sign of tumor.
Figure 2.
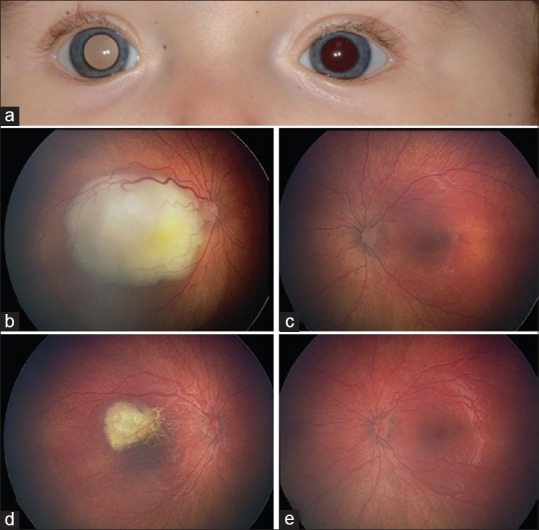
Case 2 - a 10-month-old girl with 13q syndrome who presented with (a) right leukocoria, secondary to (b) a macular retinoblastoma measuring 12 mm in largest diameter and 5 mm thickness. (c) Her normal left eye at baseline. (d) The tumor completely regressed following one cycle of intra-arterial chemotherapy (melphalan 5 mg) while (e) the left eye remained normal
Discussion
A half-century after chemotherapy was first injected into the internal carotid to treat RB, Yamane et al. refined the technique using a balloon catheter to indirectly shunt the infusion into the ophthalmic artery.[3] Later, the advent of microcatheters allowed for the infusion of chemotherapy directly into the ophthalmic artery.[4] One study elaborating this technique reported a 32/39 (82%) 2-year ocular salvage rate for primary IAC-treated eyes, and a 33/56 (58%) salvage rate for secondary IAC-treated eyes.[5] Complications included significant neutropenia in 18 of 78 patients (23%), forehead erythema in 14 of 95 eyes (15%), eyelash thinning or loss in 12/95 eyes (13%), periocular erythema and edema in ten eyes (11%), and avascular retinopathy with total vision loss in four eyes (4%).[5] Two patients (3%) developed metastatic disease.[5]
After 5 years of IAC experience, our team reported complete tumor control in 26 of 36 eyes (72%) treated with primary IAC and 21/34 (62%) treated with secondary IAC.[6] The most frequent complications included ipsilateral eyelid edema (10/198 catheterizations, 5%) and ptosis (10/198 catheterizations, 5%). Vitreous hemorrhage, ophthalmic artery spasm with reperfusion, and partial choroidal ischemia, all of which are potentially blinding, were noted in four catheterizations (2%) each. Ophthalmic artery obstruction and transient forehead erythema occurred in three catheterizations (2%) each.
Several centers have attempted to minimize the number of necessary IAC cycles to achieve RB control.[5,7] Initially, we observed that three cycles yielded satisfactory outcomes in most cases. We subsequently reported experience with patients, often with less advanced disease, receiving fewer than three cycles, and demonstrated complete tumor control in seven of eight patients (88%).[7]
Only one other case of RB in the setting of 13q syndrome treated with minimal-exposure IAC has been reported, after the failure of systemic IVC.[8] To fully appreciate the importance of minimizing chemotherapy exposure and invasive procedures in patients with 13q syndrome, one must understand its long-term health implications. In addition, to the aforementioned multitissue pathology, patients with 13q syndrome may experience increased risk of secondary nonocular malignancies, such as Burkitt lymphoma,[9] and treatment-related acute myeloid leukemia.[10] They are also predisposed to osteosarcoma and other tumors experienced by hereditary RB patients as a result of hemizygosity of RB1 and other tumor suppressor genes in its vicinity. While long-term sequelae of 13q syndrome have not been well documented, in individuals with any variety of renal, cardiac, pulmonary, gastrointestinal, otologic, hematologic, or neural abnormalities, it is prudent to minimize systemic exposure to potentially harmful chemotherapeutic agents. For this patient population, minimal-exposure IAC presently represents an ideal treatment option.
In summary, we report two cases of successful treatment of unilateral RB in patients with 13q syndrome and multiple congenital anomalies. This represents an optimal treatment for this relatively vulnerable population as it allows for maximal effective tumor eradication with minimal systemic toxic exposure.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Allderdice PW, Davis JG, Miller OJ, Klinger HP, Warburton D, Miller DA, et al. The 13q-deletion syndrome. Am J Hum Genet. 1969;21:499–512. [PMC free article] [PubMed] [Google Scholar]
- 2.Bunin GR, Emanuel BS, Meadows AT, Buckley JD, Woods WG, Hammond GD. Frequency of 13q abnormalities among 203 patients with retinoblastoma. J Natl Cancer Inst. 1989;81:370–4. doi: 10.1093/jnci/81.5.370. [DOI] [PubMed] [Google Scholar]
- 3.Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9:69–73. doi: 10.1007/s10147-004-0392-6. [DOI] [PubMed] [Google Scholar]
- 4.Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol. 2011;129:732–7. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA, Caywood EH, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: Outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–60. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Kaliki S, Shah SU, Bianciotto CG, Liu D, Jabbour P, et al. Minimal exposure (one or two cycles) of intra-arterial chemotherapy in the management of retinoblastoma. Ophthalmology. 2012;119:188–92. doi: 10.1016/j.ophtha.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Roche A, Mora J, Perez Mdel M, Gean E, Perez B, O'Callaghan M, et al. Axenfeld-Rieger ocular anomaly and retinoblastoma caused by constitutional chromosome 13q deletion. Pediatr Blood Cancer. 2010;54:480–2. doi: 10.1002/pbc.22354. [DOI] [PubMed] [Google Scholar]
- 9.Hongeng S, Parapakpenjun S, Pakakasama S, Rerkamnuaychoke B, Pornkul R. Secondary Burkitt lymphoma in a retinoblastoma patient with 13q deletion syndrome. Pediatr Blood Cancer. 2006;46:524–6. doi: 10.1002/pbc.20372. [DOI] [PubMed] [Google Scholar]
- 10.Hon C, Chan GC, Ha SY, Ma SK, Wong KF, Au WY. Bone marrow transplantation for therapy-related acute myeloid leukemia in congenital retinoblastoma associated with 13q deletion syndrome. Ann Hematol. 2004;83:481–3. doi: 10.1007/s00277-004-0884-5. [DOI] [PubMed] [Google Scholar]


