Abstract
Rationale
Anti-angiogenesis therapies such as bevacizumab, the monoclonal antibody to vascular endothelial growth factor (VEGF), have been used against ovarian cancer, but transient and low peritoneal drug levels are likely a factor in treatment failure. We hypothesized that a single administration of adeno-associated virus (AAV)-mediated intraperitoneal expression of bevacizumab would direct persistent expression and suppress growth and metastasis of ovarian cancer.
Methods
AAVrh10.BevMab, a rhesus serotype 10 adeno-associated viral vector coding for bevacizumab, was evaluated for the capacity of a single intraperitoneal administration to persistently suppress peritoneal tumor growth in an intraperitoneal model of ovarian carcinomatosis with human ovarian cancer cells in nude immunodeficient mice.
Results
The data demonstrates that AAVrh10.BevMab mediates persistent and high levels of bevacizumab in the peritoneal cavity following a single intraperitoneal administration in mice. In AAVrh10.BevMab treated A2780 human ovarian cancer-bearing mice, tumor growth was significantly suppressed (p<0.05) and the area of blood vessels in the tumor were decreased (p<0.04). Survival of mice with A2780 xenografts or SK-OV3 xenografts was greatly prolonged in the presence of AAVrh10.BevMab (p<0.001). Administration of AAVrh10.BevMab 4 days after A2780-luciferase cell implantation reduced tumor growth (p<0.01) and increased mouse survival (p<0.0001). Combination of AAVrh10.BevMab with cytotoxic reagents paclitaxel or topotecan proved to be more effective in increasing survival than treatment with cytotoxic reagent alone.
Conclusion
A single administration of AAVrh10.BevMab provides sustained and high local expression of bevacizumab in the peritoneal cavity, and significantly suppresses peritoneal carcinomatosis and increases survival in an ovarian cancer murine model.
Introduction
Ovarian cancer is the most lethal gynecologic cancer and fifth leading cause of cancer death in the United States [1]. It is generally asymptomatic in the early stages and no effective screening approach is available. Approximately 75% of women with ovarian cancer when diagnosed are already at an advanced stage with peritoneal dissemination. Current treatment strategies for advanced ovarian cancer include tumor debulking by surgery and chemotherapy with platinum and taxane [2]. Despite progress in treatment, the 5 yr survival for advanced ovarian cancer is only 27% [1], and the majority of patients eventually develop drug resistance and experience disease recurrence [3].
One strategy to treat ovarian cancer is inhibition of angiogenesis, the growth of blood vessels from pre-existing vasculature, a process essential for tumor growth [4]. Angiogenesis is regulated by a number of factors of which vascular endothelial growth factor (VEGF) is key [5]. Ovarian cancer patients with high expression of VEGF show poor prognosis and the overexpression of VEGF is associated with poor progression-free survival and overall survival [6].
Bevacizumab (Avastin®) is a humanized monoclonal IgG1 antibody that targets VEGF-A, a member of VEGF family involved in tumor angiogenesis [7]. This antibody prevents activation of VEGF receptors through binding to and neutralizing all active isoforms of VEGF-A. It has been approved by FDA to treat metastatic colorectal cancer, non-small cell lung cancer, glioblastoma multiforme and metastatic renal cell carcinoma. Based on encouraging preclinical data on bevacizumab treatment of ovarian cancer [8], efficacy of bevacizumab on ovarian cancer has been studied as a single agent or combined with cytotoxic agents in clinical trials. As a single agent to treat patients with recurrent ovarian cancer, bevacizumab is well tolerated and effective with a responsive rate of 21% [9]. Four randomized trials have assessed the use of bevacizumab in three different clinical studies of ovarian cancer, including primary treatment, platinum-sensitive and platinum-resistant recurrences [10-13]. All report improved progression-free survival without an effect on overall survival. Consistent with these findings, other anti-angiogenic therapies have demonstrated measures of efficacy for ovarian cancer. In phase III trials, pazopanib, an angiogenic inhibitor that binds to the VEGF receptor, improved progression-free survival [14] and other anti-angiogenic small molecules, including sorafenib and sunitinib, which also inhibit the activation of VEGF, have shown modest effects but with relatively high toxicity in phase II trials [15,16]. Another VEGF inhibitor, aflibercept, did not meet the primary endpoint of a radiographic response in clinical trials [17].
One challenge for treatment of ovarian cancer with bevacizumab is that the tumor cells constantly express VEGF [18]; thus, to prevent revascularization associated with anti-VEGF therapy withdrawal, persistent delivery of bevacizumab may be needed. Maintenance bevacizumab treatment has better efficacy with acceptable toxicity in clinical trials [10,11], but requires repetitive bevacizumab therapy [19].
Our previous studies demonstrate that administration of AAVrh10.BevMab, a Rhesus serotype 10 adeno-associated viral vector coding for bevacizumab, leads to sustained bevacizumab expression and inhibits the growth of prostate carcinoma in lung [20]. AAVrh10.BevMab was also proved to effectively suppress retinal neovascularization [21]. Based on these findings, we hypothesized that persistent suppression of VEGF in the peritoneal cavity with AAVrh10.BevMab would be efficacious in suppressing the growth of ovarian cancer peritoneal carcinomatosis. We observed that intraperitoneal administration of AAVrh10.BevMab provided high local expression of bevacizumab in the peritoneal cavity and a single administration of AAVrh10.BevMab produces long-term persistent expression of bevacizumab. Importantly, intraperitoneal administration of AAVrh10.BevMab significantly inhibits tumor angiogenesis and tumor growth and prolongs survival of mice bearing ovarian cancer either as a single reagent or combined with cytotoxic reagent in a murine xenograft model mimicking advanced ovarian cancer.
Methods
Adeno-associated Virus Vectors
All AAV vectors were based on the non-human primate-derived AAV serotype rh.10 capsid with the expression cassette comprised of the AAV serotype 2 5’ and 3’ inverted terminal repeats flanking the promoter and transgene. For the AAVrh10.BevMab vector, the expression cassette included the cytomegalovirus enhancer chicken β-actin promoter, the anti-human VEGF heavy-chain and light-chain sequences separated by a furin 2A self-cleavage site and rabbit β-globin gene polyadenylation signal. As a control, an irrelevant antibody against anthrax protective antigen (AAVrh10.αPA) was used [21].
AAV vectors were produced using 2 plasmids: (1) an expression cassette plasmid pAAVαVEGF or pAAVαPA carrying the transgene (humanized anti-human VEGF antibody cDNA for pAAVαVEGF, anti-anthrax protective antigen antibody cDNA for pAAVαPA); (2) pPAK help plasmid. To produce the AAVrh.10 vectors, pAAVαVEGF or pAAVαPA (600 μg) and pPAK help plasmid (1200 μg) were co-transfected into human embryonic kidney 293 cells in 40 dishes of 150 mm petri-dish using pEI”max”. Seventy-two hr after transfection, cells were harvested and a crude viral lysate was prepared using 5 cycles of freeze/thaw followed by centrifugation. The AAV vector was purified by iodixanol (Sigma-Aldrich, Norway) gradient and QHP anion exchange chromatography (GE Healthcare, Uppsala, Sweden). The purified AAV vector was concentrated using an Amicon Ultra-15 100K centrifuge filter devices (Millipore, Billerica, MA) and stored in phosphate-buffered saline (PBS, pH=7.4) at −80°C. Vector genome titers were determined by quantitative TaqMan real-time PCR using a chicken β-actin promoter-specific primer-probe set (Applied Biosystems, Foster City, CA).
Cell Culture
All cells were cultured at 37°C in a humidified 5% CO2 incubator. SK-OV-3 cells (American Type Culture Collection, ATCC) were cultured in McCoy's 5, a Medium Modified supplemented with 10% fetal bovine serum. A2780 cells (Sigma-Aldrich, St. Louis, MO) and luciferase-labeled A2780-luciferase cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum and 2 mM glutamine. Human embryonic kidney 293 cells were cultured in DMEM with 10% fetal bovine serum, 2 mM glutamine and 10 mM sodium pyruvate. Cells were passaged every three days by using 0.05% porcine trypsin-ethylenediaminetetraacetic acid (EDTA). All medium components for cell culture were purchased from Gibco (Life Technologies, Grand Island, NY).
Mice
Female Balb/c or Blab/c nude mice (Taconic, Germantown, NY) were housed in filter-capped cages and kept in a sterile facility upon arrival. At least 1 wk quarantine was imposed on all animals before starting the study. All procedures were performed according to protocols approved by the Research Animal Resource Center, Weill Cornell Medical College.
Chemicals
Topotecan (Sigma-Aldrich, St. Lois, MO) was dissolved in 1% dimethyl sulfoxide (DMSO; Sigma-Aldrich) at concentration of 62.5 μg/ml. Paclitaxel (Sigma-Aldrich) was dissolved in DMSO at 33.3 mg/ml and diluted to 2 mg/ml with phosphate buffered saline, pH 7.4 (PBS) before use.
Local Bevacizumab Expression in the Peritoneal Cavity after In Vivo Administration of AAVrh10.Bevmab
AAVrh10.BevMab (1011 gc) in 200 μl PBS was administered intraperitoneally or intravenously to 14 to 15 wk old female Balb/c mice. After 9 wk, peritoneal lavage was carried out with 1 ml PBS and omentum, mesentery and diaphragm were collected. Protein in tissue was extracted by homogenizing 10 mg of tissue in 200 μl of T-PER® tissue protein extraction reagent (Thermo Scientific, Rockford, IL). The levels of bevacizumab in lavage, tissue and organs were determined by a human VEGF-specific enzyme-linked immunosorbent assay (ELISA) as previously described with minor modifications [21]. Flat-bottomed 96-well EIA/RIA plates (Corning Life Sciences, Lowell, MA) were coated with 0.02 μg human VEGF-A165 (R&D Systems, Minneapolis, MN) per well in a total volume of 100 μl of 0.05 M carbonate buffer overnight at 4°C. The plates were washed three times with PBS and blocked with 5% dry milk in PBS for 30 min. The plates were washed 3 times with PBS containing 0.05% Tween-20 (Bio-Rad Laboratories, Hercules, CA). Serial dilution of lavage or tissue protein lysates in PBS containing 1% dry milk were added to each well and incubated for 60 min at room temperature. Bevacizumab (Genentech, San Francisco, CA) was used as standard. The plates were washed three times with PBS/Tween-20, and then 100 μl/well of 1:5000 diluted peroxidase conjugated goat anti-human kappa light chain (Sigma-Aldrich) in PBS containing 1% dry milk was added and incubated for 60 min, 23°C. The plates were washed four times with PBS/Tween-20 and once with PBS. Peroxidase substrate (100 μl/well, Bio-Rad, Hercules, CA) was added. After 15 min, the reaction was stopped by addition of 100 μl/well of 2% oxalic acid (Sigma-Aldrich). Absorbance at 415 nm was measured. Bevacizumab concentrations were calculated according to the standard curve based on a log (OD)-log (concentration) interpolation model and a cutoff value equal to 2-fold the absorbance of background. Bevacizumab expression was standardized by total protein concentration using a bicinchoninic acid assay (Bio-Rad) following the manufacturer's instructions.
Murine Ovarian Cancer Models
Six to 8 wk old female Balb/c nude mice were used for all experiments. Xenografts of human ovarian cancer cells (A2780 or SK-OV3) were established by intraperitoneal administration of the tumor cells into Balb/c nude mice. AAVrh10.BevMab or AAVrh10.α-PA (1011 gc in 200 μl of PBS) or PBS was administered intraperitoneally 1 day or 4 days after tumor cell implantation. Mice were sacrificed at different time points and samples were collected. To establish the topotecan-AAVrh.10BevMab combination treatment model, mice were administered A2780 cells intraperitoneally. After 2 wk, the mice were treated with topotecan at 0.625 mg/kg body weight/day, 5 times/wk for 3 wk [22]. Five wk after tumor cell inoculation, AAVrh10.BevMab or AAVrh10.α-PA (1011 gc in 200 μl of PBS) was administered intraperitoneally. To establish the paclitaxel-AAVrh.10BevMab combination treatment model, mice were administered A2780 cells intraperitoneally. After 1 wk, the mice were treated with paclitaxel at 20 mg/kg body weight, once/wk for 2 wk [23]. Three wk after tumor cell inoculation, AAVrh10.BevMab or AAVrh10.α-PA (1011 gc in 200 μl of PBS) was administered intraperitoneally.
Tumor Vasculature
Tumor tissue was freshly harvested and embedded into optimum cutting temperature compound (Sakura Finetek, Torrance, CA). Tissue sections were stained with rat polyclonal antibodies against mouse CD31 (Abcam, Cambridge, MA). Sections were incubated in a blocking solution (PBS containing 10% normal goat serum and 2% bovine serum albumin) for 1 hr, 23°C. Primary antibody diluted with blocking solution (1:300) was applied to the sections and incubated overnight at 4°C. After washing with PBS, the slides were incubated with a secondary antibody, goat anti-rat IgG conjugated with cy3 (Invitrogen, Eugene, OR) diluted with blocking buffer (1:1000) for 2 hr, 23°C. After 3 washes, sections were mounted with prolong gold anti-fade reagent with DAPI (Invitrogen, Eugene, OR). Sections were examined with an Olympus IX71 microscope, and representative areas were photographed using a 20X objective. Five fields from each section containing the highest frequency of blood vessels were examined. The quantification of vascular area was analyzed using ImageScope (Aperio, Vista, CA).
Assessment of Tumor Burden by Human DNA
Quantitative TaqMan real-time PCR analysis for Alu was used to quantify human DNA as tumor burden in ovarian cancer mouse xenografts [20]. Genomic DNA from mouse tissue was extracted with a DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) and quantified by Nanodrop (Qiagen). All genomic DNA samples were diluted to a working concentration of 1 ng/μl and 1ul of genomic DNA was amplified. Human DNA standard series containing 0.01, 0.1, and 1 ng of human DNA per microliter was prepared with mouse DNA to make the total DNA concentration 1 ng/μl. The following primer-probe sets specific to human Alu coding sequence were used: Alu-P1 5′- CGGGTTCACGCCATTCTC -3′ and Alu-P2 5′- AAAAATTAGCCGGGCGTAGTG -3′ and Alu Probe 5′FAM- AGCTGGGACTACAGGCGCCCG –TAMRA 3′. The amount of human DNA in tumor tissue was normalized per total DNA.
Assessment of Tumor Burden In Vivo
In vivo luciferase activity was measured with Xenogen IVIS imaging system (Xenogen, Alameda, CA) composed of a charge-coupled device connected to a light-tight black chamber. Mice with A2780-Luciferase xenografts were injected intraperitoneally with d-luciferin (Caliper LifeSciences, Hopkinton, MA) in PBS at a dose of 150 mg/kg body weight and anesthetized with 2.5% isoflurane (Piramal Healthcare, Andhra Pradesh, India). Mice were placed prone in the chamber. Fifteen min after d-luciferin injection, bioluminescence images were taken and gray scale reference images were obtained under dim illumination. Pseudocolor images representing bioluminescent intensity were acquired with LivingImage software (Caliper LifeSciences, Hopkinton, MA). These images were superimposed on the gray scale images for analysis with LivingImage 3.2. The data were expressed as photo emission (photons/sec-cm2 steradian).
Statistical Analysis
All data are presented as mean ±standard error. Statistical comparison was made by two-tailed Student's t test. When the mice died or were sacrificed because the abdominal circumference was over 9 cm or mice became moribund, this was recorded as the date of death. Survival evaluation was calculated by Kaplan-Meier method and compared by log-rank test. A value of p<0.05 was considered as significant.
Results
Persistent Expression of Bevacizumab in Peritoneal Cavity of Mice with Single Administration of AAVrh10.BevMab
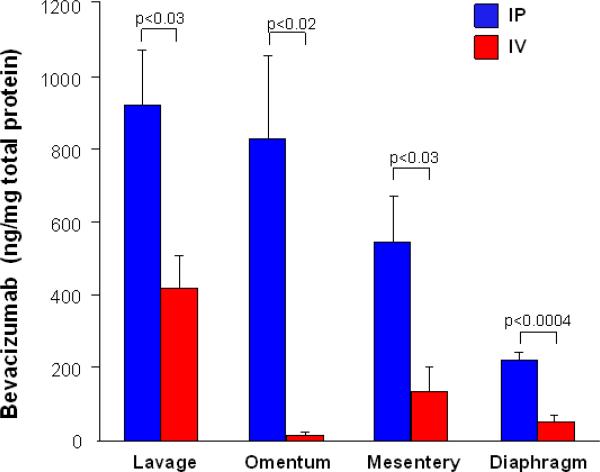
To determine the best administration route of AAVrh10.BevMab, 1011 genome copies of AAVrh10.BevMab were administered intraperitoneally or intravenously into Balb/c mice. Nine wk later, bevacizumab levels were analyzed by peritoneal lavage and in the omentum, mesentery and diaphragm. Overall, there was higher bevacizumab expression in intraperitoneal organs in mice receiving AAVrh10.BevMab intraperitoneally than intravenously (Figure 1). Thus, a single administration of AAVrh10.BevMab provides persistent expression of bevacizumab and intraperitoneal administration of AAVrh10.BevMab provides higher local bevacizumab expression in the peritoneal cavity than intravenous administration (serum levels of 66.5 μg/ml at 36 wk; not shown). Based on this data, we chose intraperitoneal administration for all the following experiments.
Figure 1.
Local expression of bevacizumab in peritoneal cavity following intraperitoneal or intravenous administration of AAVrh10.BevMab vector. AAVrh10. BevMab (1011 gc) was administered intraperitoneally or intravenously to Balb/c mice (n=4 or 5). After 9 wk, the mice were sacrificed. Peritoneal lavage, omentum, mesentery and diaphragm were collected. Bevacizumab levels were assayed by ELISA and normalized by total protein.
Effects on Tumor Growth
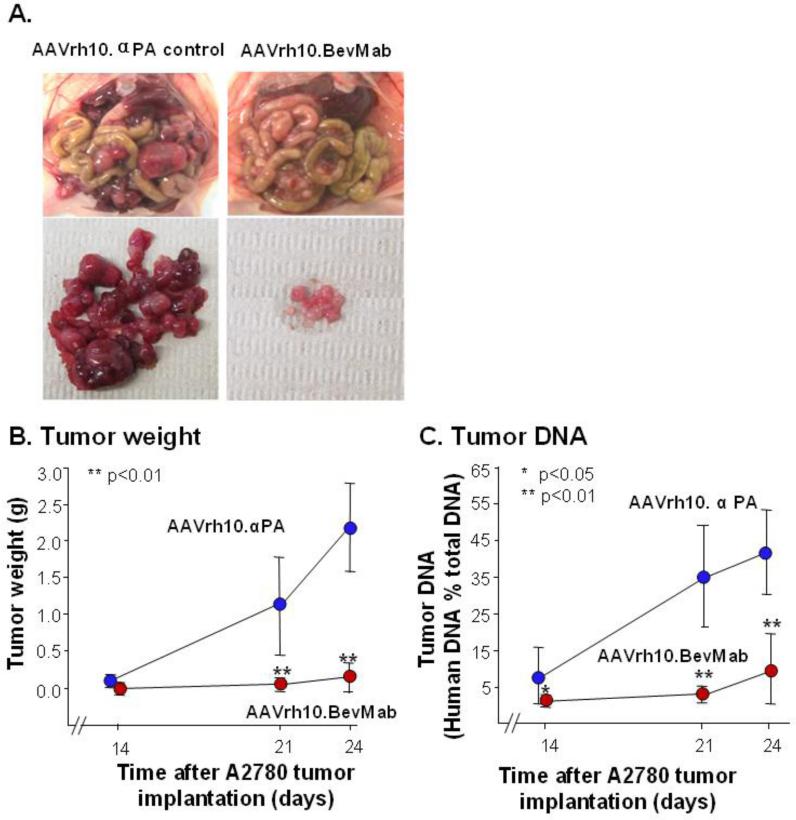
To determine the effects of intraperitoneal administration of AAVrh10.BevMab on tumor growth of ovarian cancer, we established a model of ovarian cancer with 5×106 A2780 human ovarian cancer cells administered intraperitoneally in Balb/c nude immunodeficient mice, followed by administration of AAVrh10.BevMab, or AAVrh10.αPA as the control. Treatment with AAVrh10.BevMab resulted in a marked reduction of peritoneal carcinomatosis of the ovarian cancer (Figure 2A). Tumor weight and A2780-derived human DNA were measured on day 14, 21 and 24. On day 14, AAVrh10.αPA-treated mice showed detectable tumor growth, whereas the AAVrh.10BevMab treated-mice had no visually detectable tumors (control 0.11±0.8 g vs treated not detectable). By day 21, the difference was 55-fold greater tumor weight in the control group (control 1.1±0.7 g vs treated 0.02±0.01 g, p<10−3) and on day 24, the difference was 15-fold (control 2.2±0.6g vs treated 0.15±0.2, p<10−7; Figure 2B). Tumor burden was also quantified by quantitative PCR for the tumor-derived human DNA in tissue in mouse peritoneal cavity. In the control vector-treated group, human DNA accounted for 8, 35 and 42% of the total DNA (from both mouse and human) on day 14, 21, and 24, respectively, whereas in the AAVrh10.BevMab-treated group, human DNA was only 1, 3 and 10% on day 14, 21 and 24, respectively (Figure 2C). These data indicate that AAVrh10.BevMab treatment significantly slows tumor growth in the peritoneal cavity.
Figure 2.
Effects of AAVrh10.BevMab on ovarian cancer peritoneal carcinomatosis. Five million A2780 human ovarian cancer cells were inoculated intraperitoneally into Balb/c nude mice. One day after tumor cell inoculation, mice were treated with 1011 gc AAVrh10. BevMab or, as control, AAVrh10.αPA, an AAVrh10. vector coding for an irrelevant IgG antibody. Mice were sacrificed at different time points for tumor burden measurement. A. Images of peritoneal carcinomatosis of ovarian cancer 24 days after intraperitoneal administration of A2780 cells. B. Tumor weight. Tumor nodules in peritoneal cavity were isolated and weighed. C. Tumor DNA. All tissue and organs in peritoneal cavity were homogenized and genomic DNA was extracted. Human genomic DNA (Alu) was quantified by quantitative real-time PCR. Data (means ± standard error) were obtained from n=10 mice. AAVrh.10BevMab vs AAVrh10.αPA, *p<0.05, ** p<0.01.
Effects on Tumor Angiogenesis
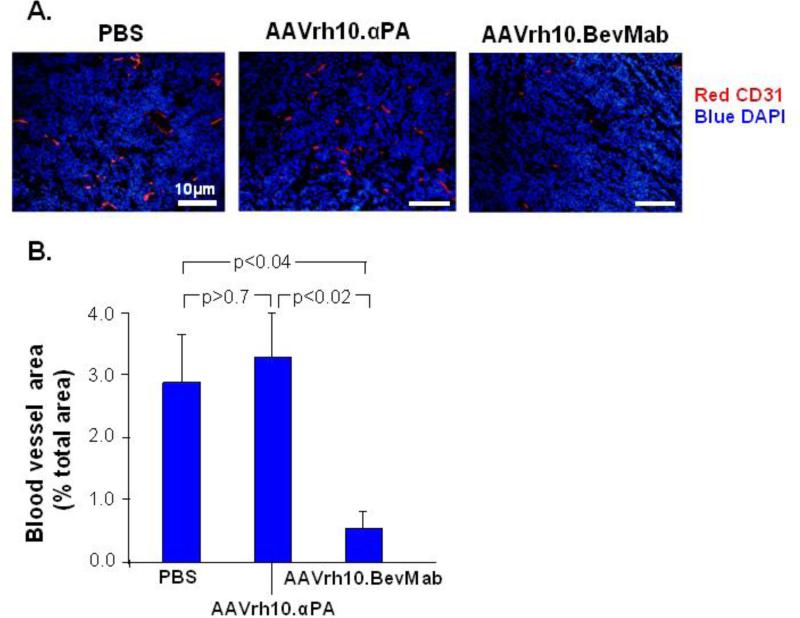
To evaluate the effects of intraperitoneal administration of AAVrh10.BevMab on angiogenesis of ovarian cancer, the area of blood vessels in tumors after AAVrh10.BevMab administration was quantified by immunohistochemistry staining for CD31, an endothelial cell marker. The result showed significant reduction of vasculature in AAVrh10.BevMab-treated tumors as compared with PBS-, and AAVrh10. αPA-treated tumors (Figure 3). Blood vessels accounted for 0.63% tumor area in AAVrh10.BevMab-treated tumors; there was 4.6-fold and 5.3-fold more vasculature in tumors treated with PBS or AAVrh10.αPA, respectively (Figure 3B). This data demonstrates a correlation between suppressed tumor growth and reduced vasculature in AAVrh10.BevMab-treated mice.
Figure 3.
Effects of AAVrh10.BevMab on angiogenesis of ovarian cancer. A2780 cells (5×106) were administered intraperitoneally into Balb/c nude mice. One day after tumor cell inoculation, mice were treated with 1011 gc AAVrh10. BevMab or, as controls, AAVrh10.αPA or PBS. On day 24, tumor nodules were collected. Frozen sections were stained with rat anti-mouse CD31 followed by goat anti-rat IgG conjugated with cy3. Nuclei were stained with DAPI. A. CD31+ tumors for PBS control, AAVrh.10αPA control and AAVrh.10BevMab. B. Quantification of blood vessel area. Data were obtained from n=4 mice per group.
Effects on Survival
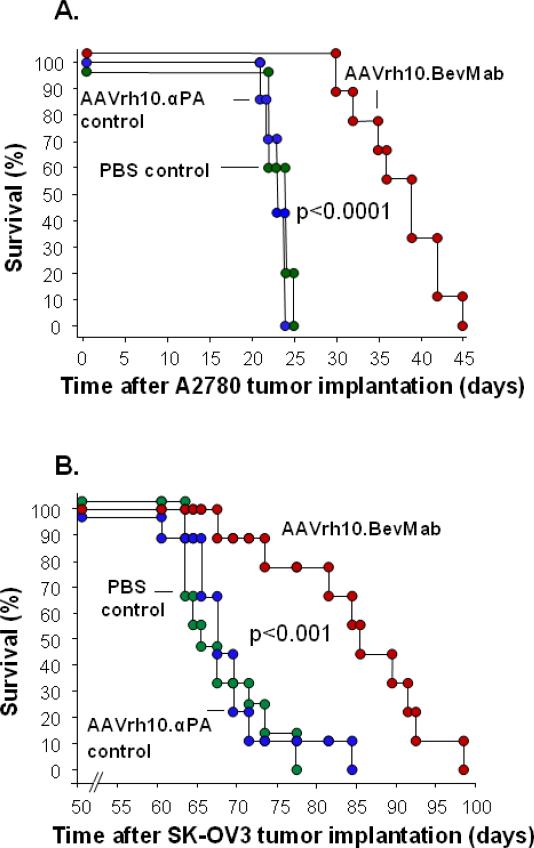
AAVrh10.BevMab treatment significantly increased survival of mice with A2780 tumors (Figure 4A). The median survival days are 24 days, 23 days and 39 days for mice treated with PBS, AAVrh10.αPA control and AAVrh10.BevMab, respectively (p<0.001, AAVrh10.BevMab vs all other groups). Consistent with this result, AAVrh10.BevMab also prolonged survival of mice bearing SK-OV3 xenografts (Figure 4B, p<0.001, AAVrh10.BevMab vs all other groups), suggesting the effects of AAVrh10.BevMab are not tumor cell specific.
Figure 4.
Effects of AAVrh10.BevMab on survival of ovarian cancer-bearing mice. Five million A2780 cells or SK-OV3 cells were administered intraperitoneally into Balb/c nude mice. One day after tumor cell inoculation, mice were treated with 1011 gc AAVrh10. BevMab or, as controls, AAVrh10.PA or PBS. Survival is presented as the percentage of surviving mice in each group. Data were obtained from n=7 to 10 animals per group. AAVrh.10BevMab vs all other groups, A. A2780 tumors, p<0.0001; and B. SK-OV3 tumors p<0.001.
Effects on Pre-established Ovarian Cancer
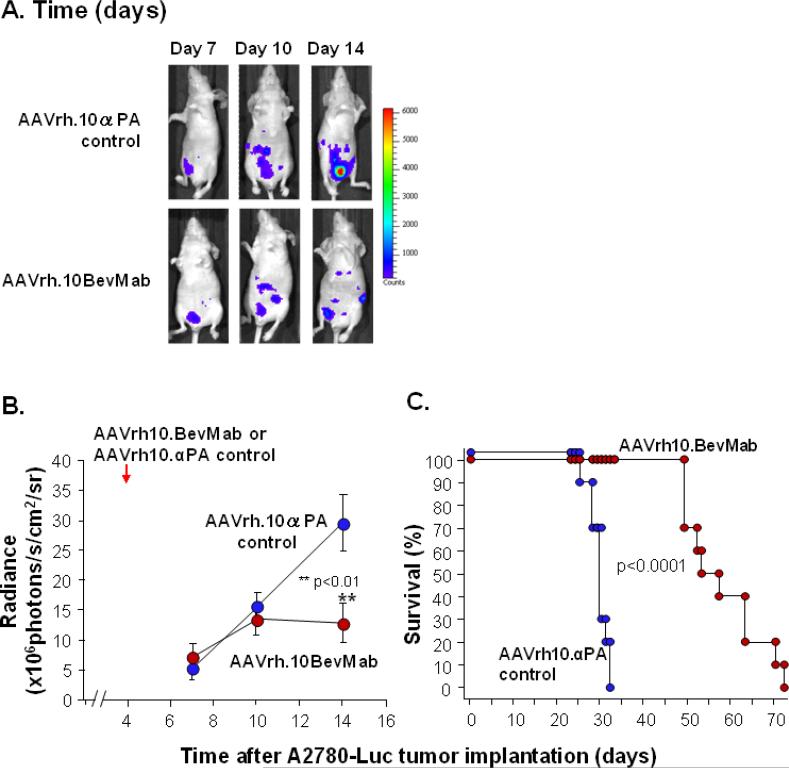
To evaluate the effects of AAVrh10.BevMab on established tumors, AAVrh10.BevMab treatment was initiated 4 days after tumor cell inoculation. A2780 cells were genetically modified and tagged with luciferase so that peritoneal dissemination of the ovarian cancer could be evaluated by in vivo imaging. There was no difference in luciferase expression between AAVrh10.BevMab-treated mice and control vector-treated mice until 14 days after tumor cell implantation (Figure 5 A,B). On day 14, there was significantly higher luciferase expression in the control group than in the AAVrh10.BevMab-treated group, indicating higher tumor burden in the control group. Consistent with the imaging data, AAVrh10.BevMab-treated mice exhibited a statistically significant survival advantage in contrast to mice that received the AAVrh10.αPA control vector (Figure 5C, p<0.0001).
Figure 5.
Effects of AAVrh10. BevMab on mice with established A2780 xenografts. Two million A2780-Luciferase cells were administered intraperitoneally into Balb/c nude mice (n=10). Four days after tumor cell inoculation, mice were treated with 1011 gc AAVrh10. BevMab or AAVrh10.αPA control vector. Tumor growth was assayed by luciferase activity. A. Images day 7, 10 and 14 for AAVrh.10αPA control and AAVrh.10BevMab. B. Quantified luciferase activity, AAVrh.10BevMab vs AAVrh10.αPA, **p<0.01. C. Survival presented as the percentage of surviving mice in each group, AAVrh.10BevMab vs AAVrh10.αPA, p<0.0001.
Effects of AAVrh10.BevMab in Mice Pre-treated with Cytotoxic Drugs
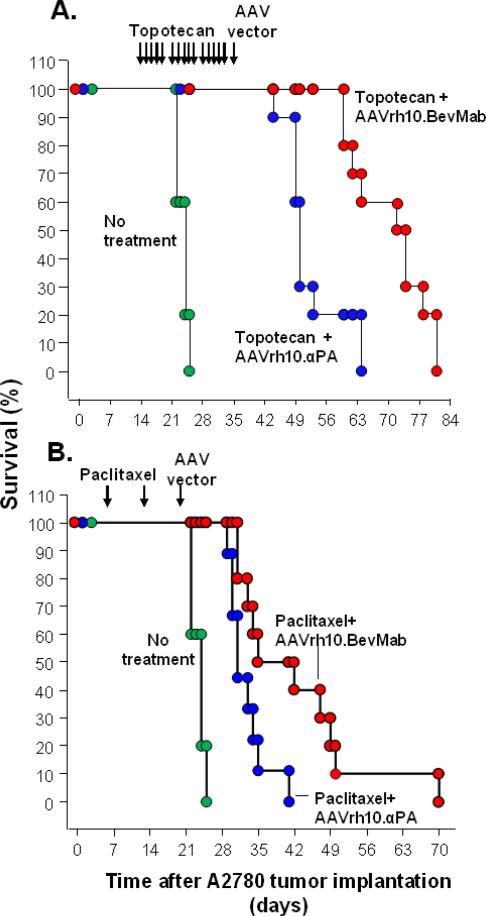
To validate the therapeutic efficacy of AAVrh10.BevMab in a more clinically relevant model, A2780 human ovarian cancer cell xenografts were treated with cytotoxic reagents, topotecan or paclitaxel, before the AAV vectors were administered. At the doses and schedules used, topotecan and paclitaxel were efficacious as single agents, but the addition of the AAV vector coding for bevacizumab further prolonged survival of the ovarian cancer-bearing mice (Figure 6). The median survival was 24 days for mice without treatment. Topotecan treatment prolonged the median survival to 50 days, and the combination of topotecan and AAVrh10.BevMab treatment increased the median survival to 73 days (Figure 6A, topotecan+AAVrh10.BevMab vs all other group, p<0.001). Mice receiving paclitaxel treatment only showed a median survival of 31 days, while addition of AAVrh10.BevMab increased it to 39 days (Figure 6B, paclitaxel + AAVrh10.BevMab vs no treatment, p<0.0001, AAVrh10.BevMab vs AAVrh10.αPA, p<0.02). These data demonstrate the feasibility of the combination of AAVrh10.BevMab with chemotherapy and the advantage of the addition of AAVrh10.BevMab to chemotherapy in ovarian cancer treatment.
Figure 6.
Effects of AAVrh10. BevMab on A2780-bearing mice pretreated with cytotoxic reagents. A. Survival of mice pre-treated with topotecan. A2780 cells (5×106) were administered intraperitoneally into Balb/c nude mice. One group received no treatment. Two wk after tumor cell inoculation, topotecan was intraperitoneally injected into mice at 0.625 mg/kg-day, 5 days/wk for 3 wk. Five wk after tumor cell inoculation, mice were administered intraperitoneally with 1011 gc AAVrh10. BevMab or AAVrh10.αPA control vector. Survival is presented as the percentage of surviving mice in each group. Data were obtained from n=10 animals per group AAVrh.10BevMab vs all other groups, p<0.001. B. Survival of mice pre-treated with paclitaxel. A2780 cells (5×106 cells) were administered intraperitoneally into Balb/c nude mice. One group received no treatment. One wk after tumor cell inoculation, paclitaxel was intraperitoneally injected into mice at 20 mg/kg, once a week for 2 wk, 3 wk after tumor cell inoculation, mice were administered intraperitoneally with 1011 gc AAVrh10. BevMab or AAVrh10.αPA control vector. Survival is presented as the percentage of surviving mice in each group. Data were obtained from n=9-10 animals per group AAVrh.10BevMab vs no treatment, p<0.0001, AAVrh.10BevMab vs AAVrh10.αPA, p=0.01.
Discussion
The tropism of ovarian cancer for the peritoneum at initiation and during recurrences proposes a role for peritoneal therapies. The present study demonstrates that a single intraperitoneal delivery of the genetic sequence of bevacizumab using an AAVrh10 gene transfer vector provides long term, high level of bevacizumab in the peritoneal cavity, as well as target organs such as omentum. Intraperitoneal administration of AAVrh10.BevMab alone or following chemotherapy significantly reduced ovarian cancer growth through inhibition of angiogenesis, extending the survival of tumor-bearing mice.
The peritoneal niche which supports ovarian cancer enables proliferation and tissue invasion and provides resistance to current therapies [24]. Studies over the past few years have identified local intraperitoneal therapies, including standard chemotherapy and hyperthermic chemotherapy as potentially efficacious [25]. Further, the identification of activated endothelial cells at the ovarian cancer peritoneal niche [26] suggests that an anti-angiogenic strategy could have an important impact in blocking tumor growth, resulting in numerous multifaceted approaches, including small molecule drugs and monoclonal antibodies designed to block VEGF or its receptor. The challenge with these approaches is the specific delivery of relatively high concentrations to the peritoneum and maintaining consistent levels of the therapeutic without the peaks and troughs associated with typical pulsatile drug delivery [27].
Overall, the data for the numerous anti-angiogenic strategies suggest a positive impact for efficacy with concerns of toxicology for some that are likely unrelated to the anti-angiogenic mechanism; but a host of questions remain for optimal efficacy, including dose, regimen, duration of treatment, and route of delivery. Based on this experience with anti-angiogenic therapies for ovarian cancer, we hypothesized and evaluated the concept that high, consistent, persistent and localized bevacizumab levels would suppress ovarian tumor growth. The present study demonstrates that a single intraperitoneal delivery of the genetic sequence of bevacizumab using an AAVrh10 gene transfer vector provides long term, high level of bevacizumab in the peritoneal cavity, as well as target organs such as the omentum. Intraperitoneal administration of AAVrh10.BevMab alone or following chemotherapy significantly reduced ovarian cancer growth through inhibition of angiogenesis, extending the survival of tumor-bearing mice.
Viral-based gene therapy is an ideal therapeutic approach for ovarian cancer that can provide sustained expression of anti-angiogenic factors at a therapeutic level focal to the peritoneum, thus overcoming the challenges specific to protein and small molecule-based therapies. To date, adenovirus and AAV vectors have been used to deliver anti-angiogenic factors to treat ovarian cancer in murine models. Adenovirus-mediated gene transfer of angiostatin or endostatin, two angiogenesis inhibitors, effectively inhibited malignant ascites and blood vessel formation and inhibited tumor growth in ovarian cancer but the transient expression characteristic of adenovirus to provide vectors long term resolution is unlikely [28].
In contrast to adenovirus vectors, AAV vectors provide stable gene expression and have an excellent safety profile with relatively silent host defense response documented in numerous clinical trials and the persistent, long term expression from a single administration obviates concerns for neutralizing antibody production which would only impact a second vector administration [29]. Specific to ovarian cancer, AAV delivered endostatin has been shown to inhibit blood vessel formation and tumor growth [30]. In the present study AAVrh.10, a nonhuman primate AAV serotype 10, was used for the following reasons: (1) expected low human seroprevalence of neutralizing anti-AAVrh10 antibodies based on that observed for closely related nonhuman primate AAV8 [31]; and (2) AAVrh.10-mediated delivery of bevacizumab demonstrates robust expression of bevacizumab in mice as well as other transgenes in monkeys [20,21,32]. The current study demonstrates that a single intraperitoneal administration of AAVrh10.BevMab mediates persistent bevacizumab expression up to 9 wk (the longest time point evaluated) in mice. The concentration of bevacizumab in the peritoneal cavity was higher than that in intravenous-injected mice. Importantly, high levels were measured in the omentum, mesentery and diaphragm, the common metastatic sites of ovarian cancer. A murine xenograft model was established by intraperitoneal injection of ovarian cancer cells to mimic human ovarian cancer with wide intra-abdominal metastasis [33]. The AAVrh10.BevMab expression reduced tumor growth rates and tumor vessel numbers with concomitant extension of survival. Combined treatments of cytotoxic therapies with AAVrh.10BevMab establish an additive effect, facilitating extended survival greater than either treatment individually. These results indicate that AAVrh10.BevMab could be an effective therapeutic for ovarian cancer and when used in conjunction with cytotoxic reagents and suggest the possibility of combinations with other vector expressed anti tumor antibodies, to provide synergistic value. Pretreatment with cytotoxic drugs provides a model for the residual tumor burden thought to be the source of tumor resurgence in the clinical setting. Post-cytotoxic AAVrh10.BevMab treatment delayed the expanded tumor burden resulting from residual disease.
One concern for the AAVrh.10BevMab strategy is the potential to drive metastatic disease. Long-term anti-VEGF antibody treatment has been reported not to increase tumor metastasis either as a single agent or combined with chemotherapy [34]. Consistent with these findings, we did not observe any metastasis outside of the peritoneal cavity. Systemic bevacizumab treatment designed to provide long term anti-angiogenic activity results in a range of adverse effects, including hypertension, gastrointestinal perforation, proteinuria, hemorrhage, and impaired wound healing [35]. Our strategy of providing high local peritoneal bevacizumab concentration with low systemic concentration should reduce these side effects.
In summary, AAVrh10.BevMab provides high focal levels of bevacizumab, which significantly inhibit tumor angiogenesis and growth of ovarian cancer. The vector prolongs survival of mice bearing ovarian cancer as a single agent and had an additive effect when combined with chemotherapy. The data support additional studies with AAVrh10.BevMab with the goal of translation into clinical practice.
Research highlights.
Local administration of AAVrh.10BevMab provides persistent bevacizumab expression
AAV-mediated therapy reduces tumor burden and prolongs overall survival
AAV-mediated therapy, combined with chemotherapy provides additive survival outcome
Acknowledgments
We thank A Kwaa and BP De for help with these studies; E Peguero and JH Shieh for help with in vivo imaging; and N. Mohamed and D.N. McCarthy for help with the manuscript. These studies were supported, in part, by The Starr Cancer Consortium; the Qatar Foundation, Qatar, NPRP 4-640-1-096, 6-1131-3-268, 09-1174-3-291; and the Weill Cornell Medical College in Qatar, Doha, Qatar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest exists.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Markman M. New developments in the anti-neoplastic drug management of ovarian cancer. F1000Prime Rep. 2013;5:48. doi: 10.12703/P5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;128:391–6. doi: 10.1016/j.ygyno.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 8.Mabuchi S, Terai Y, Morishige K, Tanabe-Kimura A, Sasaki H, Kanemura M, et al. Maintenance treatment with bevacizumab prolongs survival in an in vivo ovarian cancer model. Clin Cancer Res. 2008;14:7781–9. doi: 10.1158/1078-0432.CCR-08-0243. [DOI] [PubMed] [Google Scholar]
- 9.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 10.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 11.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 12.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Aurelia: a randomized phasee III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrant ovarian cancer (OC). Journal of Clinical Oncology. 2012;30(18_suppl) Ref Type: Abstract. [Google Scholar]
- 14.Du Bois A, Floquet A, Kim JW, Rau J, Del Campo JM, Friedlander M, et al. Randomized, double-blind, phase III trial of pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (AEOC): results of an international intergroup trial (AGO OVAR16). J Clin Oncol. 2013;31(18_suppl) Ref Type: Abstract. [Google Scholar]
- 15.Herzog TJ, Scambia G, Kim BG, Lhomme C, Markowska J, Ray-COquard I, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol Oncol. 2013;130:25–30. doi: 10.1016/j.ygyno.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Baumann KH, du BA, Meier W, Rau J, Wimberger P, Sehouli J, et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann Oncol. 2012;23:2265–71. doi: 10.1093/annonc/mds003. [DOI] [PubMed] [Google Scholar]
- 17.Tew WP, Colombo N, Ray-COquard I, Del Campo JM, Oza A, Pereira D, et al. Intravenous aflibercept in patients with platinum-resistant, advanced ovarian cancer: Results of a Randomized, Double-Blind, Phase 2, Parallel-Arm Study. Cancer. 2013 doi: 10.1002/cncr.28406. [DOI] [PubMed] [Google Scholar]
- 18.Lambrechts D, Lenz HJ, de HS, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–30. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 19.Monk BJ, Pujade-Lauraine E, Burger RA. Integrating bevacizumab into the management of epithelial ovarian cancer: the controversy of front-line versus recurrent disease. Ann Oncol. 2013;24(Suppl 10):x53–x58. doi: 10.1093/annonc/mdt472. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Boyer JL, Crystal RG. AAVrh.10-mediated genetic delivery of bevacizumab to the pleura to provide local anti-VEGF to suppress growth of metastatic lung tumors. Gene Ther. 2010;17:1042–51. doi: 10.1038/gt.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao Y, Kiss S, Boyer JL, Hackett NR, Qiu J, Carbone A, et al. Persistent suppression of ocular neovascularization with intravitreal administration of AAVrh.10 coding for bevacizumab. Hum Gene Ther. 2011;22:1525–35. doi: 10.1089/hum.2011.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delord JP, Allal C, Canal M, Mery E, Rochaix P, Hennebelle I, et al. Selective inhibition of HER2 inhibits AKT signal transduction and prolongs disease-free survival in a micrometastasis model of ovarian carcinoma. Ann Oncol. 2005;16:1889–97. doi: 10.1093/annonc/mdi405. [DOI] [PubMed] [Google Scholar]
- 23.Vassileva V, Allen CJ, Piquette-Miller M. Effects of sustained and intermittent paclitaxel therapy on tumor repopulation in ovarian cancer. Mol Cancer Ther. 2008;7:630–7. doi: 10.1158/1535-7163.MCT-07-2117. [DOI] [PubMed] [Google Scholar]
- 24.Rafii A, Mirshahi P, Poupot M, Faussat AM, Simon A, Ducros E, et al. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One. 2008;3:e3894. doi: 10.1371/journal.pone.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 26.Pasquier J, Guerrouahen BS, Al TH, Ghiabi P, Maleki M, bu-Kaoud N, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–86. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 28.Hampl M, Tanaka T, Albert PS, Lee J, Ferrari N, Fine HA. Therapeutic effects of viral vector-mediated antiangiogenic gene transfer in malignant ascites. Hum Gene Ther. 2001;12:1713–29. doi: 10.1089/104303401750476221. [DOI] [PubMed] [Google Scholar]
- 29.Calcedo R, Wilson JM. Humoral Immune Response to AAV. Front Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian IV, Bui Nguyen TM, Truskinovsky AM, Tolar J, Blazar BR, Ramakrishnan S. Adeno-associated virus-mediated delivery of a mutant endostatin in combination with carboplatin treatment inhibits orthotopic growth of ovarian cancer and improves long-term survival. Cancer Res. 2006;66:4319–28. doi: 10.1158/0008-5472.CAN-05-3297. [DOI] [PubMed] [Google Scholar]
- 31.Vandenberghe LH, Breous E, Nam HJ, Gao G, Xiao R, Sandhu A, et al. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther. 2009;16:1416–28. doi: 10.1038/gt.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiuchiolo MJ, Kaminsky SM, Sondhi D, Hackett NR, Rosenberg JB, Frenk EZ, et al. Intrapleural Administration of an AAVrh.10 Vector Coding for Human alpha1-Antitrypsin for the Treatment of alpha1-Antitrypsin Deficiency. Hum Gene Ther Clin Dev. 2013;24:161–73. doi: 10.1089/humc.2013.168. [DOI] [PubMed] [Google Scholar]
- 33.Castells M, Milhas D, Gandy C, Thibault B, Rafii A, Delord JP, et al. Microenvironment mesenchymal cells protect ovarian cancer cell lines from apoptosis by inhibiting XIAP inactivation. Cell Death Dis. 2013;4:e887. doi: 10.1038/cddis.2013.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh M, Couto SS, Forrest WF, Lima A, Cheng JH, Molina R, et al. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumour models. J Pathol. 2012;227:417–30. doi: 10.1002/path.4053. [DOI] [PubMed] [Google Scholar]
- 35.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117:497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]