Abstract
Diabetic neuropathies (DNs) differ in clinical course, distribution, fiber involvement (type and size), and pathophysiology, the most typical type being a length-dependent distal symmetric polyneuropathy (DSP) with differing degrees of autonomic involvement. The pathogenesis of diabetic DSP is multifactorial, including increased mitochondrial production of free radicals due to hyperglycemia-induced oxidative stress. Mechanisms that impact neuronal activity, mitochondrial function, membrane permeability, and endothelial function include formation of advanced glycosylation end products, activation of polyol aldose reductase signaling, activation of poly(ADP ribose) polymerase, and altered function of the Na+/K+-ATPase pump. Hyperglycemia-induced endoplasmic reticulum stress triggers several neuronal apoptotic processes. Additional mechanisms include impaired nerve perfusion, dyslipidemia, altered redox status, low-grade inflammation, and perturbation of calcium balance. Successful therapies require an integrated approach targeting these mechanisms. Intensive glycemic control is essential but is insufficient to prevent onset or progression of DSP, and disease-modifying treatments for DSP have been disappointing. Atypical forms of DN include subacute-onset sensory (symmetric) or motor (asymmetric) predominant conditions that are frequently painful but generally self-limited. DNs are a major cause of disability, associated with reduced quality of life and increased mortality.
Keywords: Diabetic neuropathy, Diabetic polyneuropathy, Diabetic radiculoplexus neuropathy, Treatment-induced neuritis, Diabetic small-fiber neuropathy
Introduction
Diabetes is the commonest cause of neuropathy worldwide, producing a wide spectrum of conditions involving different types of nerves and pathologic mechanisms (e.g., metabolic, ischemic, immunologic, and compressive) [1, 2••, 3]. The different forms of diabetic neuropathy (DN) can be classified in terms of their anatomical distribution (e.g., proximal or distal, symmetric or asymmetric, focal or multifocal or diffuse), clinical course (e.g., acute, subacute, or chronic), characteristic features (painful or nonpainful, sensory, motor, or autonomic), or pathophysiology. Classification into “typical” or “atypical” forms is based on their occurrence [2••], with the most typical form of DN being a chronic, distal (length-dependent) symmetric polyneuropathy (DSP) that accounts for about 75% of DNs [4]. Any variation from this description suggests an “atypical” form [5].
Distal Symmetric Polyneuropathy
DSP is the commonest chronic complication of both type 1 and type 2 diabetes, with an estimated lifetime prevalence exceeding 50 % [5, 6]. Diabetic DSP develops in response to long-standing hyperglycemia [7••], but about 20 % of newly diagnosed diabetes patients show evidence of DSP [2••]. A DSP involving a patient with abnormal glucose metabolism that is nonetheless insufficient to diagnose diabetes is sometimes classified as “impaired glucose tolerance” or “prediabetic” neuropathy [4]. Sensory loss in diabetic DSP is generally restricted to a stocking distribution. Large-fiber involvement results in tingling paresthesias and a perception that the feet feel “numb or asleep” in the setting of distally impaired vibration, joint position, and touch-pressure sensations and abnormal (unequivocally diminished or absent) ankle reflexes [4]. The stocking distribution “asleep or numb” sensation is usually not particularly painful. In contrast, prickling, stabbing, and burning sensations likely reflect small-fiber involvement that ultimately results in persistent neuropathic pain affecting about 20 % of diabetic patients [8, 9]. Foot ulceration is a consequence of severe diabetic DSP that sometimes leads to amputation [9]. When the DSP is severe, distal weakness with foot drop may develop, as may variable amounts of autonomic dysfunction [1, 7••].
A diagnosis of diabetic DSP is based on the symptoms and signs of a length-dependent neuropathy in a patient with diabetes in whom other causes of neuropathy have been excluded [4]. Diabetic DSP is commonly accompanied by retinal and renal involvement, as part of the diabetic “triopathy” of neuropathy, retinopathy, and nephropathy. The 2009 Toronto Consensus Panel on Diabetic Neuropathies updated the definitions and diagnostic criteria for DSP to include possible, probable, confirmed, and subclinical categories [5]. Possible DSP was defined to include appropriate sensory symptoms or signs (symmetric decrease in distal sensation or unequivocally abnormal ankle reflexes). Probable DSP was defined as at least two abnormalities among sensory symptoms, sensory signs, and ankle reflexes. Confirmed DSP required symptoms or signs (sensory or ankle reflexes) and abnormal nerve conduction study (NCS) findings, whereas subclinical DSP was defined as abnormal NCS findings, without signs or symptoms.
It is generally assumed that physicians can accurately diagnose DSP. This assumption was challenged by a recent study in which 12 physicians (diabetologists and neurologists) experienced in the evaluation of DSP evaluated 24 diabetic subjects on two occasions [10]. These evaluators showed unsatisfactory diagnostic proficiency (based on accuracy and intra- or interevaluator variability), overestimating DSP compared with 75 % group diagnosis and a summated NCS score. In a second study, instructions to base clinical diagnoses on unequivocally abnormal symptoms and signs, while taking age, sex, and physical variables into account, greatly improved proficiency and avoided overreporting of signs [11••].
Abnormal NCS findings are considered one of the first quantitative indicators of diabetic DSP [5]. Early in the course of asymptomatic DSP, motor conduction velocity slowing near the lower limit of normal frequently leads to the diagnosis of diabetes [2••]. The 2009 Toronto Consensus Panel concluded that composite sum scores based on normal deviates (from percentiles) using NCS attributes sensitive to DPS (e.g., fibular and tibial conduction velocity, sural amplitude) performed best in diagnosing DSP [7••]. This approach is not readily available in most medical practices, but more easily applied criteria (e.g., use of one or more abnormal attributes in two separate lower extremity nerves based on the first and the 99th percentile values) also performed well [7••]. Requiring that the sural nerve be included among the nerves studied (American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation recommendations) produced similar results. To be reliable, however, NCSs must be done rigorously. This requires measuring and maintaining adequate limb temperature, proper electrode placement, “just supramaximal” stimulation levels, accurate measurement of distances, and determination of abnormality using reference values corrected for appropriate variables [5, 7••]. The quantitative nature of NCSs might suggest they are reliable indicators of abnormality. However, just as clinical proficiency was below expectations, an extension of the diabetic DSP study [10] to include an evaluation of NCS proficiency showed similarly disappointing results. Despite high intraobserver agreement on repeated testing, statistically significant interobserver differences were observed for most NCS attributes [12••]. Use of a uniform NCS technique, standard references, and broader categorization of abnormality reduced but did not eliminate small but statistically significant interobserver differences [13•]. Whereas the interexaminer differences were unlikely to be clinically meaningful for use in medical practice, they raised concern for use in therapeutic trials and supported the conclusion of Johns Hopkins University investigators [14] that the ideal results were obtained when the same clinical electrophysiologists performed serial NCSs for a given subject.
Diagnosing a DSP that preferentially involves small nerve fibers (small-fiber neuropathy, SFN) can be challenging [2••]. Emerging markers of SFN include nerve biopsy (invasive and highly specialized), skin biopsy measurement of intraepidermal nerve fiber density (IENFD) (minimally invasive and good sensitivity and specificity), corneal confocal microscopy (potential surrogate for SFN), and nerve axon reflex/flare response (requires further validation) [5]. The 2009 Toronto Consensus Panel defined SFN [5] to include three categories: possible (length-dependent symptoms and/or signs of small-fiber damage), probable (length-dependent symptoms, clinical signs, and normal sural NCS findings), and definite (fulfilling definition of probable plus abnormal IENFD at the ankle or quantitative thermal threshold at the foot) [5].
Diabetic autonomic neuropathy frequently accompanies diabetic DSP [1], but rarely occurs in isolation. Autonomic symptoms and deficits are usually mild until late stages of the disease [15]. Diabetic autonomic neuropathy potentially involves all organs receiving autonomic innervation, resulting in a variety of cardiovascular (reduced heart rate variability, resting tachycardia, exercise intolerance, silent cardiac ischemia, orthostasis), gastrointestinal (esophageal dysfunction, gastroparesis, nausea, diarrhea/constipation), genitourinary (erectile dysfunction, retrograde ejaculation, reduced vaginal lubrication, neurogenic bladder), pupillary (Argyll Robertson pupil), cutaneous sudomotor (heat intolerance, sweating disturbance, gustatory sweating), and other (hypoglycemia unawareness, reduced hypoxia-induced ventilatory drive) disturbances [2••, 4]. Cardiovascular autonomic neuropathy, in particular, was shown to be an independent predictor of mortality [5].
Mechanisms of Diabetic DSP
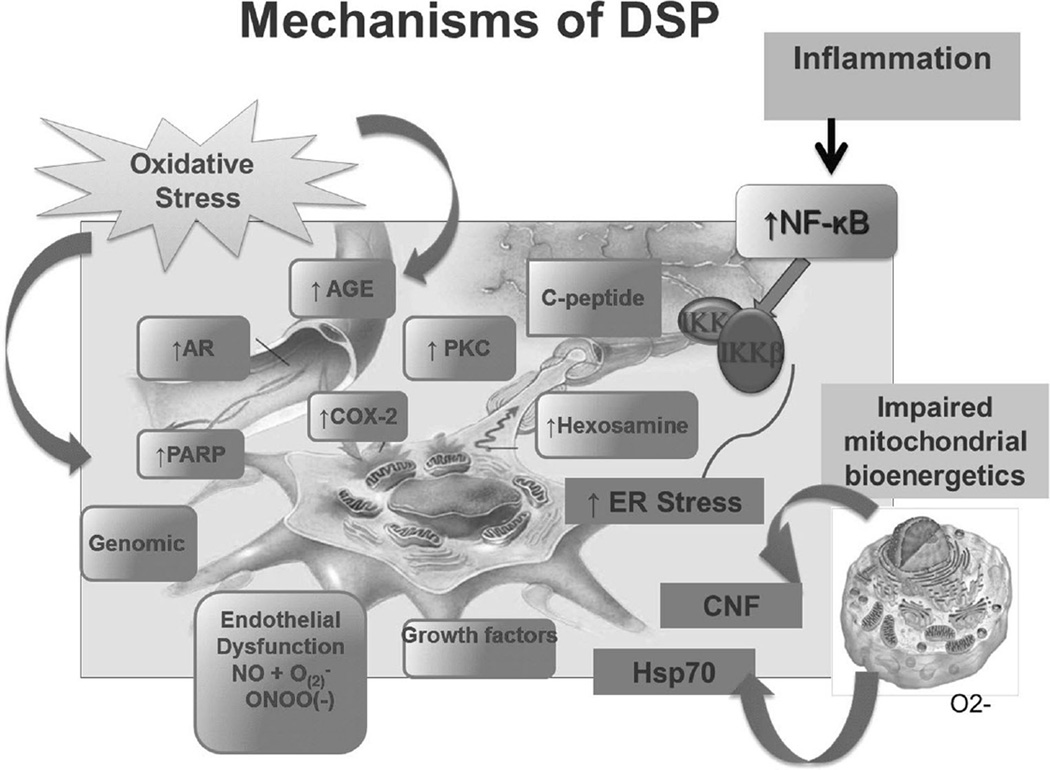
The specific mechanisms contributing to diabetic DSP are not completely understood. It is generally accepted that the pathogenesis of diabetic DSP is multifactorial, involving complex interactions between the degree of glycemic control, diabetes duration, age-related neuronal attrition, and other factors such as blood pressure, lipid levels, and weight [16–20]. Experimental evidence shows that hyperglycemia, glucotoxicity, and impaired insulin signaling act in concert with other risk factors and activate several biochemical pathways that affect cellular metabolism (Fig. 1). These alterations promote structural changes such as segmental demyelination, Wallerian degeneration, and microangiopathy, and induce dorsal root ganglia neuronal apoptosis, resulting in subsequent damage to and loss of myelinated and unmyelinated fibers [21–23].
Fig. 1.
Proposed mechanisms of diabetic distal symmetric polyneuropathy (DSP). AGE advanced glycation end products, AR aldose reductase, CNF ciliary neurotrophic factor, COX-2 cyclooxygenase 2, ER endoplasmic reticulum, Hsp70 heat shock protein 70, IKKβ inhibitor of nuclear factor κB kinase subunit β, NF-kB nuclear factor κB, PARP poly(ADP ribose) polymerase, PKC protein kinase C. The neuron displayed in the figure was drawn by the Juvenile Diabetes Research Foundation (JDRF) for the University of Michigan Center for Diabetes Complications, and it is reproduced here with permission from Helen Nickerson, PhD, Senior Scientific Program Manager JDRF
Hyperglycemia induces increased mitochondrial production of free radicals [24], which in concert with inadequate antioxidant defenses are responsible for activating additional damaging pathways [24–27]. These additional mechanisms include increased formation of advanced glycation end products [26, 27], downregulation of the soluble receptor for advanced glycation end products [28], activation of polyol aldose reductase signaling with accumulation of protein kinase C [24, 29], activation of poly(ADP ribose) polymerase [30], cyclooxygenase 2 activation [31], endothelial dysfunction [26], peroxynitrite and protein nitration [32], and altered function of the Na+/K+-ATPase pump [26, 33], all having direct impact on neuronal activity, mitochondrial function, membrane permeability, and endothelial function. Hyperglycemia also induces endoplasmic reticulum (ER) stress and results in accumulation of unfolded or misfolded proteins within the ER lumen, activating the unfolded protein response, a signaling cascade responsible for restoring normal ER function. During times of extreme or long-term stress, the unfolded protein response may become overwhelmed, triggering several apoptotic processes [22]. These include tumor-necrosis-factor-receptor-associated factor 2 and apoptosis-signal-regulating kinase 1, with the resultant activation of c-Jun N-terminal kinase [34]; release of calcium stores into the cytosol, depolarization of the mitochondrial membrane, and cytochrome c release [35]; and cleavage of procaspase 12 [22, 36]. Additional mechanisms include impaired nerve perfusion [33, 37, 38], impaired C-peptide-related signaling pathways [39], dyslipidemia with increases in the levels of unsaturated fatty acids and high levels of circulating fatty acids [27, 40, 41], decreased levels of glycolytic and tricarboxylic acid cycle intermediates [42], altered redox status, and perturbation of calcium balance [22, 27]. Additional evidence suggests that low-grade inflammation mediated by nuclear factor κB activation and its downstream effects [31, 43, 44], and altered mitochondrial bioenergetics in dorsal root ganglia neurons [45–47], which appears to be modulated by heat shock protein 70 [34] and ciliary neurotrophic factor [21], are also important to DSP.
In humans, observational studies have shown that hyperglycemia is critical for development of DSP in both type 1 and type 2 diabetes. Surprisingly, the importance of hyperglycemia as an independent risk factor for diabetic DSP was not confirmed in a randomized controlled trial until 1993, when the Diabetes Control and Complications Trial strongly demonstrated that intensive glycemic control is essential to preventing DSP in patients with type 1 diabetes [48••, 49, 50••, 51]. The evidence associating glycemic control and the risk of diabetic DSP is less conclusive for type 2 diabetes [52]. Emerging data suggest that DSP may occur before development of diabetic-range hyperglycemia in individuals with features of metabolic syndrome or in patients with impaired glucose tolerance [2••].
The pathogenesis of diabetic DSP is multifactorial and involves many interrelated mechanisms. Successful therapeutic interventions will require an integrated approach targeting these mechanisms.
Emerging Treatments for DSP
Glycemic control, lifestyle interventions, and disease-modifying treatments specific for DSP are discussed in the following sections. Treatments related to pain control or the atypical DNs are beyond the scope of this review.
Glycemic Control
Tight and stable glycemic control is, to date, the only proven-effective pathogenic treatment for DSP. The Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study strongly demonstrated that intensive glycemic control designed to achieve near-normal blood glucose level, implemented early in the course of diabetes, delays development of DSP in patients with type 1 diabetes [48••, 49, 50••]. The beneficial effects of glycemic control on DSP are less conclusive in patients with type 2 diabetes. For example, some trials suggest that glycemic control in type 2 diabetes is most beneficial if implemented early in the disease, when patients have fewer microvascular complications [53], whereas other studies failed to confirm this finding [54–56]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study of patients with type 2 diabetes reported significant reductions in the development of DSP after 5 years of intensive versus conventional glycemic control [57•]. The Bypass Angioplasty Revascularization Intervention 2 Diabetes (BARI 2D) trial reported that specific glucose-lowering strategies and medications used to reach glycemic goals also have different effects in preventing DSP in type 2 diabetes [58•].
Lifestyle Interventions
Diet and exercise have been shown to improve neuropathic symptoms and IENFD among patients with DSP associated with impaired glucose tolerance [59•]. This observation was extended to patients with diabetic DSP, and improvements in IENFD measures in association with a 10-week exercise program consisting of moderately intense aerobic and resistance training were described [60•].
Disease-Modifying Treatments
Treatments that specifically target pathogenetic DSP mechanisms exist, including many that have proven to be efficacious in animal models of DSP but ineffective in randomized human trials using objective measures of DSP. These include aldose reductase inhibitors, other antioxidants, recombinant nerve growth factors, and acetylcarnitine [61, 62•, 63, 64••, 65]. Additional mechanistic treatments undergoing evaluation include the following:
-
–
α-Lipoic acid (ALA). The antioxidant ALA has been proposed to slow development and reduce painful symptoms of DSP by reducing hyperglycemia-induced oxidative stress [61, 62•, 63]. Several trials have associated ALA with clinically meaningful symptomatic improvement relative to placebo, although no significant differences were identified for primary DSP composite end points, NCS results, or quantitative sensory testing [62•, 63, 66, 67]. Most recently, a small randomized controlled comparison of a triple antioxidant combination (ALA, allopurinol, and nicotinamide) with placebo among patients with type 1 diabetes found no significant group differences on objective DSP measures that included NCSs and IENFD [68].
-
–
C-peptide. Proinsulin C-peptide is a bioactive peptide having potential importance to peripheral nerve structure and other cellular functions. The observation that C-peptide is lacking in patients with type 1 diabetes has resulted in several small clinical studies and reports of beneficial effects of the C-peptide on measures of DSP [69, 70], effects likely mediated via Na+/K+-ATPase and endothelial nitric oxide synthase pathways. A phase 3 clinical trial evaluating the effects of pegylated C-peptide in patients with type 1 diabetes and mild to moderately severe DSP is ongoing (ClinicalTrials.gov identifier NCT01681290).
-
–
Actovegin. This deproteinized hemoderivative mixture of low molecular weight compounds produced from calf blood is thought to stimulate oxygen utilization, cellular energy metabolism, glucose transport, and glucose oxidation. In a multicenter, randomized controlled trial of patients with type 2 diabetes and symptomatic DSP, Actovegin improved neuropathic symptoms, vibration perception threshold, and sensory function compared with placebo [71].
Maintaining near-normal blood glucose level and sustaining lifestyle interventions are important but difficult long-term DSP treatment options. Clinical studies of pharmaceutical disease-modifying DSP treatments have generally been disappointing. One possibility is that an integrated pharmacologic strategy, combining several agents targeting the various mechanisms important to DSP, will be required to supplement glycemic control and lifestyle interventions before DSP can be prevented or effectively controlled in the long term.
Diabetic Neuropathy Subtypes
Several atypical DNs have subacute onset (days to weeks), a monophasic or relapsing course, and preferentially involve small sensory and autonomic or motor nerve fibers. Some are associated with chronic hyperglycemia or treatment onset, whereas others are not [7••]. Their characteristic features are contrasted with those of diabetic DSP in Table 1 and are further described in the following sections.
Table 1.
Comparison of diabetic polyneuropathy and atypical diabetic neuropathy subtypes
| Features/symptoms/signs | DSP | DRPN | CIDP | PSFN-WL | PSFN-TI | DMN |
|---|---|---|---|---|---|---|
| Symptoms | ||||||
| Acute/subacute onset | No | Yes | Yes | Yes | Yes | At times |
| Progression rate | Years | Weeks/months | Weeks/months | Days | Days | Variable |
| Pain/allodynia | <20 % (feet) | Yes | No | Yes | Yes (with or without diffuse pain) | Yes (focal) |
| Numbness/tingling | Yes | At times | Yes | No | At times | Yes (focal) |
| Weakness | Late | Yes | Yes | No | No | Motor nerve |
| Dysautonomia | Yes | Yes (~50 %) | No | Yes | Yes (>85 %) | No |
| Signs | ||||||
| Decreased vibration, joint position | Yes | Variable | Yes | Uncommon | Uncommon | No |
| Decreased pin-pain, temperature | Yes | Variable | At times | Mild or absent | Yes | Yes (focal) |
| Weakness/atrophy, proximal | No | Yes | Yes | No | No | At times |
| Weakness/atrophy, distal | Late | At times | Yes | No | No | At times |
| Absent knee reflexes | Late | Yes | Yes | No | No | No |
| Absent ankle reflexes | Yes | Variable | Yes | No | No | No |
| Orthostatic hypotension | Mild | Yes | No | No | Yes (~33 %) | No |
| Anatomical pattern | ||||||
| Symmetric | Yes | No | Yes | Yes | Yes | No |
| Proximal predominant | No | Yes | When severe | No | No | Variable |
| Stocking-glove | Yes | No | Yes | Yes | Yes | No |
| Associated features | ||||||
| Weight loss | No | Yes | No | Yes | At times | No |
| Onset after DM treatment | No | No | No | No | Yes | No |
| Immunotherapy response | No | Anecdotal | Yes | No | No | No |
| Diagnostic results | ||||||
| Abnormal NCS findings | Yes | Common | Yes | No | No | Common |
| Abnormal needle EMG | Yes | Yes | Yes | No | No | At times |
| Abnormal autonomic tests | Late | Common | No | Yes | Yes | No |
| Elevated ESR | No | Yes | No | No | No | No |
| Elevated CSF protein level | At times | Yes | Yes | No | No | No |
| Prognosis | ||||||
| Chronic/progressive | Yes | No | ~33 % | No | No | Yes |
| Monophasic/resolution | No | Yes (partial) | Often | Yes (months) | Yes (months) | No |
| Pathophysiology | ||||||
| Metabolic/microvascular | Yes | No | No | No | No | No |
| Immune-mediated | No | Yes | Yes | Yes | No | No |
| Compressive/ischemic | No | No | No | No | No | Yes |
CIDP chronic inflammatory demyelinating polyneuropathy, CSF cerebrospinal fluid, DM diabetes mellitus, DMN diabetic mononeuropathy, DRPN diabetic radiculoplexus neuropathy, DSP distal symmetric polyneuropathy, EMG electromyography, ESR erythrocyte sedimentation rate, NCS nerve conduction study, PSFN-TI treatment-induced painful small-fiber neuropathy, PSFN-WL painful small-fiber neuropathy with weight loss
Diabetic Radiculoplexus Neuropathy
Diabetic radiculoplexus neuropathy (DRPN) includes cervical, thoracic, and/or lumbosacral presentations. The commonest is a lumbosacral DRPN (diabetic amyotrophy), affecting about 1 % of diabetic patients, who are typically middle aged or older and have type 2 diabetes [1, 72••]. The risk of developing DRPN is unrelated to the level of glycemic control, the duration of diabetes, treatment, or the severity of a coexisting DSP [1, 2••, 4, 73]. DRPN presents subacutely with unilateral or asymmetric proximal pain involving the back, hip, or anterior thigh [7••, 74]. The pain may be difficult to manage. The pain is followed by asymmetric proximal leg weakness and profound atrophy, with pain and weakness spreading in a progressive or stepwise manner over weeks to months to nearby and contralateral segments, with some patients becoming wheelchair-dependent or developing an asymmetric quadriparesis [75]. Despite an initial proximal predilection, distal limb segments become involved to some extent in most patients [2••]. Weight loss and dysautonomia are common, with about half of the patients experiencing combinations of orthostatic intolerance and change in sexual, bladder, and bowel function [1, 2••].
NCSs typically identify an underling DSP upon which the DPRN is superimposed, with asymmetric denervation that may be profound depending on the timing of the evaluation relative to the onset of weakness. Needle EMG abnormalities involve nerve roots, the plexus, and individual nerves, and autonomic testing confirms the presence of small-fiber involvement. Laboratory abnormalities include nonspecific indicators of an immune-mediated response, such as an elevated erythrocyte sedimentation rate, reactive rheumatoid factor, or a positive antinuclear antibody test; CSF abnormalities include an elevated protein level indicating extension to the nerve root level [1, 2••].
DRPN has a monophasic course, with improvement beginning within 9–12 months [4], although recovery can be incomplete and protracted over years [72••]. DRPN reflects a multifocal ischemic injury thought to reflect an immunemediated microvasculitis involving motor, sensory, and autonomic fibers [76, 77•]. This presumed pathogenesis has resulted in use of immunotherapy treatments, and anecdotal reports suggest that intravenous immunoglobulin [78] or intravenously administered methylprednisolone [3] reduces pain and weakness. However, efficacy is unproven, and no evidence convincingly supports the use of any immunotherapy treatment [72••, 79]. A painless form of DRPN resembles painful DRPN in all other ways aside from greater symmetry [80].Whether this is a variant of DRPN, chronic inflammatory demyelinating polyneuropathy (CIDP), or another disorder is uncertain [80]. A painful radiculoplexus neuropathy indistinguishable from DRPN exists in the absence of diabetes [81], but may be the presenting problem leading to a diagnosis of diabetes [72••].
Chronic Inflammatory Demyelinating Polyradiculoneuropathy
CIDP is an immune-mediated neuropathy. The purported association between CIDP and diabetes is controversial [2••], possibly representing the chance occurrence of two common disorders or unmasking of mild diabetes among some CIDP patients treated with corticosteroids. One study concluded that patients with diabetes were 11-fold more likely to fulfill EMG and clinical criteria for CIDP compared with those without diabetes [82], but more recent studies have failed to confirm an increased risk of CIDP in diabetic populations [83, 84]. Nevertheless, the coexistence of diabetes in a patient with prominent weakness, slow motor nerve conduction, and an elevated CSF protein level makes the distinction between a severe diabetic DSP and CIDP difficult. Even if the relationship between diabetes and CIDP is spurious, their coexistence poses a therapeutic challenge. As a general rule, marked weakness and pronounced slowing of motor conduction velocity with abnormal temporal dispersion and/or partial conduction block rarely occurs in diabetic DSP. Such findings should prompt additional testing for conditions associated with CIPD, such as an underlying monoclonal gammopathy. At times, a trial of therapeutic plasma exchange, corticosteroids, or intravenous immunoglobulin results in unequivocally improved strength and confirms the presence of an immune-mediated CIDP in a patient with diabetes.
Acute Painful SFNs
Several atypical DNs involve small nerve fibers. They are characterized by subacute onset of painful sensations in the legs, progressing over days to weeks to unremitting burning dysesthesias and allodynia [1, 9], with occasional spread to proximal sites involving the trunk or more diffusely [2••, 85]. Autonomic features may be prominent. Sensory loss is mild or absent, and there is no weakness [9]. Ankle reflexes are preserved unless there is an underlying DSP. The course is monophasic [2••, 86], usually resolving after 3–18 months [9, 85]. These neuropathies involve small nerve fibers that are not evaluated by conventional neurophysiological studies [85, 86], and documentation of an SFN requires additional testing, including measures of thermal thresholds, R-R variation, sweat production, orthostatic blood pressure, and skin biopsy [87]. The monophasic course and lack of correlation with diabetes duration and symptom onset make it unlikely that acute SFNs represent one end of the spectrum of length-dependent neuropathy involving pain fibers [1]. Treatment is symptomatic [88]. Two closely-related subtypes of diabetic SFN differ in the degree of autonomic involvement; one is associated with weight loss and the other develops shortly after initiation of intensive glycemic treatment [9].
SFN with Weight Loss
Profound weight loss is a prominent feature of this painful SFN, referred to as diabetic cachexia. Weight loss usually precedes the onset of severe burning pain and allodynia, but sometimes develops at the onset of pain or even as the pain subsides [1, 2••]. It most often involves patients with type 2 diabetes. Autonomic features other than impotence are uncommon [3]. On occasion, the onset of pain and profound weight loss (e.g., exceeding 25 %) develop after starting intensive glycemic treatment [89], but most cases are unrelated to initiation or change in treatment or degree of glycemic control. The mechanism by which weight loss produces a severe diabetic SFN is unknown. The prognosis is good, and pain resolves in the setting of adequate glycemic control and weight gain [2••].
Treatment-induced SFN
Treatment-induced SFN (“insulin neuritis”) develops 2–4 weeks (occasionally up to 8 weeks) after rapid and sustained glycemic control with insulin, oral hypoglycemic agents, or diet [87, 90]. Aside from the temporal association with rapid glycemic control, no abnormal laboratory results suggest other causes. Weight loss is not a prominent feature, but some patients have a remote history of withholding insulin for weight loss (diabetic anorexia) [87], and some patients experience profound weight loss after initiating intensive treatment but before the onset of pain [89], blurring the distinction between diabetic cachexia and treatment-induced SFN. Dysautonomia is frequently prominent, and in a recent study all patients with treatment-induced SFN had autonomic symptoms, including orthostatic hypotension and parasympathetic dysfunction in two thirds of them [87]. Although treatment-induced SFN is generally self-limited with continued glycemic control [90], some patients experience residual pain and dysautonomia, especially those with type 2 diabetes [87]. The risk of developing treatment-induced diabetic SFN should not discourage patients from attaining hemoglobin A1c levels approaching normal [91]. The mechanism by which rapid normalization of high blood glucose level causes neuropathy is unknown. It does not appear to be explained by hypoglycemia, nor is it associated with symptoms of hypoglycemia [85], although it may recur if diabetic control lapses and is again rapidly initiated [2••]. Sural nerve biopsy showing arteriolar attenuation and epineural arteriovenous shunting with proliferating “new vessels” similar to those found in the retina suggest a “steal” effect rendering the endoneurium ischemic [90]. Worsening of retinal examination findings in parallel with treatment-induced DN supports a common pathophysiology [87].
Diabetic Focal Peripheral Neuropathies
A number of focal peripheral neuropathies involving cranial, thoracic, or extremity nerves are associated with diabetes. Oculomotor palsy is the commonest cranial neuropathy, presenting with acute onset of unilateral headache, ptosis, and impaired extraocular movements but with a pupil that responds normally to light (partial third nerve palsy with pupillary sparing) [1]. The association between diabetes and other cranial neuropathies, including trochlear and facial nerves, is less clear [2••]. A common complication of diabetes is a unilateral truncal (thoracic) radiculopathy, presenting with acute abdominal pain sometimes suggesting an intra-abdominal process, herpes zoster, or a structural (spinal) process [2••].
Nerves susceptible to compression or cumulative trauma, including the median, ulnar, radial, lateral femoral cutaneous, fibular, and plantar nerves, are frequently injured in patients with diabetes (“sick” nerves are prone to injury) [4]. The explanation for the predisposition to injury is undoubtedly multifactorial, involving metabolic and ischemic factors, impaired reinnervation, and even obesity, as body mass index is an independent risk factor for carpal tunnel syndrome (CTS). Although the frequency of “entrapment” mononeuropathies is undoubtedly increased in diabetes, this may reflect the interpretation of NCS results. A diagnosis of median mononeuropathy does not indicate CTS, a clinical diagnosis requiring appropriate symptoms. When standard electrophysiological criteria for median mononeuropathy are applied to patients with mild diabetic DSP, over one fifth show “positive results” despite being asymptomatic for CTS [92], a frequency of false-positive results indicating that diabetic patients require special consideration when one is diagnosing entrapment neuropathy. A diabetic patient with a mononeuropathy should, of course, be investigated for entrapment [2••, 4], and patients with diabetes and CTS (but not necessarily diabetic DSP) are thought to have the same beneficial outcome after carpal tunnel release as nondiabetic patients [93]. However, robust agreement between clinical and electrophysiological findings, not blind reliance on mild NCS abnormalities, should guide treatment decisions.
Conclusion
The most typical neuropathy associated with diabetes mellitus is a length-dependent DSP with differing degrees of dysautonomia. The precise mechanisms producing DSP are unknown, but are undoubtedly multifactorial and include pathological alterations due to impaired glycemic control, the most prominent of which involves increased production of free radicals due to hyperglycemia-induced oxidative stress. The only proven treatment that effectively delays the onset or progression of DSP is intensive glycemic control. Nevertheless, DSP eventually develops and progresses in most patients despite intensive glycemic control. This observation, together with the ineffectiveness in human clinical trials of targeted therapies but effectiveness in animal models of DSP, supports the multifactorial pathogenesis of DSP. Atypical forms of DN reflect rare conditions, some of which present with subacute onset of pain and dysautonomia, associated either with a symmetric SFN (e.g., insulin neuritis or diabetic cachexia) or asymmetric proximal weakness (DRPN). Despite the frequent association of CIDP and diabetes mellitus, it is unclear if this represents a causal relationship or is coincidental. Diabetes predisposes individuals to focal peripheral neuropathies involving individual nerves and nerve roots. The oculomotor nerve is the most frequently involved cranial nerve, presenting as a partial third nerve palsy with pupillary sparing. Another common complication is a unilateral truncal (thoracic) radiculopathy, presenting with acute chest or abdominal pain. Extremity nerves susceptible to compression or cumulative trauma (e.g., the median, ulnar, radial, lateral femoral cutaneous, fibular, and plantar nerves) are frequently injured in patients with diabetes (“sick” nerves are prone to injury).
Acknowledgments
Conflict of Interest Within the past 36 months, James W. Albers’ institution has received support from Eli Lilly and Company/Amylin Pharmaceuticals for his participation as a clinical investigator in a diabetes drug trial. He has also received personal compensation from Eli Lilly and Company (NZ), an affiliate of Eli Lilly and Company, Alnylam Pharmaceuticals/Veristat, Dow Chemical Company, Dow AgroSciences, and PeriphaGen (formerly Diamyd), and from firms representing these companies. These activities have been as a consultant, data monitoring committee member, advisory board member, or expert witness.
Rodica Pop-Busui’s institution has received grant support from Eli Lilly and Company/Amylin Pharmaceuticals (now Bristol-Myers Squibb) for an investigator-initiated trial testing the effects of exenatide on measures of diabetic neuropathy in which she is the principal investigator, and she receives grant support from the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the American Diabetes Association. She has also received personal compensation from Acorda Therapeutics, Astra Zeneca, and Janssen Pharmaceuticals as a consultant.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
James W. Albers, Email: jwalbers@umich.edu, Neuromuscular Section, Department of Neurology, University of Michigan Health System, 1C325 University Hospital, 1500 East Medical Center Drive, Ann Arbor, MI 48109-0032, USA.
Rodica Pop-Busui, Email: rpbusui@umich.edu, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, 5329 Brehm Tower, 1000 Wall Street, Ann Arbor, MI 48105, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Sinnreich M, Taylor BV, Dyck PJ. Diabetic neuropathies. Classification, clinical features, and pathophysiological basis. Neurologist. 2005;11:63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- 2. Smith AG, Singleton JR. Diabetic neuropathy. Continuum. 2012;18:60–84. doi: 10.1212/01.CON.0000411568.34085.3e. This is an excellent and detailed review of DN.
- 3.Tracy JA, Engelstad JK, Dyck PJ. Microvasculitis in diabetic lumbosacral radiculoplexus neuropathy. J Clin Neuromuscul Dis. 2009;11:44–48. doi: 10.1097/CND.0b013e3181b1eb6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82:95–100. doi: 10.1136/pgmj.2005.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol. 2012;25:536–541. doi: 10.1097/WCO.0b013e328357a797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dyck PJ, Albers JW, Anderson H, et al. Diabetic polyneuropathies: update on research definitions, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27:620–628. doi: 10.1002/dmrr.1226. This is an important 2009 Toronto consensus statement updating research on and clinical definitions of diabetic DSP.
- 8.Mackenzie K, DeLisa JA. Distal sensory latency measurement of the superficial radial nerve in normal adult subjects. Arch Phys Med Rehabil. 1981;62:31–34. [PubMed] [Google Scholar]
- 9.Tesfaye S, Kempler P. Painful diabetic neuropathy. Diabetologia. 2005;48:805–807. doi: 10.1007/s00125-005-1721-7. [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJ, Overland CJ, Low PA, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. N Phys trial. Muscle Nerve. 2010;42:157–164. doi: 10.1002/mus.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dyck PJ, Overland CJ, Low PA, et al. “Unequivocally abnormal” vs “usual” signs and symptoms for proficient diagnosis of diabetic polyneuropathy: Cl vs N Phys trial. Arch Neurol. 2012;69:1609–1614. doi: 10.1001/archneurol.2012.1481. This is a follow-up Mayo Clinic study showing how clinical proficiency may be improved.
- 12. Dyck PJ, Albers JW, Wolfe J, et al. A trial of proficiency of nerve conduction: greater standardization still needed. Muscle Nerve. 2013;48:369–374. doi: 10.1002/mus.23765. This presents the electrophysiological component of the Mayo Clinic-based study of physician proficiency.
- 13. Litchy WJ, Albers JW, Wolfe J, Bolton CF, Walsh N, Klein CJ, et al. Proficiency of nerve conduction using standard methods and reference values. Muscle Nerve. 2014 doi: 10.1002/mus.24243. This is a follow-up Mayo Clinic study showing how nerve conduction proficiency may be improved.
- 14.Chaudhry V, Cornblath DR, Mellits ED. Inter- and intra-examiner reliability of nerve conduction measurements in normal subjects. Ann Neurol. 1991;30:841–843. doi: 10.1002/ana.410300614. [DOI] [PubMed] [Google Scholar]
- 15.Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27:2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 16.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 17.Wiggin TD, Sullivan KA, Pop-Busui R, et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58:1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearsall AW, Russell GV., Jr Ipsilateral clavicle fracture, sternoclavicular joint subluxation, and long thoracic nerve injury: an unusual constellation of injuries sustained during wrestling. Am J Sport Med. 2000;28:904–908. doi: 10.1177/03635465000280062301. [DOI] [PubMed] [Google Scholar]
- 19.Witte DR, Tesfaye S, Chaturvedi N, et al. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48:164–171. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 20.Stella P, Ellis D, Maser RE, et al. Cardiovascular autonomic neuropathy (expiration and inspiration ratio) in type 1 diabetes. Incidence and predictors. J Diabetes Complicat. 2000;14:1–6. doi: 10.1016/s1056-8727(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 21.Saleh A, Roy Chowdhury SK, Smith DR, et al. Ciliary neurotrophic factor activates NF-B to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology. 2013;65:65–73. doi: 10.1016/j.neuropharm.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien PD, Hinder LM, Sakowski SA, Feldman EL. ER stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5807. [DOI] [PubMed] [Google Scholar]
- 23.Vincent AM, McLean LL, Backus C, et al. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 25.Vincent AM, Russell JW, Low P, et al. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JL, Vincent AM, Cheng HT, et al. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 28.Witzke KA, Vinik AI, Grant LM, et al. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. 2011;34:1617–1621. doi: 10.2337/dc10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Liaudet L, Soriano FG, et al. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 31.Kellogg AP, Wiggin TD, Larkin DD, et al. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes. 2007;56:2997–3005. doi: 10.2337/db07-0740. [DOI] [PubMed] [Google Scholar]
- 32.Stavniichuk R, Shevalye H, Lupachyk S, et al. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2014 doi: 10.1002/dmrr.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron NE, Cotter MA, Jack AM, et al. Protein kinase C effects on nerve function, perfusion, Na+, K+-ATPase activity and glutathione content in diabetic rats. Diabetologia. 1999;42:1120–1130. doi: 10.1007/s001250051280. [DOI] [PubMed] [Google Scholar]
- 34.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 35.Timmins JM, Ozcan L, Seimon TA, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 37.Cameron NE, Cotter MA, Low PA. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am J Physiol. 1991;261:E1–E8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- 38.Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997;46(Suppl 2):S38–S42. doi: 10.2337/diab.46.2.s38. [DOI] [PubMed] [Google Scholar]
- 39.Sima AA, Zhang W, Grunberger G. Type 1 diabetic neuropathy and C-peptide. Exp Diabesity Res. 2004;5:65–77. doi: 10.1080/15438600490424541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinder LM, Vincent AM, Hayes JM, et al. Apolipoprotein E knockout as the basis for mouse models of dyslipidemia-induced neuropathy. Exp Neurol. 2013;239:102–110. doi: 10.1016/j.expneurol.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent AM, Hayes JM, McLean LL, et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinder LM, Vivekanandan-Giri A, McLean LL, et al. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J Endocrinol. 2013;216:1–11. doi: 10.1530/JOE-12-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets. 2008;9:60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Schmeichel AM, Iida H, et al. Enhanced inflammatory response via activation of NF-κB in acute experimental diabetic neuropathy subjected to ischemia-reperfusion injury. J Neurol Sci. 2006;247:47–52. doi: 10.1016/j.jns.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Chowdhury SK, Zherebitskaya E, Smith DR, et al. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes. 2010;59:1082–1091. doi: 10.2337/db09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma J, Farmer KL, Pan P, et al. Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J Pharmacol Exp Ther. 2014;348:281–292. doi: 10.1124/jpet.113.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernyhough P, Roy Chowdhury SK, Schmidt RE. Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev Endocrinol Metab. 2005;5:39–49. doi: 10.1586/eem.09.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. This pivotal trial demonstrated the benefit of intensive glucose control in delaying neuropathy in type 1 diabetes and changed the standard of diabetes care.
- 49.Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 50. Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Diabetes Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. The importance of early and tight glucose control is reflected in long-term protection against DSP in type 1 diabetes.
- 51.Martin CL, Albers JW, Pop-Busui R, et al. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–38. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callaghan BC, Little AA, Feldman EL, et al. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6 doi: 10.1002/14651858.CD007543.pub2. CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 54.Azad N, Emanuele NV, Abraira C, et al. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM) J Diabetes Complicat. 1999;13:307–313. doi: 10.1016/s1056-8727(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 55.Charles M, Fleischer J, Witte DR, et al. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013;56:101–108. doi: 10.1007/s00125-012-2744-5. [DOI] [PubMed] [Google Scholar]
- 56.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 57. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. Largest trial demonstrating benefit of intensive glucose control in patients with type 2 diabetes.
- 58. Pop-Busui R, Lu J, Brooks MM, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36:3208–3215. doi: 10.2337/dc13-0012. This is the first large trial of patients with type 2 diabetes demonstrating that different glucose-lowering strategies have different effects on neuropathy prevention.
- 59. Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. Initial study of the efficacy of lifestyle intervention.
- 60. Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complicat. 2012;26:424–429. doi: 10.1016/j.jdiacomp.2012.05.007. This trial demonstrates the effects of exercise and objective measures of DSP.
- 61.Ziegler D. Treatment of diabetic neuropathy and neuropathic pain: how far have we come? Diabetes Care. 2008;31(Suppl 2):S255–S261. doi: 10.2337/dc08-s263. [DOI] [PubMed] [Google Scholar]
- 62. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054–2060. doi: 10.2337/dc11-0503. This is a summary of all trials involving α-lipoic acid.
- 63.Ziegler D, Nowak H, Kempler P, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: a meta-analysis. Diabet Med. 2004;21:114–121. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 64. Boulton AJ, Kempler P, Ametov A, et al. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013;29:327–333. doi: 10.1002/dmrr.2397. This is an excellent review of pathogenic treatments for DSP.
- 65.Sima AA, Calvani M, Mehra M, et al. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89–94. doi: 10.2337/diacare.28.1.89. [DOI] [PubMed] [Google Scholar]
- 66.Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alphalipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 67.Ziegler D, Reljanovic M, Mehnert H, et al. α-Lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp Clin Endocrinol Diabetes. 1999;107:421–430. doi: 10.1055/s-0029-1212132. [DOI] [PubMed] [Google Scholar]
- 68.Pop-Busui R, Stevens MJ, Raffel DM, et al. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia. 2013;56:1835–1844. doi: 10.1007/s00125-013-2942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ekberg K, Brismar T, Johansson BL, et al. Amelioration of sensory nerve dysfunction by C-peptide in patients with type 1 diabetes. Diabetes. 2003;52:536–541. doi: 10.2337/diabetes.52.2.536. [DOI] [PubMed] [Google Scholar]
- 70.Ekberg K, Brismar T, Johansson BL, et al. C-peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care. 2007;30:71–76. doi: 10.2337/dc06-1274. [DOI] [PubMed] [Google Scholar]
- 71.Ziegler D, Movsesyan L, Mankovsky B, et al. Treatment of symptomatic polyneuropathy with actovegin in type 2 diabetic patients. Diabetes Care. 2009;32:1479–1484. doi: 10.2337/dc09-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chan YC, Lo YL, Chan ES. Immunotherapy for diabetic amyotrophy. Cochrane Database Syst Rev. 2012;6 doi: 10.1002/14651858.CD006521.pub3. CD006521. This is an excellent review highlighting the limited number of controlled studies of DRPN treatments.
- 73.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 74.Barohn RJ, Sahenk Z, Warmolts JR, et al. The Bruns-Garland syndrome (diabetic amyotrophy). Revisited 100 years later. Arch Neurol. 1991;48:1130–1135. doi: 10.1001/archneur.1991.00530230038018. [DOI] [PubMed] [Google Scholar]
- 75.Taylor BV, Dunne JW. Diabetic amyotrophy progressing to severe quadriparesis. Muscle Nerve. 2004;30:505–509. doi: 10.1002/mus.20107. [DOI] [PubMed] [Google Scholar]
- 76.Massie R, Mauermann ML, Staff NP, et al. Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain. 2012;135:10–88. doi: 10.1093/brain/aws244. [DOI] [PubMed] [Google Scholar]
- 77. Younger DS. Diabetic lumbosacral radiculoplexus neuropathy: a postmortem studied patient and review of the literature. J Neurol. 2011;258:1364–1367. doi: 10.1007/s00415-011-5938-8. This is a case report of DRPN, but one of the few to include a postmortem evaluation.
- 78.Wada Y, Yanagihara C, Nishimura Y, et al. A case of diabetic amyotrophy with severe atrophy and weakness of shoulder girdle muscles showing good response to intravenous immune globulin. Diabetes Res Clin Pract. 2007;75:107–110. doi: 10.1016/j.diabres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 79.Donofrio PD, Berger A, Brannagan TH, III, et al. Consensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve. 2009;40:890–900. doi: 10.1002/mus.21433. [DOI] [PubMed] [Google Scholar]
- 80.Garces-Sanchez M, Laughlin RS, Dyck PJ, et al. Painless diabetic motor neuropathy: a variant of diabetic lumbosacral radiculoplexus Neuropathy? Ann Neurol. 2011;69:1043–1054. doi: 10.1002/ana.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25:477–491. doi: 10.1002/mus.10080. [DOI] [PubMed] [Google Scholar]
- 82.Sharma KR, Cross J, Farronay O, et al. Demyelinating neuropathy in diabetes mellitus. Arch Neurol. 2002;59:758–765. doi: 10.1001/archneur.59.5.758. [DOI] [PubMed] [Google Scholar]
- 83.De Sousa EA, Chin RL, Sander HW, et al. Demyelinating findings in typical and atypical chronic inflammatory demyelinating polyneuropathy: sensitivity and specificity. J Clin Neuromuscul Dis. 2009;10:163–169. doi: 10.1097/CND.0b013e31819a71e1. [DOI] [PubMed] [Google Scholar]
- 84.Laughlin RS, Dyck PJ, Melton LJ, III, et al. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73:39–45. doi: 10.1212/WNL.0b013e3181aaea47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dabby R, Sadeh M, Lampl Y, et al. Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients. Biomed Pharmacother. 2009;63:707–709. doi: 10.1016/j.biopha.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Gemignani F. Acute painful diabetic neuropathy induced by strict glycemic control (“insulin neuritis”): the old enigma is still unsolved. Biomed Pharmacother. 2009;63:249–250. doi: 10.1016/j.biopha.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 87.Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67:534–541. doi: 10.1002/ana.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bril V, England JD, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy. Report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve. 2011;43:910–917. doi: 10.1002/mus.22092. [DOI] [PubMed] [Google Scholar]
- 89.Grewal J, Bril V, Lewis GF, et al. Objective evidence for the reversibility of nerve injury in diabetic neuropathic cachexia. Diabetes Care. 2006;29:473–474. doi: 10.2337/diacare.29.02.06.dc05-2177. [DOI] [PubMed] [Google Scholar]
- 90.Tesfaye S, Malik R, Harris N, et al. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis) Diabetologia. 1996;39:329–335. doi: 10.1007/BF00418349. [DOI] [PubMed] [Google Scholar]
- 91.American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2002;23(Suppl 1):S28–S31. doi: 10.2337/diacare.26.2007.s28. [DOI] [PubMed] [Google Scholar]
- 92.Albers JW, Brown MB, Sima AAF, et al. Frequency of median mononeuropathy in patients with mild diabetic neuropathy in the Early Diabetes Intervention Trial (EDIT) Muscle Nerve. 1996;19:140–146. doi: 10.1002/(SICI)1097-4598(199602)19:2<140::AID-MUS3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 93.Thomsen NO, Cederlund R, Rosen I, et al. Clinical outcomes of surgical release among diabetic patients with carpal tunnel syndrome: prospective follow-up with matched controls. J Hand Surg [Am] 2009;34:1177–1187. doi: 10.1016/j.jhsa.2009.04.006. [DOI] [PubMed] [Google Scholar]