Abstract
Diabetic peripheral and autonomic neuropathies are common complications of diabetes with broad spectrums of clinical manifestations and high morbidity. Studies using various agents to target the pathways implicated in the development and progression of diabetic neuropathy were promising in animal models. In humans, however, randomized controlled studies have failed to show efficacy on objective measures of neuropathy. The complex anatomy of the peripheral and autonomic nervous systems, the multitude of pathogenic mechanisms involved, and the lack of uniformity of neuropathy measures have likely contributed to these failures. To date, tight glycemic control is the only strategy convincingly shown to prevent or delay the development of neuropathy in patients with type 1 diabetes and to slow the progression of neuropathy in some patients with type 2 diabetes. Lessons learned about the role of glycemic control on distal symmetrical polyneuropathy and cardiovascular autonomic neuropathy are discussed in this review.
Keywords: Distal symmetrical sensorimotor polyneuropathy, Cardiovascular autonomic neuropathy, Clinical trials, Glucose control, Diabetic neuropathy
Introduction
Diabetic neuropathy (DN) complicates type 1 and type 2 diabetes [1, 2]. The spectrum of clinical manifestations is broad, although a large number of patients with DN may be asymptomatic in various stages of the disease [1, 2, 3•]. Numerous classifications have been proposed both for clinical care and for research purposes [1, 2, 3•].
Among peripheral DNs, the most studied in clinical trials is distal (length-dependent) symmetrical sensorimotor polyneuropathy (DSP), and among autonomic neuropathies, the most studied is cardiovascular autonomic neuropathy (CAN). Evidence pertinent to these two forms of DN is included in this review.
Measures of DSP Used in Large Clinical Trials
The 2009 Toronto Consensus Panel on Diabetic Neuropathies updated the definitions and diagnostic criteria for DSP for use in clinical trials to represent several different categories of diagnostic “certainty,” including possible, probable, confirmed, and subclinical categories [2, 3•]. Table 1 provides a comprehensive summary of DSP measures used in large observational and randomized glucose control trials. The majority of these trials were designed/conducted prior to the above recommendations, which may partly explain the lack of uniformity of DSP measures and criteria. DSP assessments in some of the largest trials are briefly described below.
Table 1.
Summary of Trials Reporting Impact of Glucose Control on DSP Measures
| Type 1 Diabetes Observational Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study Name Author/year | DSP Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| Ziegler et al. 1991(36) | NCS measured by EMG and thermal discrimination threshold by Marstock stimulator | N=32 Mean age 20 years |
N/A Group 1: Mean HbAIc<8.3 % Group 2: Mean HbAIc>8.3 % |
5 years |
Baseline Group 1: 10.6 % Group 2: 11.6 % Follow-up Group 1: 7 % Group 2: 10 % |
Prevalence of DSP | ||
| Group 1 | Group 2 | |||||||
| Baseline | 0 | 3.5 % | ||||||
| 24 month | 5.6 % | 14.6 % | ||||||
| 48 months | 2.6 % | 22 % | ||||||
| 60 months | 6.1 % | 21 % | ||||||
| P <0.05 | ||||||||
| Pittsburg Epidemiology of Diabetic Complications (EDC) Study Maser RE et al./1989 (37) |
Two or more of the following: symptoms, sensory and/or motor signs, and/or absent tendon reflexes. | N=400 DSP+= 135 DSP −=228 Mean age 28 years |
N/A | N/A | DSP + : 10.2 % DSP − : 9.8 % |
OR for DSP HbA1c 10 vs. 9 %=1.36 (1.16–1.61) P <0.05 |
||
| Seattle Prospective Diabetic Foot Study Adler et al./1997(60) |
Monofilament testing | N=775 DSP+= 388 DSP−=387 Mean age 62 years |
N/A | 2.5 years | Baseline DSP+= 11.6 % DSP-=10.9 % Follow-up DSP+ : 8.8 % DSP− : 8.3 % |
OR for DSP per 1 % HbA1c =1.06 (1.01–1.11) P=0.03 |
||
| EURODIAB IDDM Complications Study. Tesfaye S et al./ 1996 (57) |
Neuropathic symptoms and physical signs, vibration perception threshold, abnormal autonomic function | N=3250 Mean age 32 years |
N/A | 7.3 years | Baseline : 6.7 % Follow -up : 8.3 % |
Prevalence of DSP: Overall Prevalence: 28 % DSP Prevalence by HbA1c |
||
| HbA1c | DSP | |||||||
| <5.4 | 15 % | |||||||
| 5.4–6.4 | 26 % | |||||||
| 6.5–7.7 | 30 % | |||||||
| >7.8 | 40 % | |||||||
| OR=2.48 (1.50– 4.11) P<0.001 | ||||||||
| Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) study Klein R et al. /1996 (39) |
Loss of tactile sensation or temperature sensitivity | N=1210 mean age 29 years |
N/A | 10 years | Baseline : 10.8 % Follow- up: 10.1 %; |
2 % change in HbA1c from baseline to 4 years results in 19 % decrease in the 10-year incidence of loss of tactile sensation P <0.005 |
||
| Randomized Controlled Trials | ||||||||
| Study Name Author/year | DSP Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| Reichard et al./1993(61) | Composite of symptoms of neuropathy and NCS | N=102 Mean age 31 years |
INT N=48 Intensified insulin therapy, individual education, tutoring and home glucose monitoring CON N=54 Standard insulin therapy, routine diabetes care |
7.5 years. | Baseline INT: 9.5 % CON : 9.4 % Follow-up INT : 7.1 % CON: 8.5 % |
Prevalence of DSP at follow-up INT : 14 % CON : 28 % P=NS |
||
| Oslo study Amthor et al./1994(62) |
Motor and sensory NCS | N=45 Mean age 26 years |
INT N=33 c Continuous insulin infusion/ multiple injections (4–6 daily) CON N =12 Twice daily insulin therapy |
8 years | Baseline :11.2 % Follow-up : 9.5 % |
Every 1 % change in HbA1c resulted in a 1.3 m/s change in nerve conduction velocity during 8 years. Change in peroneal NCS from baseline to 8 years by mean HbA1c groups | ||
| HbA1c | NCS | |||||||
| < 9 %= | −2.2 m/s | |||||||
| 9.1–10 %= | −0.2 m/s | |||||||
| > 10 %= | −4.8 m/s | |||||||
| P <0.01 | ||||||||
| Diabetes Control and Complications Trial (DCCT) DCCT study group/1993, 1995(5; 6) | Abnormal neurological examination plus abnormal NCS in at least 2 peripheral nerves | N =1441 Mean age 26 years |
INT N=711 either external insulin pump or by three or more daily insulin injections guided by frequent blood glucose monitoring CON N=730 one or two daily insulin injections |
6.5 years | Baseline INT : 9.1 % CON: 9.1 % End of DCCT INT : 7.2 % CON: 9. 1 % |
Prevalence of DSP INT : 6.8 % CON : 5.6 % INT : 9.3 % CON:17.51 % P <0.002 |
||
| Epidemiology of Diabetes Interventions and Complications (EDIC) Albers et al./2010(11) |
Abnormal neurological examination plus abnormal NCS in at least 2 peripheral nerves | N=1186 Mean age 34 years |
N/A Former INT N=603 Former CON N=583 |
13/14 years | EDIC year 13/14 INT : 8.0 % CON: 8. 0 % |
Prevalence of DSP INT : 23.6 % CON:32.7 % P <0.05 |
||
| Type 2 Diabetes Observational Studies | ||||||||
| Study Name Author/year | DSP Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| San Luis Valley Diabetes Study Sands et al./ 1997(48) |
Two or more of the following: bilateral paresthesia in legs or feet; bilateral decreased or absent ankle reflexes; and/or bilateral decreased or absent cold temperature discrimination in feet to an iced tuning fork | N=231 Age 20–74 years |
N/A | 4.7 years | Baseline DSP + : 11.2 % DSP − : 10.2 % Subgroups: HbA1c <9.0 % HbA1c =9.0 % |
Unadjusted incidence rate (IR) : 6.1 /100 person-yrs Adjusted IR 4.71 /100 person/yrs 5.60 /100 person/yrs P=NS |
||
| Taiwan Study Chao et al./2007(46) |
Neuropathy Symptom Score, Neuropathy Disability Score, Total Neuropathy Score | N=498 Mean age 62 years |
N/A | N/A | DSP + : 8.2 % DSP – : 7.8 % |
Significant correlations between A1c and warm/ cold thresholds. (r=0.451 and r=0.380 respectively, P <0.0001) | ||
| Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) study Klein R et al. 1996(39) |
Loss of tactile sensation or temperature sensitivity | N=1780 Mean age 65 years |
N/A | 4 years | Baseline : 10.2 % Follow-up : 9.7 % |
2 % change in A1c from baseline to 4 years resulted in 23 % decrease in the 10-year incidence of loss of tactile sensation P <0.005 |
||
| Perkins et al./2010(63) | NCS median sensory nerve, bilateral sural nerve. | N=110 Mean age 56 years |
N/A Placebo cohort analysis |
1 year | Baseline 8.3 % | Improvement in A1c by −0.8 % associated with 2.9 m/s improvement in NCS A1c worsening by +1 % associated with decrease of −2.6 m/s NCS P =0.02 |
||
| Randomized Controlled Trials | ||||||||
| Study Name Author/year | DSP Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| KUMAMOTO Trial Ohkubo et al./1995(12) |
NCS and VPT | N=110 Mean age 49 years |
INT N=50 3 or more injections of insulin daily CON N =52 1or 2 daily injections of insulin |
6 years | Baseline INT: ~9.2 % CON: ~9 % Follow-up INT: 7.1 % CON : 9.4 % |
Median Motor NCS | ||
| Baseline | 6 years | |||||||
| INT | 50.8 m/s | 53.2 m/s | ||||||
| CON | 51.6 m/s | 50.2 m/s | ||||||
| P <0.05 | ||||||||
| United Kingdome Prospective Diabetes Study (UKPDS) UKPDS group/1998(22) |
Either one of loss of knee/ ankle reflexes or abnormal VPT | N=3867 Mean age 54 years |
INT N=2729 Oral agents or with insulin CON N=1138 Diet or oral agents or with insulin |
10–15 years | Baseline INT : 7.05 % CON : 7.09 % Follow-up INT : 7.0 % CON : 7.9 % |
Prevalence of abnormal VPT INT : 30.2 % CON : 51.7 % P =0.005 Prevalence of absent ankle reflexes INT : 35 % CON : 37 % P=NS |
||
| VA Cooperative Study on Type II Diabetes Mellitus (VA CSDM) Azad et al./1999(18) |
Symptoms and signs of DSP by cranial neuropathy, muscle strength, deep tendon reflexes, touch sensation, prickling sensation, vibratory sensation, proprioceptive sensation | N =153 Mean age 60 years |
INT N=75 four-step plan, daily self- monitoring CON N=78 1 morning injection/ day |
2 years | Baseline INT: 9.3 % CON: 9.5 % Follow-up INT=7.3 % CON=9.5 % |
Prevalence of DSP INT : 48 % CON : 53 % Prevalence of DSP INT : 64 % CON : 69 % P=NS |
||
| Veteran Affairs Diabetes Trial (VADT) Duckworth et al. /2009(24) |
Self -reported radiculoneuropathy, polyneuropathy, diabetic amyotrophy, or neuropathic ulcer. | N =1791 Mean age 60 years |
INT N=892 started on oral drugs, then insulin if HbA1c not<6 % CON N=899 started on half the maximal doses, insulin if HbA1c not less than 9 % |
5.6 years | Baseline INT: 9.4 % CON: 9.4 % Follow-up INT: 6.9 % CON: 8.4 % |
Incidence of DSP at follow-up INT: 38.4 % CON: 40 % P=NS |
||
| ADDITION Denmark Study (ADDITION) Charles et al./2011(17) |
Either one abnormality in the following: vibration detection threshold and light touch sensation, and the MNSI | N=1,533 Mean age 60 years |
INT N=702 Glucose target-driven intervention using multiple medications and lifestyle interventions CON N=459 Standard of care for diabetes at the time in Denmark |
6 years | Baseline INT : 6.4 % CON: 6.4 % Follow-up INT: 6.4 % CON: 6.4 % |
Prevalence of DSP at follow-up INT : 30.1 % CON: 34.8 % P=NS |
||
| Action to Control Cardiovascular Risk in Diabetes (ACCORD) Ismail-Beigi et al./2010(15) |
MNSI>2, vibratory sensation, ankle reflex, monofilament test | N=10,251 Mean age 62 years |
INT N=5128 intensive glycemic therapy target HbA1c<6 % CON N=5123 Standard glycemic therapy target HbA1c 7–7.9 % |
5 years | Baseline INT: 8.1 % CON: 8.1 % Follow- up INT: 6.3 % CON: 7.6 % |
Prevalence of DSP at follow-up MNSI>2 INT:55·6 % CON: 58·6 % HR=0.92 CI (0.86–0.99) ; P=0.02 Loss of ankle jerk INT : 45.7 % CON: 49.3 % HR=0.90 CI (0.84–0.97); P=0.005 Loss of light touch INT: 12·1 % CON: 14·1 % HR=0·85 CI (0.76–0.95); P=0.004 |
||
| STENO 2 Gaede et al./2003 (23) | VPT | N =160 Mean age 55 years |
INT N=80 stepwise behavior modification and pharmacologic therapy targeting glucose and other risk factors CON N =80 Denmark national guidelines |
7.8 years | Baseline INT: 8.4 % CON: 8.8 % Follow-up INT :7.9 % CON: 8.6 % |
Relative Risk of DSP with intervention 1.09 (0.54–2.22) P=NS |
||
| Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI2D Pop-Busui et al./ 2013(16) |
MNSI clinical examination score>2 | N=2159 DSP - IS =530 IP=545 DSP + IS=550 IP=534 Mean age 62 years |
IS: metformin, thiazolidinedies (TZDs), or both IP :sulfonylureas/ meglitinides, insulin or both |
4 years | Baseline DSP + IS =7.7 % IP =7.8 % DSP - IS =7.6 % IP =7.6 % Follow-up IS=7.1 % IP=7.6 % |
4-year cumulative incidence rate of DSP in subjects free of DSP at baseline IS=66 % IP=72 % P <0.05 |
||
Footnotes: NCS: nerve conduction studies, INT-intensive treatment, CON-conventional treatment, DSP-distal symmetrical sensorimotor polyneuropathy, CAN- cardiovascular autonomic neuropathy, RCT-randomized control trial, VPT-vibration perception threshold, OR-odds ratio, N-number, MNSI- Michigan Neuropathy Screening Instrument, IS-insulin sensitizing, IP-insulin providing, N/A-not applicable, HR-hazard ratio, CI-confident interval, NS-non significant
The Diabetes Control and Complications Trial (DCCT), a randomized controlled trial in patients with type 1 diabetes (T1D), and its follow-up, the observational Epidemiology of Diabetes Interventions and Complications (EDIC) study [25], used robust measures of DSP [11, 12]. Briefly, DSP was assessed by board-certified neurologists using a standardized evaluation to identify symptoms, signs, and temperature-controlled nerve conduction abnormalities consistent with DSP, at DCCT baseline, after 5 years of DCCT participation and/or at the end of the DCCT and again during EDIC year 13–14 [12, 26•, 27]. The Michigan Neuropathy Screening Instrument (MNSI) was introduced at the start of the EDIC follow-up as an annual measure of DSP [27, 28]. Vibration perception threshold (VPT) testing was performed at EDIC year 13–14 [26•, 29].
The primary DSP outcome in DCCT and EDIC was confirmed clinical neuropathy, defined as the presence of clinically evident neuropathy and nerve conduction studies (NCSs) abnormalities [11–13, 26•]. Clinically evident neuropathy was defined as at least two positive findings among sensory symptoms, signs, or reflex abnormalities consistent with a distal symmetrical polyneuropathy as assessed clinically by a neurologist; abnormal NCSs were defined by the presence of at least one abnormal NCS attribute >2 nerves among the median, peroneal, and sural nerves [11–13, 26•]. Other DSP outcomes measured during DCCT/EDIC included abnormal VPT (defined as >2.5 SD age-adjusted mean of nondiabetic referents), abnormal MNSI (score of ≥7 on the symptom questionnaire or a score of >2 on the structured examination) [27, 28], and neuropathy related quality of life measures [12, 13, 26•, 27].
A trial in Japanese participants with type 2 diabetes (T2D) randomized to either intensive or conventional insulin treatment used relatively objective DSP evaluations that included temperature-controlled NCS, but at the median nerve only, and VPT at baseline and after 6 years of follow-up [17].
The rest of the large trials used less robust DSP measures, often limited to clinical instruments (Table 1). Among these, the MNSI, a two-step instrument comprising a 15-item yes/no symptom questionnaire and a clinical examination (MNSIc) [30], has been used extensively. A cutoff of >2 on MNSIc has been shown to have the highest sensitivity and specificity for correctly classifying DSP defined by symptoms, signs, and NCS abnormalities [30, 31]. The MNSIc served as the primary DSP measures in several large randomized clinical trials evaluating the role of glucose control or glucose-lowering strategies in patients with T2D, including Action to Control Cardiovascular Risk in Diabetes (ACCORD) [22], Bypass Angioplasty Revascularization Investigation 2 Diabetes (BA-RI 2D) [24•], and the ADDITION-Denmark Study [21]. The VA Cooperative Study on Type II Diabetes Mellitus (VA-CSDM) [19] used abbreviated versions of the Neuropathy Disability Score and Neuropathy Symptom Profile [32, 33]. The European IDDM Prospective Complications Study (EURODIAB), an observational study that included >3,000 participants with T1D across Europe, used self-reported symptoms, some physical signs, and VPT [34]. The United Kingdom Prospective Diabetes Study (UKPDS) included assessment of knee and ankle reflexes and VPT [18], the Steno-2 trial used VPT [23], while the Veteran Affairs Diabetes Trial (VADT) used annual self-reported neuropathy [20].
Thus, the evaluation of the impact of glucose control on neuropathy outcomes in many large randomized trials is limited by the lack of uniformity of DSP definitions and measures used. DSP was a secondary outcome in most of these trials, and the costs and burden to sponsors, sites, and participants could have prohibited more comprehensive DSP evaluations. Confounding the assessment of DSP in clinical trials is that many clinical scales may be insufficiently sensitive to reliably capture small, incremental DSP worsening or show monotonic change over relatively short periods (e.g., 2–4 years) of follow-up [35]. Furthermore, the reproducibility of a clinical diagnosis of DSP, even among experienced physicians, is rather poor, as has been recently shown [36].
None of the trials considered previously assessed small-fiber DSP. Neither NCSs nor any other of the commonly used tools/instruments provide direct information on deficits preferentially involving small fibers [37, 38]. Diagnosing a small-fiber DSP is challenging, and many of the currently available tests are invasive, not sufficiently validated, and expensive [2, 37]. However, the lack of small-fiber DSP measures in these trials precludes objective estimations of the true effects of glucose control on neuropathy, since the earliest and, presumably, most susceptible to therapeutic interventions stages of DSP were not evaluated.
Measures of CAN Used in Large Clinical Trials
A summary of CAN measures used in large glucose control trials is provided in Table 2. Brief descriptions are provided for some representative trials, many of which were already discussed above.
Table 2.
Summary of Trials Reporting Impact of Glucose Control on CAN measures
| Type 1 Diabetes Observational Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study Name Author/year | CAN Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| Ziegler et al./1991(36) | Heart rate variation at rest and during deep breathing | N=32 Group 1=13 Group 2=19 Mean age 20 years |
N/A | 5 years | Baseline Group 1: 10.6 % Group 2: 11.6 % Follow-up Group 1:< 8.3 % Group 2: >8.3 % |
Prevalence of CAN | ||
| Group 1 | Group2 | |||||||
| Baseline | 0 | 3 % | ||||||
| 24 month | 0 | 17 % | ||||||
| 48 months | 0 | 8.3 % | ||||||
| 60 months | 5 | 23.3 % | ||||||
| P <0.05 | ||||||||
| EURODIAB IDDM Complications Study Tesfaye S et al./1996 (57) Witte et al. 2005 (34) |
Changes in R–R and systolic blood pressure with standing Changes in R–R and systolic blood pressure with standing |
N=3250 Mean age 33 years N=956 Mean age 31 years |
N/A N/A |
7.3 years 7.3 years |
Baseline : 6.7 % Follow- up : 8.3 % CAN+ : 6.9 % CAN -: 6.4 % |
OR CAN per % A1c increase 1.20 (1.09–1.32) P<0.001 20 % increase in risk of CAN per percentage point increase in HbA1c. P <0.001 |
||
| Randomized Controlled Trials | ||||||||
| Study Name Author/year | CAN Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| Oslo study Amthor et al./1994(62) |
Cardiovascular reflex tests obtained only at follow-up | N=45 Mean age 26 years |
INT N=33 continuous insulin infusion, multiple injections (4–6 daily) CON N=12 twice daily insulin therapy |
8 years | Baseline : 11.2 % Follow-up : 9.5 % |
RR Variation A1c>10 %=19.4 A1c<10 %=27.4 P=0.03 |
||
| Diabetes Control and Complications Trial (DCCT) DCCT study group/1993(5; 29) | Cardiovascular reflex tests. One of the following conditions: R-R variation<15; R-R variation <20 in combination with Valsalva ratio =1.5 or postural hypotension |
N =1441 Mean age 26 years |
INT N=711 either external insulin pump or by three or more daily insulin injections and guided by frequent blood glucose monitoring CON N=730 one or two daily insulin injections |
6.5 years | Baseline INT : 9.1 % CON: 9.1 % End of DCCT INT : 7.2 % CON: 9. 1 % |
CAN Prevalence INT : 4 % CON: 5 % CAN Prevalence INT: 3 % CON: 10 % P =0.001 45 % risk reduction in incident CAN with INT |
||
| Epidemiology of Diabetes Interventions and Complications (EDIC) Pop-Busui et al./ 2009(31) |
Cardiovascular reflex tests. One of the following conditions: R-R variation<15; R-R variation <20 in combination with Valsalva ratio =1.5 or postural hypotension |
N=1211 Mean age 48 years |
N/A Former INT N=620 Former CON N =591 |
13–14 years | EDIC year 13/14 INT : 8.0 % CON: 8. 0 % |
CAN Prevalence and Incidence Prevalence INT: 29 % CON: 35 % P <0.05 Incidence 30 % risk reduction in incident CAN at year 13–14 with INT |
||
| Type 2 diabetes | ||||||||
| Study Name Author/year | CAN Measures | Study Population N/age | Intervention | Follow-up | HbA1c | Outcomes | ||
| KUMAMOTO Trial Ohkubo et al./ 1995(12) |
Coefficient of variation (CV) of R-R interval and postural hypotension | N=110 Mean age 49 years |
INT N=50 3 or more injections of insulin daily CON N=52 1or 2 daily injections of insulin |
6 years | Baseline INT: ~9.2 % CON : ~9 % Follow-up HbA1c INT: 7.1 % CON : 9.4 % |
CV of R-R interval Baseline INT : 5.3 % CON : 5.2 % Follow-up INT : 5.7 CON : 4.9 P=NS |
||
| United Kingdome Prospective Diabetes Study (UKPDS) UKPDS study Group/1998(22) |
R-R interval and postural hypotension | N=3867 Mean Age 53±9 years |
INT N=2729 Oral agents or with insulin CON N=1138 Diet or oral agents or with insulin |
10 years | Baseline INT : 7.05 % CONV : 7.09 % Follow-up INT : 7.0 % CONV : 7.9 % |
No differences between groups P=NS |
||
| VA Cooperative Study on Type II Diabetes Mellitus (VA CSDM) Azad et al./1999(18) |
Valsalva ratio<1.2 R-R variation <10 |
N =153 Mean age 60 years |
INT N=75 four-step plan, daily self-monitoring CON N =78 1 morning injection/ day |
2 years | Baseline INT: 9.3 % CON : 9.5 % Follow-up INT =7.3 % CON =9.5 % |
Prevalence abnormal Valsalva ratio and/ or R-R variation Baseline INT : 31 % CON: 38 % Follow-up INT : 48 % CON: 55 % P=NS |
||
| Veteran Affairs Diabetes Trial (VADT) Duckworth et al. /2009(24) |
Self-reported symptomatic orthostatic hypotension, gastroparesis, neurogenic bladder, or diabetic diarrhea | N =1791 Mean age 60 years |
INT N=892 started on oral drugs, or insulin if HbA1c>6 % CON N=899 started on half the maximal doses, insulin if HbA1c>9 % |
5.6 years | Baseline INT: 9.4 % CON: 9.4 % Follow-up INT: 6.9 % CON: 8.4 % |
Prevalence of self-reported autonomic symptoms at follow-up INT: 8.2 % CON: 5.2 % P=NS |
||
| STENO 2 Gaede et al./2003(23) |
R-R interval during deep breathing and orthostatic hypotension | N =160 Mean age 55 years |
INT N=80 stepwise behavior modification and pharmacologic therapy CON N =80 Denmark national guidelines |
7.8 years | Baseline INT: 8.4 % CON: 8.8 % Follow-up INT :7.9 % CON: 8.6 % |
Relative Risk for CAN at follow-up OR 0.37 (0.18–0.79) with INT P=0.002 |
||
Footnotes: T1D –type 1 diabetes, T2D- type 2 diabetes, HbA1c- hemoglobin A1c; INT-intensive treatment, CON-conventional treatment, CAN- cardiovascular autonomic neuropathy, RCT-randomized control trial, OR-odds ratio, N-number, N/A-not applicable, HR-hazard ratio, CI-confident interval, NS-non significant, VR- Valsalva ratio
Rigorous and comprehensive CAN evaluations were performed in DCCT/EDIC. CAN was assessed during DCCT at baseline every 2 years and at DCCT-end and in EDIC during years 13–14 and 16–17, using standard cardiovascular reflex testing (CARTs) that included R–R variation to paced breathing (RRV), R–R response to Valsalva maneuver (VR), and postural changes in blood pressure [26•, 27, 40]. These tests are objective, standardized, simple to use, highly reproducible [41, 42], and endorsed by the Toronto Consensus on Diabetic Neuropathies as the gold standard [2, 43]. The primary CAN outcome in DCCT/EDIC was as either RRV<15 or RRV=15–19.9 plus VR≤1.5 or a decrease of >10 mmHg in diastolic blood pressure [26•, 27, 40, 41]. Secondary CAN outcomes included changes in age-adjusted continuous measures of RRV and VR [26•, 27]. Participants were questioned about autonomic symptoms at EDIC year 13–14 [27, 41]. VA-CSDM reported CAN measures similar to those used in DCCT [19].
Less standardized assessments were used in other large trials (Table 2). EURODIAB queried symptoms and changes in heart rate and blood pressure with standing [39, 44], while the UKPDS assessed R–R intervals with deep breathing and standing [18]. Steno-2 assessed R–R interval during paced breathing and presence of orthostatic-hypotension [23], ADDITION obtained resting heart rate variability and some CARTs, but only at the 6-year follow-up visit. The VADT relied on self-reported autonomic symptoms [20]. The ACCORD trial identified CAN using indices of heart rate variability derived from short ECG recordings [45].
Thus, as with the DSP evaluations, a major limitation in correctly estimating the effects of glucose control interventions on CAN is a lack of standardized measures used in these trials.
Lessons from Type 1 Diabetes Trials
Despite limitations in assessments and outcome-measure standardization, beneficial effects of glucose control on measures of DSP and/or CAN in T1D have been shown (Tables 1 and 2). In a small observational trial, DSP prevalence increased from ~12 % to ~60 %, and CAN prevalence increased from ~3 % to 23 % after 5 years of follow-up in the group with poor glucose control, as compared with better glucose control where DSP and CAN prevalence were static [4]. The Pitts-burgh Epidemiology of Diabetes Complications (EDC) Study, a prospective cohort study of childhood-onset T1D, reported significant baseline associations between higher A1c and DSP [5]. A decline in the incidence of DSP and CAN associated with declining A1c levels was reported in a subset of EDC participants in whom DSP and CAN were reassessed after 20–25 years [46]. The Wisconsin Epidemiologic Study of Diabetic Retinopathy reported 20 % decrease in the incidence of DSP for a 2 % decrease in A1c from baseline to the 4th year of follow-up [8]. EURODIAB reported a substantial increase in DSP prevalence with increasing A1c levels among ~3,000 patients with T1D (<15 % in participants with A1c <5.4 % vs. 40 % in those with A1c >7.8 %) [34]. Strong correlations were also observed between A1c and the prevalence of CAN at baseline [44]. Hypertension, smoking, obesity, and triglycerides were independent risk factors for neuropathy in the EURODIAB T1D cohort [34].
None of these studies were designed specifically to measure the effects of glucose control on DSP or CAN, yet their data further support the benefits of blood glucose control on DSP and CAN.
The DCCT and the follow-up observational EDIC (DCCT/EDIC), which recently marked its 30th year, stands as the pivotal trial demonstrating clear and persistent benefits of tight glucose control for both DSP and CAN in patients with T1D [26•, 47]. DCCT enrolled 1,441 patients with T1D who were randomly assigned to intensive or conventional insulin therapy [11, 12, 26•, 27]. Both DSP and CAN were uncommon at DCCT start, in part owing to the intentional exclusion of persons with symptomatic neuropathy [26•, 27]. After an average of 6.5 years of follow-up, A1c was 7.4 % in the intensive group and 9.1 % in the conventional group [11]. The prevalence of confirmed DSP increased substantially in the conventional participants (from 5 % to 17 %; p<.001) and only slightly among the intensive group participants (from 7 % to 9 %) [11, 12, 26•, 27]. Adjusting for the presence of confirmed DSP at baseline, the risk reduction for incident DSP with intensive glucose control during DCCT was 64 % (95 % CI 45–76) [11, 12, 26•]. The prevalence of CAN almost doubled in the conventional group by DCCT-end, while remaining static in the intensive group [11, 40]. The risk reduction in incident CAN with intensive therapy during DCCTwas 45 % [11, 26•, 27, 40].
The DCCT/EDIC has furthered the understanding of the role of glucose control in the development and progression of neuropathy [26•, 27]. At DCCT closeout, all participants were encouraged to adopt intensive treatment, and diabetes care was transitioned to community-based providers. Most DCCT participants (94 %) agreed to participate in the EDIC follow-up [11, 25]. The A1c separation between former intensive and conventional treatment groups observed through the DCCT-end quickly waned; by the 5th year of EDIC follow-up, no statistically significant A1c separation remained [41]. Prevalence of DSP increased during EDIC in both groups, and despite no measureable difference in glucose control, a 30 % reduction in the risk of developing confirmed that DSP with prior intensive glucose control persisted [26•, 27]. Similar trends were observed on several NCS measures [13, 26•, 27]. After adjusting for NCS results at DCCT closeout, the risk reduction attenuated to 17 % (not significant), suggesting that different levels of subclinical DSP (as documented by differences in NCS at DCCT-end) contributed to the finding of a persistent benefit [13, 26•, 48]. Logistic regression analyses to evaluate the effects of glycemic control on prevalent and incident DSP demonstrated that the odds of all DSP-related outcomes each increased per unit (percentage) increase in DCCT and EDIC mean A1c [13, 26•, 27]. Likewise, the incidence of any DSP outcomes measured at EDIC year 13–14 among participants free of DSP at DCCT closeout increased per unit increase in mean EDIC A1c [13, 26•, 27, 41]. CAN prevalence also increased by EDIC year 13–14 (29 % in the intensive vs. 35 % in the conventional group), with treatment group differences remaining significant [26•, 41]. After adjusting for important covariates, including age, sex, cohort assignment, and the level of RRV at DCCT closeout, intensive glucose control during DCCT reduced the risk of incident CAN during EDIC by 31 % and of abnormal RRV by 30 % [41]. The adjusted RRV was significantly higher in the former intensive group, as compared with the conventional group [41]. The persistent beneficial effects of intensive glucose therapy on CAN after 13–14 years of follow-up in EDIC were explained by the differences in the DCCT and EDIC mean A1c levels between groups [26•, 27, 41]. Although one third of DCCT/EDIC participants had CAN by the 13–14th year of EDIC follow-up, few participants reported autonomic symptoms. No group differences in the prevalence of autonomic symptoms reported were observed, supporting the notion that autonomic neuropathies may remain largely asymptomatic, even after many years of T1D.
To summarize findings from the DCCT/EDIC study, the differences in the incidence and prevalence of both DSP and CAN reflect differences in glucose control, favoring A1c levels that are closer to nondiabetic levels. These findings parallel the reported long-term benefits of prior intensive glycemic control on retinopathy, nephropathy [49–51], and cardiovascular disease [52], a phenomenon termed metabolic memory [51].
Lessons from Type 2 Diabetes Trials
Data on the beneficial effects of glucose control on neuropathy in T2D emerged from observational trials summarized in Tables 1 and 2 [8, 14, 15, 53]. The Kumamoto trial was the first randomized controlled trial to report beneficial effects of tight glucose control (A1c of 7.4 % at trial end), as compared with conventional glucose control (A1c 9.4 % at trial end), in preventing NCS deterioration in 110 Japanese participants with T2D followed for a mean of 6 years; no differences were observed on CAN measures [17].
The UKPDS trial enrolled 3,867 relatively young patients with newly diagnosed T2D. By the end of the trial (10-year average), intensive glucose control (A1c 7 %) had no effect on DSP or CAN, as compared with standard control (A1c 7.9 %), although some marginal benefit was observed in those followed for 15 years [18]. The use of insensitive DSP or CAN measures, the rather narrow difference in the A1c between arms, and potential differences in other risk factors for T2D DSP could have contributed to these findings. Since no measures of neuropathy were assessed during the observational follow-up of the UKPDS, unveiling a potential long-term beneficial “legacy effect,” as observed for cardiovascular outcomes [54], was not possible.
The VA-CSDM achieved as much as a 2 % A1c difference between the intensive and standard arms but reported no differences in DSP (64 % intensive vs. 69 % standard) or CAN (48 % intensive vs. 55 % standard) prevalence after 2 years [19].
The VADT randomized 1,791 veterans with T2D (baseline A1c 9.4 %) to either intensive or standard glucose control. After approximately 5.6 years of follow-up, there were no differences in the rates of new DSP in the intensive versus standard arm (38 % vs. 40 %, respectively), despite significant differences in the mean A1c between groups (6.9 % vs. 8.4 %, respectively) [20]. The study reported a nonsignificant increase in CAN in the intensive group and 8.2 % versus 5.2 % [20]. The effects of glucose control on DSP/CAN development in this elderly cohort with multiple comorbidities are difficult to interpret, especially given that reliance on self-report to define neuropathy outcomes has a large potential for bias.
The ADDITION-Denmark study enrolled more than 1,500 individuals with newly diagnosed T2D. No difference in the prevalence of DSP or CAN between the intensively treated and standard groups (see Tables 1 and 2 for a description) was observed after 6-year follow-up [55]. Although the participants enrolled in the ADDITION trial were very early in their disease course and, theoretically, more susceptible to glucose control intervention, important limitations are to be considered. ADDITON did not obtain baseline evaluations for DSP or CAN, preventing objective evaluations of change in DSP or CAN with intervention [21, 55]. Per trial design, ADDITION randomized the health-care providers and their respective practices, and not the participants, to intensive or standard treatment. At the end of the trial, it was evident that most providers intensified treatment as much as possible; as a consequence, the A1c was identical in both groups (6.4 %) [21, 55].
The ACCORD trial enrolled ~10,000 patients with T2D at high risk for CVD events and examined the effects of intensive glucose control (target A1c <6 %) versus standard of care (A1c ~7 %) on cardiovascular disease and other complications [56]. The main DSP measure was the MNSIc, with individual components of the MNSI included in the prespecified analytic outcomes [22]. Although the intensive glycemic intervention arm of ACCORD was stopped early due to an increase in all-cause mortality [56] and glycemic control was relaxed in this arm, follow-up continued for the entire 5 years as originally planned. A treatment group difference in the A1c of 1 % was maintained at 3.8 years, and at study end, the incidence of DSP was significantly reduced in the intensive, as compared with the standard, treatment group (HR 0.92, CI 0.86–0.99; p=.027; NNT=33) [22]. Incidence of loss of ankle reflexes was also lower in the intensive, as compared with the standard, arm; however, the loss of VPT (the DSP measure evaluated in UKPDS) was not significantly different [22].
There are several lessons to consider with regard to the ACCORD findings for DSP. The most robust and consistent data for DSP outcomes emerged from analyses that used the clinical exam portion of the MNSI, after ~5 years of follow-up [22]. However, gaps in the standardization process for MNSI training and administration across sites and lack of electro-physiology and/or small-fiber-specific measures limit our ability to assess in depth the whole benefit (or harm) of the interventions used in ACCORD on DSP. Another interesting observation from the ACCORD study, which is still under analysis, is that one parameter associated with a higher mortality risk with the intensive glycemic arm in ACCORD participants was a history of DSP at baseline [57]. Ongoing analyses from ACCORD (currently in submission) further evaluate the effects of glycemic control and the rest of the interventions in this cohort on DSP and CAN.
A recent meta-analysis of four T2D randomized trials that included the UKPDS, ACCORD, and VADT reported a 0.58 % risk reduction/year in DSP with tight glucose control [58•].
A Steno-2 trial showed that intensive multifactorial intervention targeting glucose, blood pressure, lipids, smoking, and other lifestyle factors in a European cohort of patients with T2D had no effect on DSP, although it reduced the progression/development of CAN [23].
Lastly, emerging data suggest that, in T2D, the strategies used to reach glycemic goals may be as important as the glucose control achieved. The BARI 2D trial randomized >2,000 participants with T2DM (~62 years old, ~10 years of diabetes duration) and angiographically documented coronary artery disease to either insulin-sensitizing drugs (metformin, thiazolidinediones, or both) or insulin-providing drugs (sulfonylureas/meglitinides, insulin, or both); both groups targeted an A1c <6.5 % [59]. DSP was a specified secondary outcome and was assessed at baseline and annually during the trial with the MNSIc [24•]. Among 1,075 BARI 2D participants with no DSP at baseline, the 4-year cumulative incidence rate of DSP was significantly lower in the insulin-sensitizing than in the insulin-providing strategy group, which remained significant after adjusting for the in-trial A1c. No differences were seen in the DSP remission rates in those who had already established DSP [24•] These findings suggest that some of the available agents used to treat hyperglycemia in T2D may promote additional effects that could directly interact with the development of DSP independently of their glucose lowering, such as effects on lipid metabolism, body weight, oxidative stress, or chronic inflammation [24•].
Conclusion
There are several lessons to be learned from recent large trials regarding the associations between glucose control and neuropathy.
First and foremost, many recent large trials have included DSP and CAN as only secondary outcomes, or as post hoc analyses, rather than as primary outcomes. The size of the study population, the duration of the intervention, and the neuropathy assessment used biases and limited conclusions that may be drawn from these trials. Use of outcome measures that are unlikely to show a monotonic worsening over a short duration of intervention and inclusion of study participants who are not specifically selected for either neuropathy progression or potential for regression further bias trial observations.
The lack of standardized, validated measures for DSP and CAN in most large trials limits the interpretation of the data. Trials that include DSP and CAN as defined outcomes must be especially selective about the measures used. In multicenter trials, the need to systematically train, certify, and reassess competency of staff performing tests is often overlooked, contributing to inter- and intrasite variability over time. Yet, as demonstrated by the DCCT and EDIC, well-standardized and validated measures of DSP and CAN can, in fact, be successfully implemented in large, multicenter trials [12, 13, 26•, 27, 40, 41].
Small-fiber DSP measures are not widely used in large clinical trials and were not included in any of the trials reviewed herein. Yet arguably, these are most likely to show objective change in response to interventions [37, 38], especially in trials of shorter duration or of smaller cohort size.
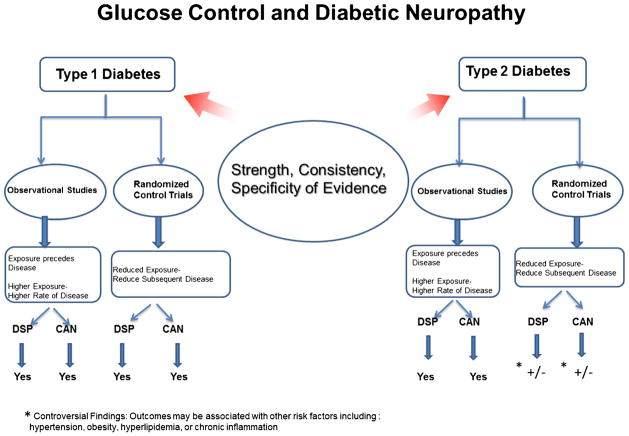
Observational studies have shown that glucose control is critical for neuropathy prevention in both T1D and T2D (Fig. 1) [8, 14, 15, 34, 60, 61]. However, different lessons are learned from randomized controlled clinical trials of glucose control in T1D and T2D (Fig. 1) [58•].
Fig. 1.
Strength, consistency, and specificity of evidence linking glucose control and neuropathy
DCCT/EDIC and other smaller trials strongly demonstrated that intensive control designed to achieve near-normal glycemia is essential to preventing or delaying progression of DSP and CAN in T1D [27]. The highly reproducible and sensitive testing protocol repeated over time in DCCT/EDIC, the robust definitions used for DSP and CAN, and the large sample size enhance the validity of the results [26•, 27]. Although the prevalence of these complications is low in young people with short T1D duration, DSP and CAN develop over time. DCCT/EDIC showed that implementing and maintaining tight glucose control as early as possible in the course of T1D prevents early neuropathy development and promotes long-time protection, especially for CAN [26•, 27]. We also learned that the emergence of DSP and CAN during EDIC was highly but not entirely glucose dependent [13, 26•, 27], suggesting that the level of glucose control achieved during DCCT is difficult to sustain over time and, perhaps, insufficient to fully prevent adverse nervous system effects [26•, 27].
The effects of glycemic control on DSP or CAN are less conclusive for T2D (Fig. 1).
Earlier data suggested that glucose control is beneficial if implemented earlier in the disease course in patients with fewer comorbidities, [17] but later studies did not confirm these findings [18, 21, 55, 58•].
Some trials showed that tighter glucose control might have a beneficial effect in preventing progression of neuropathy in some patients with T2D [17, 22, 58•]. ACCORD, the largest T2D trial, reported a significant DSP risk reduction after 5-year follow-up [22]. No effects were observed with other large trials [18, 20, 21, 55], although UKPDS reported a modest risk reduction with intensive treatment similar to that found by ACCORD, but only after 15 years [18, 58•].
The presence of multiple comorbidities and risk factors, including hypertension, hyperlipidemia, and obesity, among most T2D patients included in these trials, the polypharmacy required to reach glucose targets, and the high incidence of hypoglycemia and weight gain might have attenuated the effects of glucose control and contributed to inconsistent findings among T2D study populations. Different glucose target may also be required in T2D, as compared with T1D.
Selection of specific glucose-lowering strategies and/or combining more effectively pharmacological and lifestyle interventions may be required in future T2D trials and in clinical practice to obtain a reduction in DSP and CAN among T2D patients..
DSP and CAN are quite prevalent and confer high clinical burden, resulting in significant morbidity. Loss of protective sensation heightens the risk for foot ulceration and lower extremity amputations, and painful symptoms are frequently refractory to treatment [2, 7]. CAN, although silent in earlier stages, is an independent predictor of mortality [45, 62, 63].
Although it remains unproven whether good glycemic control can reverse preexisting DSP or CAN, the earlier and more effectively we implement intensive therapy, the more effectively we prevent neuropathy in T1D [27] and, possibly, T2D. Additional well-designed trials that consider the complexity of the neuropathic complications of diabetes are needed to answer these questions.
Acknowledgments
R.P.B. is supported by grants from NIH/NIDDK, NIH/NHLBI (1R01HL102334-01, U01-DK-094176, U01-DK-094157, U01DK098246) and the American Diabetes Association (1-14-MN-02). M.J. is supported by grants from the American Diabetes Association (1-14-MN-02). C.L.M. is supported by grants from the National Institutes of Health/NIDDK (U01-DK-094176, U01-DK-094157, and U01DK098246).
Footnotes
Conflict of Interest Lynn Ang, Mamta Jaiswal, Catherine Martin, and Rodica Pop-Busui declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article describes studies with human subjects, and Dr. Pop-Busui participated in some of them. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Lynn Ang, Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan Medical School, 5329 Brehm Tower 1000 Wall Street, Ann Arbor, MI 48105, USA.
Mamta Jaiswal, Department of Neurology, University of Michigan Medical School, Ann Arbor, MI, USA.
Catherine Martin, Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan Medical School, 5329 Brehm Tower 1000 Wall Street, Ann Arbor, MI 48105, USA.
Rodica Pop-Busui, Email: rpbusui@umich.edu, Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan Medical School, 5329 Brehm Tower 1000 Wall Street, Ann Arbor, MI 48105, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 2.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Dyck PJ, Albers JW, Andersen H, Arezzo JC, Biessels GJ, Bril V, Feldman EL, Litchy WJ, O’Brien PC, Russell JW. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011:27. doi: 10.1002/dmrr.1226. Discusses updates on diagnostic criteria for severity of diabetic polyneuropathies. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler D, Mayer P, Muhlen H, Gries FA. The natural history of somatosensory and autonomic nerve dysfunction in relation to glycaemic control during the first 5 years after diagnosis of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:822–9. doi: 10.1007/BF00408358. [DOI] [PubMed] [Google Scholar]
- 5.Maser RE, Steenkiste AR, Dorman JS, Nielsen VK, Bass EB, Manjoo Q, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1989;38:1456–61. doi: 10.2337/diab.38.11.1456. [DOI] [PubMed] [Google Scholar]
- 6.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162–7. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 7.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124:90–6. doi: 10.7326/0003-4819-124-1_part_2-199601011-00003. [DOI] [PubMed] [Google Scholar]
- 9.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–9. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 10.Amthor KF, Dahl-Jorgensen K, Berg TJ, Heier MS, Sandvik L, Aagenaes O, et al. The effect of 8 years of strict glycaemic control on peripheral nerve function in IDDM patients: the Oslo Study. Diabetologia. 1994;37:579–84. doi: 10.1007/BF00403376. [DOI] [PubMed] [Google Scholar]
- 11.DCCT. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 12.DCCT. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–80. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 13.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sands ML, Shetterly SM, Franklin GM, Hamman RF. Incidence of distal symmetric (sensory) neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1997;20:322–9. doi: 10.2337/diacare.20.3.322. [DOI] [PubMed] [Google Scholar]
- 15.Chao CC, Hsieh SC, Yang WS, Lin YH, Lin WM, Tai TY, et al. Glycemic control is related to the severity of impaired thermal sensations in type 2 diabetes. Diabetes Metab Res Rev. 2007;23:612–20. doi: 10.1002/dmrr.734. [DOI] [PubMed] [Google Scholar]
- 16.Perkins BA, Dholasania A, Buchanan RA, Bril V. Short-term metabolic change is associated with improvement in measures of diabetic neuropathy: a 1-year placebo cohort analysis. Diabet Med. 2010;27:1271–9. doi: 10.1111/j.1464-5491.2010.03110.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 18.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 19.Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, et al. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM) J Diabetes Complications. 1999;13:307–13. doi: 10.1016/s1056-8727(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 20.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 21.Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011;34:2244–9. doi: 10.2337/dc11-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 24•.Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36:3208–15. doi: 10.2337/dc13-0012. Discusses the BARI 2D trial and its findings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Martin CL, Albers JW, Pop-Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–8. doi: 10.2337/dc13-2114. Discusses neuropathy and related findings in the DCCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pop-Busui R, Herman WH, Feldman EL, Low PA, Martin CL, Cleary PA, et al. DCCTand EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Curr Diab Rep. 2010;10:276–82. doi: 10.1007/s11892-010-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–4. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin CL, Waberski BH, Pop-Busui R, Cleary PA, Catton S, Albers JW, et al. Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study. Diabetes Care. 2010;33:2635–41. doi: 10.2337/dc10-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electro-physiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 31.Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937–44. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyck PJ, Karnes J, O’Brien PC, Swanson CJ. Neuropathy Symptom Profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology. 1986;36:1300–8. doi: 10.1212/wnl.36.10.1300. [DOI] [PubMed] [Google Scholar]
- 33.Dyck PJ, Karnes JL, O’Brien PC, Litchy WJ, Low PA, Melton LJ., 3rd The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology. 1992;42:1164–70. doi: 10.1212/wnl.42.6.1164. [DOI] [PubMed] [Google Scholar]
- 34.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–50. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 35.Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30:2619–25. doi: 10.2337/dc06-2479. [DOI] [PubMed] [Google Scholar]
- 36.Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Carter RE, et al. “Unequivocally Abnormal” vs “Usual” Signs and symptoms for proficient diagnosis of diabetic polyneuropathy: Cl vs N Phys Trial. Arch Neurol. 2012;69:1609–14. doi: 10.1001/archneurol.2012.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn) 2012;18:60–84. doi: 10.1212/01.CON.0000411568.34085.3e. [DOI] [PubMed] [Google Scholar]
- 38.Malik R, Veves A, Tesfaye S, Smith G, Cameron N, Zochodne D, et al. Small fiber neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011;27:678–84. doi: 10.1002/dmrr.1222. [DOI] [PubMed] [Google Scholar]
- 39.Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48:164–71. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 40.DCCT. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41:416–23. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–93. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–8. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–53. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 44.Kempler P, Tesfaye S, Chaturvedi N, Stevens LK, Webb DJ, Eaton S, et al. Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med. 2002;19:900–9. doi: 10.1046/j.1464-5491.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 45.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–84. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–9. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 47.Cefalu WT, Ratner RE. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: the “gift” that keeps on giving! Diabetes Care. 2014;37:5–7. doi: 10.2337/dc13-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B. Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care. 2007;30:2613–8. doi: 10.2337/dc07-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DCCT. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DCCT/EDIC, Writing, Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–67. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkins BA, Orszag A, Ngo M, Ng E, New P, Bril V. Prediction of incident diabetic neuropathy using the monofilament examination: a 4-year prospective study. Diabetes Care. 2010;33:1549–54. doi: 10.2337/dc09-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 55.Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, et al. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013;56:101–8. doi: 10.1007/s00125-012-2744-5. [DOI] [PubMed] [Google Scholar]
- 56.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:721–7. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. Discusses how to prevent and treat diabetic neuropathy with enhanced glucose control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kempler P, Amarenco G, Freeman R, Frontoni S, Horowitz M, Stevens M, et al. Gastrointestinal autonomic neuropathy, erectile-, bladder- and sudomotor dysfunction in patients with diabetes mellitus: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:665–77. doi: 10.1002/dmrr.1223. [DOI] [PubMed] [Google Scholar]
- 61.Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39:1377–84. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 62.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 63.Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res. 2012;5:463–78. doi: 10.1007/s12265-012-9367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]