Abstract
Objective:
This case series was conducted to determine the clinical feasibility of a repetitive transcranial magnetic stimulation protocol for the prevention of migraine (with and without aura).
Methods:
Five patients with migraines underwent five repetitive transcranial magnetic stimulation sessions separated in 1- to 2-week intervals for a period of 2 months at a single tertiary medical center. Repetitive transcranial magnetic stimulation was applied to the left motor cortex with 2000 pulses (20 trains with 1s inter-train interval) delivered per session, at a frequency of 10 Hz and 80% resting motor threshold. Pre- and post-treatment numerical rating pain scales were collected, and percent reductions in intensity, frequency, and duration were generated.
Results:
An average decrease in 37.8%, 32.1%, and 31.2% were noted in the intensity, frequency, and duration of migraines post-repetitive transcranial magnetic stimulation, respectively. A mean decrease in 1.9±1.0 (numerical rating pain scale ± standard deviation; range: 0.4–2.8) in headache intensity scores was noted after the repetitive transcranial magnetic stimulation sessions.
Conclusion:
The tested repetitive transcranial magnetic stimulation protocol is a well-tolerated, safe, and effective method for migraine prevention.
Keywords: Repetitive transcranial magnetic stimulation, migraine, protocol
Introduction
Migraines affect approximately one in every seven Americans annually and are a leading cause of disability and suffering with significant socio-economic and health impacts.1 In addition, migraines have been found to be more prevalent in women than men with male:female ratio of 1:2.2
Studies have shown the pathophysiology of migraines to involve complex biochemical changes that lead to pain. One underlying mechanism is cortical spreading depression (CSD) of the neocortex and hippocampus, which then activates the trigeminal nucleus caudalis, causing vasodilation of blood vessels and release of calcitonin gene–related peptide (CGRP), substance P, and vasoactive intestinal polypeptide, ultimately leading to meningeal irritation and pain.2,3 Other changes include increased 5-hydroxytryptamine (5-HT) and nitric oxide (NO) levels, leading to enhanced central sensitization and pain.2
For migraines, pharmacologic therapies such as simple analgesics and non-steroidal inflammatory drugs remain the first-line treatment.4 However, many individuals continue to have headaches refractory to various prophylactic and/or abortive therapies, while others are at risk of medication overuse headaches.
Among non-pharmacologic therapies, repetitive transcranial magnetic stimulation (rTMS), a non-invasive neuromodulation technique involving repeated series of dynamic magnetic impulses, has been studied as a preventive migraine treatment.5 The mechanisms of headache relief with rTMS appear to be multifactorial. rTMS has been shown to cause various neurochemical changes including increased dopamine levels in the hippocampus, reduction in Raclopride C11 binding in the caudate nucleus, fluctuations in glutamate/glutamine levels at the site of rTMS stimulation and increased plasma β-endorphin levels.6–9 Furthermore, rTMS has been shown to stimulate weak electrical currents, causing depolarization of neurons and inhibition of CSD, which ultimately terminates the aura and reduces the duration or severity of migraine.10 Additionally, rTMS at the motor cortex is thought to reduce pain by altering the motor cortex projections to the medial thalamus and anterior cingulate/orbitofrontal cortices, thereby modulating pain.11 While prior research reveals the occurrence of migraines to be mediated by the activation of the trigeminovascular system resulting in CGRP release, the role of rTMS in CGRP modulation remains to be elucidated.
Correlating with these biochemical marker findings, a recent clinical study demonstrated that rTMS reduced the number of headache attacks and headache index when applied to the left dorsolateral prefrontal cortex at 20 Hz, 10 trains, and 90% motor threshold.12 An additional study demonstrated that rTMS when applied at 10 Hz, 600 pulses in 10 trains to the left frontal cortex, reduced migraine frequency and overall headache intensity scores in 1 month when compared to the sham stimulation group.13 On the contrary, a randomized double-blind clinical trial study revealed a reduction in headache days in the sham group as compared to those undergoing rTMS at 10 Hz, 1600 pulses in 32 trains when applied to the left dorsolateral prefrontal cortex over a period of 8 weeks.14
While the literature of rTMS in treating migraines is mixed, the feasibility of an effective rTMS long-term clinical protocol has yet to be established. To our best knowledge, this is the first case series to assess the clinical feasibility of a long-term (greater than 1 month) rTMS protocol, administered 1–2 weeks apart for the prevention of migraines (with and without aura) in the outpatient clinical setting.
Here, we report a case series of five patients with a clinical diagnosis of migraines (three patients with aura and two patients without aura) who responded favorably to a long-term clinical rTMS protocol. Almost all patients had attempted prophylactic and/or abortive migraine medications without relief of symptoms.
Materials and methods
Data were collected from five patients with migraines (with and without aura) undergoing rTMS over a period of 2 months at a single tertiary medical center. The diagnosis of migraines was based on the International Headache Society (IHS) Criteria (International Classification of Headache Disorders: 2nd edition (ICHD-II)).15 Inclusion criteria included patients >18 years of age fulfilling the diagnosis of migraines (with and without aura) as per the IHS criteria and at least two migraine prophylactic medications. Patients were excluded from the case series if they had a history of seizures or had undergone botulinum toxin therapy within the preceding 3 months. All patients were instructed to maintain their headache medication regimen during the study period and to report any change of their medications.
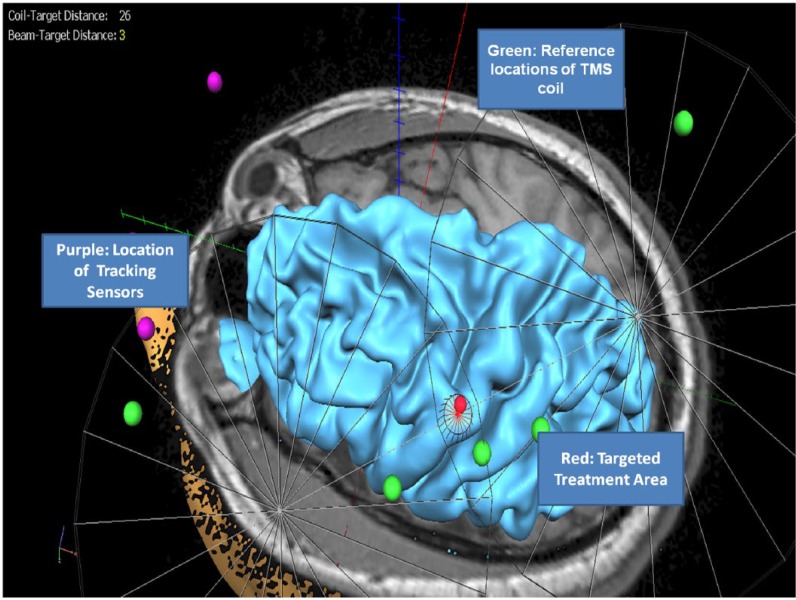
Patients included in the sample underwent a series of five rTMS sessions, separated in 1- to 2-week intervals for a period of 2 months. rTMS was applied to the left motor cortex (LMC) with 2000 pulses (20 trains with 1-s inter-train interval) delivered per session, at a frequency of 10 Hz and 80% resting motor threshold (RMT).16,17 rTMS was performed using a Magpro B-35 (MagVenture, Atlanta, GA, USA) figure-of-eight coil magnetic stimulator under brain magnetic resonance imaging (MRI) neuronavigation guidance (BrainVoyager, Maastricht, Netherlands). The location of the treatments for each subject was the same as the cortical region where the RMT was determined (Figure 1). This rTMS treatment protocol is within the safety guidelines recommended by the Food and Drug Administration (FDA).18
Figure 1.
rTMS application to the LMC at 10 Hz, 2000 pulses per session, and 80% RMT.
Pain was assessed using the numerical rating pain scale (NRS), ranging from 0 to 10.19 Pre- and post-treatment NRS scores were recorded. The pre-treatment NRS was based on headache diary from the preceding 2 weeks, while the post-treatment NRS was based on the headache diary of the preceding 1–2 weeks, after the last rTMS treatment. In the diary, patients were asked to report the intensity and duration of headaches. In addition, headache relief percent reduction was calculated between pre- and post-rTMS sessions.
Case series
Case 1
A 60-year-old male patient presented with episodic migraines with aura over 5 years. Headaches were associated with nausea, photophobia, and phonophobia and occurred once per week, lasting approximately 10 h in duration. Pain described as throbbing, beginning in the occiput and spreading to the left supraorbital region, was rated 10/10 in intensity. He reported sugar as a known trigger and slept approximately 6 h per night. Prior failed abortive medication included naproxen, and current prophylactic medication was gabapentin. Most recent brain MRI demonstrated white matter hypertensive changes.
Case 2
A 57-year-old male patient presented with episodic migraines with aura over 40 years. Headaches were associated with photophobia, phonophobia, and nausea/vomiting. Headaches occurred once per week, lasted 48 h in duration, and were described as 10/10 in intensity. Headache was described as throbbing and stabbing in quality in the bi-temporal and retro–orbital regions. Prior failed abortive medication included dihydroergotamine, and current prophylactic medication was propranolol.
Case 3
A 39-year-old male patient presented with chronic migraines with aura for 15 years. Headaches were associated with photophobia and phonophobia, however, no nausea/vomiting. Patient reported daily headache occurrences, lasting approximately 12 h at a time. He described his headache as shooting and throbbing located over the left frontotemporal region, which was rated 9/10 in intensity. Prior failed abortive medications included sumatriptan, zolmitriptan and eletriptan, while amitriptyline was the current prophylactic medication.
Case 4
A 48-year-old male patient presented with chronic migraines without aura for 21 years. Headaches were associated with photophobia and phonophobia, however, no nausea/vomiting. Headaches were described as holocephalic, throbbing in quality, occurring three times per week, and lasting 5 h per episode, and rated 8/10 in intensity. Brain MRI was unremarkable. Prior failed abortive medication included zolmitriptan, while nortriptyline was the prophylactic medication used during rTMS.
Case 5
A 32-year-old male patient presented with chronic migraines without aura for 13 years. Headaches were associated with photophobia, phonophobia, and nausea and occurred two times per week, lasting 5 h per episode. Headache was located in left temporal region, described as throbbing and pulsating, and rated 8/10 in intensity. No known triggers for the onset of the headaches. Brain MRI was unremarkable. Prior failed abortive medications included rizatriptan and sumatriptan, while desipramine was a prophylactic medication used during rTMS.
Results
With the approval of the institution’s Human Subject Protection Committee for conducting the prospective case review and patients’ informed consent for the treatments, five patients were screened and completed the treatment protocol (see Table 1). The participants were all males (5 of 5). Three of the participants had migraine with aura, while the other two experienced migraine without aura. All patients tolerated the rTMS sessions well. No adverse events, including motor weakness, numbness, or seizures, were reported. Table 1 reveals an average age ± standard deviation (SD) of 47.2 ± 11.8 (range: 32–60) years. The average duration (±years) of headache was 18.8 ± 13.1 (range: 5–40) years. Changes in NRS ± SD scores after the rTMS were seen among all subjects with a mean decrease in 1.9 ± 1.0 (range: 0.4–2.8).
Table 1.
Summary of patient characteristics, migraine type, and duration.
| Patient | Age | Gender | Migraine (episodic vs chronic) | Aura | Headache duration (years) |
|---|---|---|---|---|---|
| 1 | 60 | Male | Episodic | Yes | 5 |
| 2 | 57 | Male | Episodic | Yes | 40 |
| 3 | 39 | Male | Chronic | Yes | 15 |
| 4 | 48 | Male | Chronic | No | 21 |
| 5 | 32 | Male | Chronic | No | 13 |
| Average ± SD | 47.2 ± 11.8 | 18.8 ± 13.1 |
SD: standard deviation.
Table 2 shows the comparison between pre- and post-rTMS intensity, frequency, duration, and percent reduction. Comparison between the pre- and post-rTMS reveals a mean decrease in 2.8–1.9 headaches/week (11.2–7.6 headaches/month) or a reduction in 32.1%. A mean decrease in intensity from 9–5.6 or a reduction of 37.8% and in duration from 16–11 hours/headache or 31.2% reduction were also noted.
Table 2.
Comparison between pre- and post-rTMS intensity, frequency, duration, and percent reduction.
| Case | Pre-rTMS |
Post-rTMS |
||||
|---|---|---|---|---|---|---|
| Intensity | Frequency (Migraine/week) | Duration (hours/Migraine) | Intensity (% reduction) | Frequency (% reduction) | Duration (% reduction) | |
| 1 | 10 | 1 | 10 | 8 (20) | 1 (0) | 3 (70) |
| 2 | 10 | 1 | 48 | 10 (0) | 0.33 (67) | 48 (0) |
| 3 | 9 | 7 | 12 | 5 (44) | 4 (43) | 4 (67) |
| 4 | 8 | 3 | 5 | 5 (38) | 4 (0) | 0.17 (97) |
| 5 | 8 | 2 | 5 | 0 (100) | 0 (100) | 0 (100) |
| Average (±SD) | 9 (±1.0) | 2.8 (±2.5) | 16 (±18.2) | 5.6 (±3.8) (37.8%) |
1.9 (±1.9) (32.1%) |
11 (±20.7) (31.2%) |
SD: standard deviation; rTMS: repetitive transcranial magnetic stimulation.
Table 3 reveals the comparison between prior failed and current prophylactic and abortive headache therapies.
Table 3.
Comparison between prior failed and current prophylactic/abortive headache therapies.
| Case | Prior failed prophylactic therapies | Prior failed abortive therapies | Prophylactic therapies during rTMS | Abortive therapies during rTMS |
|---|---|---|---|---|
| 1 | Amitriptyline 100 mg qhs, topiramate 50 mg BID, Depakote 1000 mg qd | Naproxen 500 mg q8h PRN | Gabapentin 600 mg TID | Ibuprofen 600 mg q8h PRN, zolmitriptan 5 mg once weekly PRN |
| 2 | Amitriptyline 75 mg qhs, Depakote 500 mg BID, topiramate 100 mg BID | Dihydroergotamine 1 mg subcutaneously | Propranolol 20 mg TID | Sumatriptan 6 mg weekly PRN |
| 3 | Depakote 500 mg BID, nortriptyline 75 mg qhs | Sumatriptan 100 mg, eletriptan 20 mg, zolmitriptan 2.5 mg | Amitriptyline 75 mg qhs | Hydromorphone 2 mg qd |
| 4 | Gabapentin 400 mg TID, Depakote 750 mg qd | Zolmitriptan | Nortriptyline 50 mg qd | Ibuprofen 800 mg TID PRN, sumatriptan 25 mg two to three times weekly PRN |
| 5 | Amitriptyline 75 mg qhs | Rizatriptan, sumatriptan | Desipramine 50 mg qhs | Naproxen 500 mg PRN, zolmitriptan 5 mg two times weekly PRN |
Dosages (if known) are listed in table.
Discussion
In this case series, the tested rTMS protocol appeared to be clinically feasible and effective in the prevention of migraines. The tested rTMS protocol was effective in the overall reduction in immediate headache severity as evidenced by the reduction in headache NRS scores after rTMS sessions. Additionally, there was an average percent reduction in intensity (37.8%), frequency (32.1%), and duration (31.2%) of headaches post-rTMS.
The observed clinical efficacy of the tested treatment protocol is in agreement with several studies examining the effect of rTMS on migraines. In one study, a reduction in migraine frequency, headache index, and analgesic use was found in adult patients undergoing rTMS (20 Hz, 10 trains at 90% motor threshold) over 12 sessions on alternate days to the dorsolateral frontal cortex.12 Despite the similarity in clinical efficacy, the intense frequency of treatment visits in these previous study treatment protocols appeared to be not clinically feasible for a lot of patients based on the investigators’ clinical experience. On the other hand, the tested protocol with visits at weekly increments appears to be well tolerated and complied by all the patients. A similar shorter duration of rTMS study (10 Hz, 600 pulses in 10 trains) at the LMC with three sessions of treatment on alternate days also demonstrated reduced headache frequency and visual analog scale (VAS) scores at 1 month as compared to the sham stimulation group.13 Thus, instead of a long series of daily treatment sessions, rTMS given days or weeks apart can be a clinically effective and feasible way to manage migraines, particularly in the outpatient clinical setting.
This case series consists of several limitations, including small sample size (n = 5), and predominantly male population. It has been shown that migraines are more prevalent in females than the male population.2 Although all subjects were allowed to use their migraine prophylactic medications, they were asked to maintain their headache medication regimens; therefore, the interference from use of concomitant preventive and abortive headache medications during the assessment period should be minimal. In addition, prophylactic migraine medications do not appear to affect the rTMS treatment response in individuals with migraines with aura.10
In summary, the tested rTMS protocol of 2000 pulses (20 trains with 1-s inter-train interval) delivered per session to the LMC, at a frequency of 10 Hz and 80% RMT at 1- to 2-week intervals for five sessions over a 2-month period is a well-tolerated, safe and effective method for migraine prevention in individuals with migraines. Further research examining non-daily treatment rTMS protocol among larger study populations with variable migraine subtypes (episodic, chronic) and demographics is warranted.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case series was obtained from IRB VA San Diego, IRB Approval # H140222.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent for patient information to be published in this article was not obtained. Additional consent is not required by the IRB if the treating physician is simply tracking their treatment response (patients were already consented for treatment).
References
- 1. Burch R, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache 2015; 55(1): 21–34. [DOI] [PubMed] [Google Scholar]
- 2. Olese J, Goadsby PJ, Ramadan N, et al. (eds). The Headaches. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 3. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neur 2012; 15(Suppl. 1): S15–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silberstein SD, Goadsby PJ, Lipton RB. Management of migraine: an algorithmic approach. Neurology 2000; 55(9 Suppl. 2): S46–S52. [PubMed] [Google Scholar]
- 5. Lipton RB, Pearlman SH. Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics 2010; 7(2): 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keck ME, Welt T, Müller MB, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 2002; 43(1): 101–109. [DOI] [PubMed] [Google Scholar]
- 7. Strafella AP, Paus T, Barrett J, et al. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001; 21(15): RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michael N, Gösling M, Reutemann M, et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci 2003; 17(11): 2462–2468. [DOI] [PubMed] [Google Scholar]
- 9. Misra UK, Kalita J, Tripathi GM, et al. Is β endorphin related to migraine headache and its relief? Cephalalgia 2013; 33(5): 316–322. [DOI] [PubMed] [Google Scholar]
- 10. Almaraz AC, Dilli E, Dodick DW. The effect of prophylactic medications on TMS for migraine aura. Headache 2010; 50(10): 1630–1633. [DOI] [PubMed] [Google Scholar]
- 11. Wasserman EM, Epstein CM, Ziemann U, et al. The Oxford handbook of transcranial stimulation. Oxford: Oxford University Press, 2008 [Google Scholar]
- 12. Brighina F, Piazza A, Vitello G, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci 2004; 227(1): 67–71. [DOI] [PubMed] [Google Scholar]
- 13. Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J Neurol 2013; 260(11): 2793–2801. [DOI] [PubMed] [Google Scholar]
- 14. Conforto AB, Amaro E, Jr, Gonçalves AL, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia 2014; 34(6): 464–472. [DOI] [PubMed] [Google Scholar]
- 15. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl. 1): 1–160. [DOI] [PubMed] [Google Scholar]
- 16. Leung AY, Shukla S, Song DD, et al. rTMS in reducing mild TBI related headache—a pilot study. Headache 2014; 54(S1): 90. [Google Scholar]
- 17. Leung AY, Fallah A, Shukla S, et al. rTMS in alleviating mild TBI related headaches—a case series. Pain Physician 2016; 19: E347–E354. [PubMed] [Google Scholar]
- 18. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroen Clin Neuro 1998; 108(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 19. Kwong WJ, Pathak DS. Validation of the eleven-point pain scale in the measurement of migraine headache pain. Cephalalgia 2007; 27(4): 336–342. [DOI] [PubMed] [Google Scholar]