Abstract
Objective:
Dolutegravir, an HIV integrase inhibitor, is a relatively new treatment option. To assess the tolerability, side effects, and time to viral decline to non-detectable in patients newly started on dolutegravir.
Methods:
Retrospective health care record of 61 consecutive HIV treatment-naive patients started on dolutegravir was reviewed and analysed on SPSS.
Results:
The mean initial viral load was 160826.05 copies/mL (range, 79–1,126,617 copies/mL). HIV viral load became non-detectable in 63.9% of patients on dolutegravir within 3 months. In all, 60.7% of patients reported no side effects on dolutegravir; 98.4% of the patients claimed full compliance to their antiretrovirals.
Conclusion:
Dolutegravir was found to be efficacious and well tolerated in HIV-infected treatment-naive patients.
Keywords: Pharmacoepidemiology/drug safety, dolutegravir, HIV, viral load, naive, integrase inhibitor, tolerability
Introduction
The current classes of antiretrovirals include the nucleoside and nucleotide reverse-transcriptase inhibitors (NRTIs), non-nucleoside reverse-transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), entry inhibitors, and integrase strand transfer inhibitors (INSTIs).1 INSTI currently available for use are raltegravir (RAL), elvitegravir (EVG), and dolutegravir (DTG).2 RAL (approved by Food and Drug Administration (FDA) in 2007) requires twice daily dosing, EVG (FDA approved in 2012) requires pharmacological boosting to be given once daily, and significant cross resistance between RAL and EVG prevents sequential therapy with these two agents.3 DTG was approved by FDA for treatment-naive and treatment-experienced patients with HIV infection in August 2013.4 DTG can be given once a day without boosting and can overcome some previous INSTI treatment failure.3 DTG once daily is recommended for patients infected with HIV-1 without documented or clinically suspected resistance to the integrase class while twice daily is recommended for co-administration with certain medicines or for patients infected with HIV-1 with resistance to the integrase class (documented or clinically suspected).5
Different phase III studies of DTG in treatment-naive HIV-1-infected patients have demonstrated it to be efficacious and safe when compared with darunavir boosted with ritonavir (FLAMINGO study), efavirenz (SINGLE study), and RTG (SPRING-2 study).6–8 DTG has been shown to have good tolerability, infrequent drug–drug interactions, and high barrier to resistance.1 DTG is currently approved for use in European Union (EU) as a single pill (Tivicay®)5 or as combination tablet comprising DTG 50 mg and the NRTIs abacavir (ABV) 600 mg and lamivudine (3TC) 300 mg (Triumeq®).9 DTG as a single pill became available in our hospital for use in May 2014 while DTG/ABC/3TC combination pill became available for use in January 2015. Our review was undertaken to assess the tolerability, side effects, and time to viral decline to non-detectable in patients newly started on DTG.
Methods
Study design
This pharmacoepidemiologic study was commenced after obtaining approval from the Research Ethics Committee at the Mater Misericordiae University Hospital in Dublin. Informed consent was not sought for the study because it was a retrospective health care record study and there was no disclosure of personal data to any outside third parties. Retrospective health care record of patients started on DTG between May 2014 and May 2015 was reviewed.
Patients
The study sample included treatment-naive HIV-infected patients above 16 years of age who were attending the HIV clinic of the hospital and were initiated on DTG as part of HIV treatment regime between May 2014 and May 2015. All patients who were started on antiretroviral without DTG were excluded. Treatment-experienced patients who were switched to DTG were also excluded from the study.
Measurements
Health care record for all consecutive HIV-infected treatment-naive patients fulfilling the inclusion and exclusion criteria was accessed. Data was entered on Microsoft excel. Demographic information was recorded which included age, gender, duration since HIV diagnosis, duration since being on DTG, and backbone antiretroviral used alongside DTG. If patients were switched to Triumeq that was also recorded. Other variables recorded included dosing schedule of DTG, side effects reported by patients, whether patients stopped DTG after starting and reason for that, initial viral load at treatment commencement, time taken for viral load to become non-detectable, and compliance of patients to the treatment.
Statistical analysis
Data was analysed using Statistical Package for Social Sciences (SPSS), version 17. Descriptive statistics were used to report variables. Qualitative variables were presented as frequencies and percentage. Quantitative variables were either presented as mean and standard deviation or were grouped into ranges for determination of frequency and percentages for each range.
Results
A total of 61 treatment-naive HIV patients were started on DTG from May 2014 to May 2015. Mean age of the participants was 37.8. There were 44 male patients (72.1%) in the study. In all, 86.9% of total patients were diagnosed with HIV within the last 2 years. The backbone antiretroviral initially used alongside DTG included tenofovir/emtricitabine in majority of the patients (90.2%; n = 55). Kivexa was used in five patients (8.2%). In all, 26.2% (n = 16) of the total patients were switched to abacavir/lamivudine backbone when it became available as single combination pill with DTG. Only two patients were prescribed 50 mg twice daily DTG, for being on rifampicin (co-administration dose with UGT1A/CYP3A inducer) although they were INSTI naive and the rest of the patients were prescribed once daily. Totally, 96.7% (n = 59) of the patients were started on DTG within the last 12 months. The majority (60.7%) of patients reported no side effects on follow-up visits. Maximum reported side effects were rash (13.1%) and gastrointestinal disturbances (9.8%). Individual side effects as reported by patients are shown in Table 1. DTG was stopped in one patient because of side effects comprising rash, headache, and generalized aches. The mean initial viral load was 160826.05 copies/mL (range: 79 copies/mL–1,126,617 copies/mL). In total, 41% (n = 25) of patients had viral load above 100,000 copies/mL on starting DTG. HIV viral load became non-detectable in 63.9% of patients on DTG within 3 months and in 83.57% within 12 months (Figure 1). In another 9.84% patients, the viral load was not available at the time of data analysis. Overall, 98.4% of the patients reported full compliance to antiretrovirals on their follow-up clinic visits to the doctors and pharmacists.
Table 1.
Side effects reported by patients.
| Side effects reported | Frequency | Percent |
|---|---|---|
| Dizziness | 2 | 3.3 |
| GI upset | 6 | 9.8 |
| Anxiety | 2 | 3.3 |
| Insomnia | 1 | 1.6 |
| Itchy skin | 4 | 6.6 |
| Neuro-psychiatric | 1 | 1.6 |
| Nil | 37 | 60.7 |
| Rash | 8 | 13.1 |
| Total | 61 | 100.0 |
GI: gastrointestinal.
Figure 1.
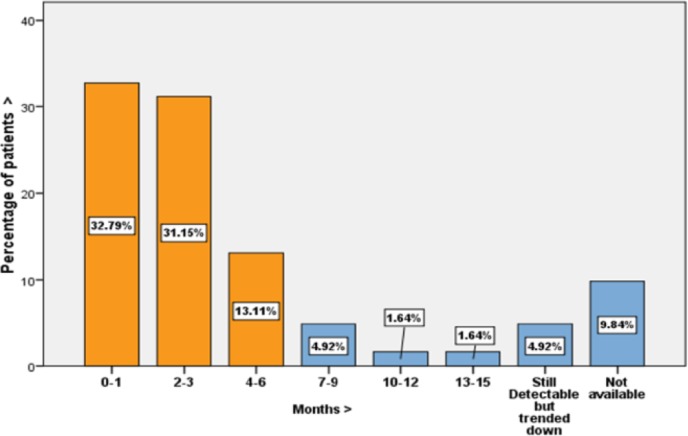
Number of months to viral load becoming non-detectable on DTG.
Discussion
In our study, DTG showed efficacy similar although lower than that reported in other efficacy studies of DTG. Overall, 83.57% of patients in our study had non-detectable viral load at 48 weeks. In SPRING-2 it was 88% at 48 weeks, in FLAMINGO it was 90% at 48 weeks, while in SINGLE study it was 88%.6,8,10 It may be noted that in 9.84% of our patients, the viral load result was not available at the time of data analysis and that could have contributed to lower apparent efficacy rate. A combined adverse effect profile of DTG based on different randomized controlled trials gives an incidence approaching 90% which is a very liberal estimate consisting predominantly of mild reactions.11 In our study, only 39.3% of patients reported some side effects to DTG, and out of the total 61 patients started on DTG, treatment had to be discontinued in only one patient (1.6%), which is similar to that reported in systematic reviews of DTG.12 A high level of compliance to the antiretrovirals was reported by our patients which could be accounted for by the single daily dosing regimen in majority (95.08%) and also by the switch to a single combination pill (26.2%) when it became available.
The main limitations of our study were small sample size, retrospective design, and single-centre analysis with no arm for comparison. All of our patients did not have the same follow-up period after starting DTG as we included all patients who were started on DTG over 1-year period and thus they had different follow-up periods.
Conclusion
DTG is well tolerated in HIV-infected treatment-naive patients, with the majority of our patients reporting no side effects. The HIV viral load among those started on DTG declined quickly. DTG can be considered an efficacious and well-tolerated drug with convenient dosing schedule in HIV treatment-naive patients. Studies of DTG should be undertaken in larger number of patients to see if similar results are obtained and its safety and efficacy should also be evaluated in special patient groups like pregnant women and elderly patients newly diagnosed with HIV.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Research Ethics Committee Mater Misericordiae University Hospital – Ethics Approval Reference Number: 1/378/1734 TMR.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not sought for this study because it was a retrospective health care record study and there was no disclosure of personal data to any outside third parties.
References
- 1. Kandel CE, Walmsley SL. Dolutegravir: a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Devel Ther 9: 3547–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Churchill D, Waters L, Ahmed N, et al. BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015, 2015, http://www.bhiva.org/HIV-1-treatment-guidelines.aspx [DOI] [PubMed]
- 3. Osterholzer DA, Goldman M. Dolutegravir: a next-generation integrase inhibitor for treatment of HIV infection. Clin Infect Dis 59: 265–271. [DOI] [PubMed] [Google Scholar]
- 4. Gu WG. Newly approved integrase inhibitors for clinical treatment of AIDS. Biomed Pharmacother 68: 917–921. [DOI] [PubMed] [Google Scholar]
- 5. EMC. Tivicay 50 mg film-coated tablets. Leatherhead: Datapharm, 2016. [Google Scholar]
- 6. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 369: 1807–1818. [DOI] [PubMed] [Google Scholar]
- 7. Raffi F, Rachlis A, Brinson C, et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naive HIV-infected individuals in three randomized trials. AIDS 29: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 13: 927–935. [DOI] [PubMed] [Google Scholar]
- 9. EMC. Triumeq 50mg, 300mg, 600mg tablets, 2016, https://www.medicines.org.uk/emc/medicine/29178
- 10. Clotet B, Feinberg J, Van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 383: 2222–2231. [DOI] [PubMed] [Google Scholar]
- 11. Curtis L, Nichols G, Stainsby C, et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor-naive patients. HIV Clin Trials 15: 199–208. [DOI] [PubMed] [Google Scholar]
- 12. Patel DA, Snedecor SJ, Tang WY, et al. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis. PLoS ONE 9: e105653. [DOI] [PMC free article] [PubMed] [Google Scholar]


