Abstract
Epilepsy is the most common neurological disorder affecting approximately 50 million people worldwide. In India, overall prevalence of epilepsy is reported to be 5.59/1000 population. Antiepileptic drugs (AEDs) constitute the main-stay of treatment with a large number of AEDs available in the market. High incidence of adverse effects is a major limitation with AEDs. One of the major concerns is significant metabolic effects on the bone. However, little attention has been paid to this issue because most of the bone effects remain subclinical for a long time and may take years to manifest clinically. The main effects include hypocalcemia, hypophosphatemia, reduced serum levels of Vitamin D, increase in parathormone (PTH) levels, and alterations in bone turnover markers. The CYP450 enzyme-inducing AEDs such as phenytoin, phenobarbital, carbamazepine, and primidone are the most common AEDs associated with bone disorders while the data regarding the effect of valproate and newer AEDs such as lamotrigine, gabapentin, vigabatrin, levetiracetam, and topiramate on bone metabolism and bone density are scanty and controversial. Deficiency of Vitamin D is commonly described as a cause for the bone loss in epileptic patients while others being decreased absorption of calcium, increased PTH levels, and inhibition of calcitonin secretion, etc. However, there are no formal practical guidelines for the management of bone disease among those taking AEDs. Evidence-based strategies regarding monitoring, prevention, and treatment of bone diseases in patients on AED therapy are needed.
Keywords: Bone disorders, bone mineral density, epilepsy, parathormone
Introduction
Epilepsy is the most common neurological disorder affecting approximately 50 million people worldwide.[1] In India, the overall prevalence of epilepsy is reported to be 5.59/1000 population.[2] There are very few incidence studies from India, and the most recent one suggests an age-standardized incidence rate of 27.3/100,000 per year.[3] Medical treatment with antiepileptic drugs (AEDs) is the main-stay of treatment. A large number of AEDs are available in the market. Use of newer AEDs is becoming increasingly prevalent due to their increased tolerability, although conventional medications are still being used in older patients.[4] It has been found that 70% of the patients become seizure-free after the institution of AED therapy.[5] The AEDs are increasingly being used for nonseizure indications such as neurological disorders such as neuropathic pain, essential tremor, prophylaxis of migraine and psychiatric disorders including anxiety, bipolar disorder, schizophrenia, and behavioral disturbance in dementia.[6]
Adverse effects of AEDs have a considerable impact on the quality of life and contribute to treatment failure.[7] One of the major concerns with the use of AEDs is the occurrence of significant metabolic effects on the bone as AED treatment is given for years or lifelong. It is very important to have knowledge and understanding of the possible metabolic derangements associated with AED use as most of the bone effects remain subclinical for a long time and may take years to manifest clinically.
The incidence of seizures and epilepsy is high in childhood and in the elderly, especially after the age of 60 years.[8] Childhood and adolescence are critical periods of skeletal bone mineralization. It has been found that peak bone mineral density (BMD) achieved by the end of adolescence will determine the risk for pathological fractures and osteoporosis in the later life.[9] Thus, any fall in BMD during this decisive period could adversely affect the bone health in later life. It is predicted that in white populations >50 years of age, ~50% of women, and ~20% of men will have a fragility fracture later in their life.[4] In India, based on 2001 census, it is predicted that by 2015, 230 million Indians will be above the age of 50 years. Of these about 10–15% of men and 20% of women will be osteoporotic leading to total affected population to be 25 million.[10]
Epilepsy is itself known to increase bone loss and the risk of fractures by a variety of mechanisms such as restrictions of physical activity imposed by seizures, coexisting neurological deficits, and seizure-related falls[11] apart from those caused by the use of AEDs, which negatively affect the bone health. An increased fracture rate of 2–6 times has been found in patients with epilepsy than seen in general population.[12]
Antiepileptic Drugs and Spectrum of Bone Disorders
A large body of evidence indicates an association between AEDs and bone abnormalities ranging from disorders of bone mineral metabolism[13,14] to decrease in BMD to an increased fracture risk.[15] Bone biopsies and dual energy X-ray absorptiometry (DEXA) which are the gold-standard technique has provided both histological as well as radiographic evidence of bone abnormalities. Bone loss associated with the use of AED is usually insidious and asymptomatic to start with and goes unrecognized for a long period and often untreated.[16]
Many biochemical abnormalities have been shown be associated with the use of AEDs such as hypocalcemia, hypophosphatemia, reduced serum levels of Vitamin D (biologically active metabolites), and increase in parathormone (PTH) levels.[17] Alkaline phosphatase, osteocalcin, and C-terminal extension peptide of Type I procollagen which are the markers of bone turnover and cross-linked carboxyterminal telopeptide of human Type I collagen and hydroxyproline which are the markers of bone resorption are found to be elevated.[15] Serum total and bone alkaline phosphatase were also significantly increased in ambulatory children with adequate sun exposure within 90 days of initiation of treatment with carbamazepine or valproic acid.[18]
A number of studies in adult patients have reported a significant decrease in the BMD at the ribs and spine,[19] neck of femur, and hip[13,14] using DEXA. Similar results have been reported in children and adolescents on AED treatment as compared with the control subjects.[20] A very high prevalence (80%) of low BMD was found in a recent study carried out in a group of inpatients with chronic epilepsy.[21]
A two- to three-fold risk increase in the risk of sustaining a fracture has been associated in patients on long-term use of AEDs.[22] Increased risk of fractures of the hip, spine, and Colles fracture has been reported.[23,24] A population-based analysis has also revealed an association between most AEDs with nontraumatic fractures in individuals aged 50 and above.[22]
Conventional Antiepileptic Drugs and Bone Disease
The AEDs most commonly associated with bone disorders are known to induce enzymes of the cytochrome P450 system; phenytoin, phenobarbital, carbamazepine, and primidone being the major culprits.[24,25] However, there is limited and conflicting data of the effect of valproate (enzyme inhibitor) on bone metabolism and bone density.[26] A cross-sectional study on 71 patients showed lower BMD in subjects, on enzyme-inducing drugs such as phenytoin, phenobarbital, carbamazepine, and primidone than those on noninducers such as valproic acid, lamotrigine, clonazepam, gabapentin, topamirate, and ethosuximide.[14]
Newer Antiepileptic Drugs and Bone Health
Over the past decade, there has been a proliferation of new AEDs which have been approved, promising a better quality of life with lesser adverse effects for many with epilepsy. However, the question now arises whether the newer AEDs, such as lamotrigine, gabapentin, vigabatrin, levetiracetam, and topiramate cause little or no adverse bone changes. Literature search reveals that the data on bone-specific effects of newer AEDs is limited with conflicting results.
Oxcarbazepine, gabapentin and for levetiracetam in preclinical studies are associated with alterations of bone metabolism.[15,27] A recent study in 108 patients concluded that newer generation (lamotrigine, topiramate, and clonazepam) AEDs are associated with low BMD. However, the patients were also on treatment with one of the conventional drugs leading to inability to arrive at conclusive evidence.[19]
Data in adult epileptic patients treated with different AEDs indicate that long-term treatment with gabapentin can lead to loss of bone at the hip and lumbar spine.[13,28] Another prospective study in older men confirmed bone loss at the femoral neck by gabapentin.[29] In addition, a significant increase in fracture risk was found to be associated with gabapentin in a retrospective, matched cohort study.[22]
Low bone mass and reduced bone formation are also reported in children aged 3–17 years treated with lamotrigine either alone or in combination with valproic acid.[30] A preclinical study in rats treated with low-dose levetiracetam resulted in decreased bone strength at the femoral neck; however, on the contrary, bone mineral content and bone mass were found to remain unchanged.[31]
In another study, administration of levetiracetam in 16 orchidectomized Wistar rats led to significant loss of BMD at the femur area, significantly decreased levels of osteoprotegerin (marker of bone formation) in serum and increased levels of carboxyterminal cross-linking telopeptide of Type I collagen (marker of bone resorption) in bone homogenate.[27]
Few studies also reported reduced 25 hydroxy Vitamin D (25[OH] D) levels, elevated markers of bone resorption,[32] and significant reductions in BMD with oxcarbazepine.[33]
Mild to moderate metabolic acidosis resulting in the development of kidney stones, osteomalacia, and/or osteoporosis has been reported in patients treated with topiramate.[33] Another study in 36 women on long-term topiramate monotherapy demonstrated lower PTH, mild hypocalcemia, and an increase in bone turnover.[34] Use of topiramate in children has also resulted in alterations in serum contents of calcium, phosphorus, and alkaline phosphatase as well as reductions in BMD.[35]
On the contrary, the data from a retrospective cohort study following 560 patients reported that patients prescribed newer, nonenzyme-inducing anticonvulsants were less expected to have osteoporosis at the lumbar spine, femoral neck, and hip suggesting that newer anticonvulsant medications are not associated with lower BMD.[6] Likewise, another study in 13 children on lamotrigine monotherapy compared to 36 control subjects and 40 patients exposed to polytherapy concluded that lamotrigine may not interfere with the bone growth.[36] In a recent study, levetiracetam monotherapy in 61 drug-naive patients did not reveal any significant association with bone metabolism and BMD.[37]
Mechanisms of Bone Loss with Antiepileptic Drugs
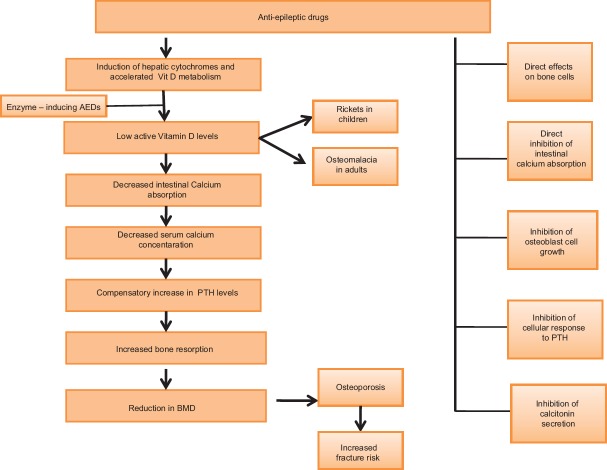
The pathogenesis of AED-associated bone abnormalities seems to be multifactorial. A large number of mechanisms have been postulated [Figure 1].
Figure 1.
Proposed mechanisms contributing to antiepileptic drug-induced bone loss
Deficiency of Vitamin D which is essential for the bone growth and remodeling is commonly described as a cause for the bone loss in epileptic patients.[13] It has been found that induction of hepatic CYP450 system accelerates the catabolism of Vitamin D into its polar inactive metabolites, decreasing biologically active forms of Vitamin D. Reduced levels of serum 25(OH)D concentrations are seen both in adults and children.[18] However, finding of Vitamin D deficiency is not consistently seen in all the studies evaluating the effect of AED on bone health and some studies have shown evidence of increased bone turnover even in the absence of Vitamin D deficiency. Valproic acid, being an enzyme inhibitor, is also associated with decrease in BMD and increased risk of fractures.[6,38] Therefore, it has been suggested that AEDs may affect the bone metabolism through mechanisms not only associated with hepatic enzyme induction.
There is decreased absorption of calcium from the gut which can be attributed to the decrease in biologically active forms of Vitamin D resulting in hypocalcemia and feedback hypersecretion of circulating PTH. Hyperparathyroidism leads to increased bone resorption and ultimately reduced BMD and increased fracture risk.[6,39,40] Inhibition of the cellular response to PTH is also thought to play a pivotal role.[12] Other mechanisms include direct effects of AEDs on bone cells, direct inhibition of calcium absorption from the intestine, inhibition of osteoblast cell growth, and inhibition of calcitonin secretion.[4,39]
Risk Factors for Bone Loss in Patients on Antiepileptic Drugs
Age, duration of epilepsy and AED treatment, dose of antiepileptics used, and polypharmacy [Table 1] are significant determinants of BMD.[14]
Table 1.
Factors contributing to bone loss and fractures in epileptic patients

Ambulatory adults with epilepsy have a greater reduction in BMD as compared to children. It has been found that increasing duration of epilepsy is associated with a progressive reduction in BMD as compared to controls.[41] The timing of deficit in BMD in children on AED is a matter of concern. Duration of therapy is one of the independent predictors of BMD in adults. Reduction in the BMD may be detected during the first 1–5 years of treatment.[16,28] The duration of AED treatment is associated with the rate of drug-induced bone loss in men on AEDs. Their long-term use is found to be associated with decreased bone mineral content in 20–65% of the patients.[41] The risk of fractures is also increased with the cumulative duration of exposure.[42]
A dose–response relationship is observed between the use of AED and fracture risk. Chronic high-cumulative doses have shown to increase the fracture risk in a dose-dependent manner.[23]
Polytherapy is found to be an independent risk factor for BMD in children.[28] It has been found that fracture risk in postmenopausal women is more commonly associated with treatment with more than one AED.[43]
Recommendations and Strategies Suggested
A number of therapeutic options are available for the prevention and treatment of reduced BMD including calcium and Vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, hormone replacement therapy, recombinant forms of PTH, and calcitonin. However, very few studies are available on the prevention and treatment of bone disease in epileptic patients on long-term AEDs.[24] Data on screening and treatment of bone diseases in patients on AED therapy are scanty. Evidence-based strategies regarding prevention and monitoring of bone diseases in patients on AED therapy are needed. Following are the recommendations and strategies suggested by various authorities and study authors:
Vitamin D and calcium supplementation: Is it effective?
The Medicines and Healthcare products Regulatory Agency recommends considering prophylactic Vitamin D supplementation for patients at risk treated with liver enzyme-inducing AEDs and valproate.[44] Prophylactic Vitamin D doses up to 2000 IU/day can be given in all patients at the beginning of treatment. Calcium intake in doses of 600–1000 mg/day should also be ensured. In case of osteopenia or osteoporosis, treatment with 2000–4000 IU/day Vitamin D is appropriate. Vitamin D doses may be increased in cases of osteomalacia.[45]
However, a recent systematic review and meta-analysis of randomized controlled trials in healthy children with normal levels of Vitamin D concluded that Vitamin D supplementation is unlikely to be effective in improving BMD in healthy children and adolescents. However, on subgroup analysis, it was found that supplementation of deficient children may be clinically useful, especially in the lumbar spine and total body bone mineral content.[46,47]
Bisphosphonates
Bisphosphonates are usually reserved for the treatment of high fracture risk patients. In addition, bisphosphonates may be required in cases where the response to Vitamin D is found to be inadequate. In one of the recent studies in patients on AEDs, supplementation of calcium and Vitamin D in addition to risedronate resulted in improvement of BMD in more than 69% of male veterans.[48]
Dietary and lifestyle modifications
Nonpharmacological measures such as alteration of lifestyle may play an important role in improving the bone health in patients on AEDs. Regular physical activity, well-balanced diet with sufficient protein intake, cessation of both smoking and excessive alcohol intake should be stressed on.[15,19] The Scottish Intercollegiate Guidelines Network recommends that patients taking both liver enzyme- and nonliver enzyme-inducing AEDs should receive dietary and lifestyle advice to reduce osteoporosis risk.[49] Seizure control is equally important and strategies to prevent falls should be introduced.
Investigations and monitoring
It is generally recommended that serum 25(OH)D levels should be measured before the initiation of treatment and then at 6–12 months in patients on long-term treatment with enzyme-inducing AEDs. Monitoring of biochemical markers of bone turnover is not recommended in routine clinical practice.[50] The National Institute for Clinical Excellence (NICE) recommends the tests of bone metabolism every 2–5 years for adults taking liver enzyme-inducing AEDs.[51] Findings from a recent meta-analysis addressing the use of AED and effect on BMD and bone metabolism in children (comprised 22 studies with 1492 subjects) indicate that AED treatment reduces the BMD in children, and BMD monitoring should be advocated for epileptic children with high risk for abnormal bone health.[52]
Due to consideration should be paid in the selection of AED for treating a newly diagnosed patient with epilepsy keeping in mind who are at risk for bone loss or have bone disease and the growing list of options available. Further investigations of newer AEDs to assess their long-term effects on bone are essential. Even in the absence of evidence, it seems reasonable to give supplements and maintain levels of 25(OH)D and further research is needed to clarify the particular subgroups, dosages, and other factors that may influence the effects of AEDs on BMD and bone metabolism. There is a compelling need of formulation of guidelines for treatment and prevention of AEDs-induced bone loss based on randomized clinical trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Epilepsy Fact Sheet; October, 2012. [Last accessed on 2015 Jun 15]. Available from: http://www.who.int/mediacentre/factsheets/fs999/en/
- 2.Bharucha NE. Epidemiology of epilepsy in India. Epilepsia. 2003;44(Suppl 1):9–11. doi: 10.1046/j.1528-1157.44.s.1.5.x. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha NE. Epidemiology and treatment gap of epilepsy in India. Ann Indian Acad Neurol. 2012;15:352–3. doi: 10.4103/0972-2327.104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RH, Lyles KW, Colón-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8:34–46. doi: 10.1016/j.amjopharm.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuji S, Akamatsu N. Treatment of epilepsy. Rinsho Shinkeigaku. 2008;48:550–5. doi: 10.5692/clinicalneurol.48.550. [DOI] [PubMed] [Google Scholar]
- 6.Lee R, Lyles K, Sloane R, Colón-Emeric C. The association of newer anticonvulsant medications and bone mineraldensity. Endocr Pract. 2012;14:1–22. doi: 10.4158/EP12119.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley RW, Penney SJ, Buckley DJ. First-drug treatment failures in children newly diagnosed with epilepsy. Pediatr Neurol. 2009;40:71–7. doi: 10.1016/j.pediatrneurol.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Sander JW, Hart YM, Johnson AL, Shorvon SD. National general practice study of epilepsy: Newly diagnosed epileptic seizures in a general population. Lancet. 1990;336:1267–71. doi: 10.1016/0140-6736(90)92959-l. [DOI] [PubMed] [Google Scholar]
- 9.Samaniego EA, Sheth RD. Bone consequences of epilepsy and antiepileptic medications. Semin Pediatr Neurol. 2007;14:196–200. doi: 10.1016/j.spen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra N, Mithal A. Osteoporosis in Indians. Indian J Med Res. 2008;127:263–8. [PubMed] [Google Scholar]
- 11.Petty SJ, O'Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int. 2007;18:129–42. doi: 10.1007/s00198-006-0185-z. [DOI] [PubMed] [Google Scholar]
- 12.Valsamis HA, Arora SK, Labban B, McFarlane SI. Antiepileptic drugs and bone metabolism. Nutr Metab (Lond) 2006;3:36. doi: 10.1186/1743-7075-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andress DL, Ozuna J, Tirschwell D, Grande L, Johnson M, Jacobson AF, et al. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol. 2002;59:781–6. doi: 10.1001/archneur.59.5.781. [DOI] [PubMed] [Google Scholar]
- 14.Farhat G, Yamout B, Mikati MA, Demirjian S, Sawaya R, El-Hajj Fuleihan G. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology. 2002;58:1348–53. doi: 10.1212/wnl.58.9.1348. [DOI] [PubMed] [Google Scholar]
- 15.Meier C, Kraenzlin ME. Antiepileptics and bone health. Ther Adv Musculoskelet Dis. 2011;3:235–43. doi: 10.1177/1759720X11410769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakken KO, Taubøll E. Bone loss associated with use of antiepileptic drugs. Expert Opin Drug Saf. 2010;9:561–71. doi: 10.1517/14740331003636475. [DOI] [PubMed] [Google Scholar]
- 17.Pack AM, Gidal B, Vazquez B. Bone disease associated with antiepileptic drugs. Cleve Clin J Med. 2004;71(Suppl 2):S42–8. doi: 10.3949/ccjm.71.suppl_2.s42. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamoorthy G, Karande S, Ahire N, Mathew L, Kulkarni M. Bone metabolism alteration on antiepileptic drug therapy. Indian J Pediatr. 2009;76:377–83. doi: 10.1007/s12098-009-0005-5. [DOI] [PubMed] [Google Scholar]
- 19.Salimipour H, Kazerooni S, Seyedabadi M, Nabipour I, Nemati R, Iranpour D, et al. Antiepileptic treatment is associated with bone loss: Difference in drug type and region of interest. J Nucl Med Technol. 2013;41:208–11. doi: 10.2967/jnmt.113.124685. [DOI] [PubMed] [Google Scholar]
- 20.Babayigit A, Dirik E, Bober E, Cakmakci H. Adverse effects of antiepileptic drugs on bone mineral density. Pediatr Neurol. 2006;35:177–81. doi: 10.1016/j.pediatrneurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Beerhorst K, Tan IY, De Krom M, Verschuure P, Aldenkamp AP. Antiepileptic drugs and high prevalence of low bone mineral density in a group of inpatients with chronic epilepsy. Acta Neurol Scand. 2013;128:273–80. doi: 10.1111/ane.12118. [DOI] [PubMed] [Google Scholar]
- 22.Jetté N, Lix LM, Metge CJ, Prior HJ, McChesney J, Leslie WD. Association of antiepileptic drugs with nontraumatic fractures: A population-based analysis. Arch Neurol. 2011;68:107–12. doi: 10.1001/archneurol.2010.341. [DOI] [PubMed] [Google Scholar]
- 23.Tsiropoulos I, Andersen M, Nymark T, Lauritsen J, Gaist D, Hallas J. Exposure to antiepileptic drugs and the risk of hip fracture: A case-control study. Epilepsia. 2008;49:2092–9. doi: 10.1111/j.1528-1167.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholas JM, Ridsdale L, Richardson MP, Grieve AP, Gulliford MC. Fracture risk with use of liver enzyme inducing antiepileptic drugs in people with active epilepsy: Cohort study using the general practice research database. Seizure. 2013;22:37–42. doi: 10.1016/j.seizure.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Verrotti A, Greco R, Latini G, Morgese G, Chiarelli F. Increased bone turnover in epileptic patients treated with carbamazepine. Ann Neurol. 2000;47:385–8. [PubMed] [Google Scholar]
- 26.Zare M, Ghazvini MR, Dashti M, Najafi MR, Alavi-Naeini AM. Bone turnover markers in epileptic patients under chronic valproate therapy. J Res Med Sci. 2013;18:338–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Fekete S, Simko J, Gradosova I, Malakova J, Zivna H, Palicka V, et al. The effect of levetiracetam on rat bone mass, structure and metabolism. Epilepsy Res. 2013;107:56–60. doi: 10.1016/j.eplepsyres.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 28.El-Hajj Fuleihan G, Dib L, Yamout B, Sawaya R, Mikati MA. Predictors of bone density in ambulatory patients on antiepileptic drugs. Bone. 2008;43:149–55. doi: 10.1016/j.bone.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Walczak TS, Blackwell TL, Ensrud ER, Barrett-Connor E, Orwoll ES Osteoporotic Fractures in Men (MrOS) Study Research Group. Antiepileptic drug use and rates of hip bone loss in older men: A prospective study. Neurology. 2008;71:723–30. doi: 10.1212/01.wnl.0000324919.86696.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo CY, Ronen GM, Atkinson SA. Long-term valproate and lamotrigine treatment may be a marker for reduced growth and bone mass in children with epilepsy. Epilepsia. 2001;42:1141–7. doi: 10.1046/j.1528-1157.2001.416800.x. [DOI] [PubMed] [Google Scholar]
- 31.Nissen-Meyer LS, Svalheim S, Taubøll E, Reppe S, Lekva T, Solberg LB, et al. Levetiracetam, phenytoin, and valproate act differently on rat bone mass, structure, and metabolism. Epilepsia. 2007;48:1850–60. doi: 10.1111/j.1528-1167.2007.01176.x. [DOI] [PubMed] [Google Scholar]
- 32.Cansu A, Yesilkaya E, Serdaroglu A, Hirfanoglu TL, Camurdan O, Gülbahar O, et al. Evaluation of bone turnover in epileptic children using oxcarbazepine. Pediatr Neurol. 2008;39:266–71. doi: 10.1016/j.pediatrneurol.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Verrotti A, Coppola G, Parisi P, Mohn A, Chiarelli F. Bone and calcium metabolism and antiepileptic drugs. Clin Neurol Neurosurg. 2010;112:1–10. doi: 10.1016/j.clineuro.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Heo K, Rhee Y, Lee HW, Lee SA, Shin DJ, Kim WJ, et al. The effect of topiramate monotherapy on bone mineral density and markers of bone and mineral metabolism in premenopausal women with epilepsy. Epilepsia. 2011;52:1884–9. doi: 10.1111/j.1528-1167.2011.03131.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wang KX, Wei Y, Xu MH, Su JM, Bao YG, et al. Effect of topiramate and carbamazepine on bone metabolism in children with epilepsy. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:96–8. [PubMed] [Google Scholar]
- 36.Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37:250–4. doi: 10.1016/j.pediatrneurol.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Koo DL, Joo EY, Kim D, Hong SB. Effects of levetiracetam as a monotherapy on bone mineral density and biochemical markers of bone metabolism in patients with epilepsy. Epilepsy Res. 2013;104:134–9. doi: 10.1016/j.eplepsyres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Boluk A, Guzelipek M, Savli H, Temel I, Ozisik HI, Kaygusuz A. The effect of valproate on bone mineral density in adult epileptic patients. Pharmacol Res. 2004;50:93–7. doi: 10.1016/j.phrs.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick LA. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004;5(Suppl 2):S3–15. doi: 10.1016/j.yebeh.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Feldkamp J, Becker A, Witte OW, Scharff D, Scherbaum WA. Long-term anticonvulsant therapy leads to low bone mineral density – Evidence for direct drug effects of phenytoin and carbamazepine on human osteoblast-like cells. Exp Clin Endocrinol Diabetes. 2000;108:37–43. doi: 10.1055/s-0032-1329213. [DOI] [PubMed] [Google Scholar]
- 41.Sheth RD, Binkley N, Hermann BP. Progressive bone deficit in epilepsy. Neurology. 2008;70:170–6. doi: 10.1212/01.wnl.0000284595.45880.93. [DOI] [PubMed] [Google Scholar]
- 42.Shiek Ahmad B, Hill KD, O'Brien TJ, Gorelik A, Habib N, Wark JD. Falls and fractures in patients chronically treated with antiepileptic drugs. Neurology. 2012;79:145–51. doi: 10.1212/WNL.0b013e31825f0466. [DOI] [PubMed] [Google Scholar]
- 43.Carbone LD, Johnson KC, Robbins J, Larson JC, Curb JD, Watson K, et al. Antiepileptic drug use, falls, fractures, and BMD in postmenopausal women: Findings from the women's health initiative (WHI) J Bone Miner Res. 2010;25:873–81. doi: 10.1359/jbmr.091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adverse Effects on Bone. Drug Safety Update. 2009. [Last accessed on 2015 Jun 15]. Available from: http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON087970 .
- 45.Drezner MK. Treatment of anticonvulsant drug-induced bone disease. Epilepsy Behav. 2004;5(Suppl 2):S41–7. doi: 10.1016/j.yebeh.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Winzenberg T, Powell S, Shaw KA, Jones G. Effects of Vitamin D supplementation on bone density in healthy children: Systematic review and meta-analysis. BMJ. 2011;342:c7254. doi: 10.1136/bmj.c7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harijan P, Khan A, Hussain N. Vitamin D deficiency in children with epilepsy: Do we need to detect and treat it? J Pediatr Neurosci. 2013;8:5–10. doi: 10.4103/1817-1745.111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazzari AA, Dussault PM, Thakore-James M, Gagnon D, Baker E, Davis SA, et al. Prevention of bone loss and vertebral fractures in patients with chronic epilepsy – antiepileptic drug and osteoporosis prevention trial. Epilepsia. 2013;54:1997–2004. doi: 10.1111/epi.12351. [DOI] [PubMed] [Google Scholar]
- 49.Edinburgh: Scottish Intercollegiate Guidelines Network; 2003. [Last accessed on 2015 Jun 16]. Scottish Intercollegiate Guidelines Network. Diagnosis and Management of Epilepsy in Adults. Available from: http://www.sign.ac.uk/pdf/sign70.pdf . [Google Scholar]
- 50.Meier C, Seibel MJ, Kraenzlin ME. Use of bone turnover markers in the real world: Are we there yet? J Bone Miner Res. 2009;24:386–8. doi: 10.1359/jbmr.090104. [DOI] [PubMed] [Google Scholar]
- 51.National Institute for Clinical Excellence. The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care. London: National Institute for Clinical Excellence; 2004. [Last accessed on 2015 Jun 16]. Available from: http://www.nice.org.uk/nicemedia/pdf/cg020fullguideline.pdf . [Google Scholar]
- 52.Zhang Y, Zheng YX, Zhu JM, Zhang JM, Zheng Z. Effects of antiepileptic drugs on bone mineral density and bone metabolism in children: A meta-analysis. J Zhejiang Univ Sci B. 2015;16:611–21. doi: 10.1631/jzus.B1500021. [DOI] [PMC free article] [PubMed] [Google Scholar]