Abstract
Chemotherapy‐induced peripheral neurotoxicity (CIPN) seriously impairs patients’ quality of life cumulatively and dose‐dependently. Because assessment of CIPN usually depends on patients’ subjective evaluation of symptoms, objective and quantitative measures are needed. We evaluated a point‐of‐care nerve conduction device (POCD), previously validated for the assessment of diabetic peripheral neuropathy. Sensory nerve action potential (SNAP) amplitude and sensory nerve conduction velocity (SNCV) of the sural nerve were measured using a portable, automated POCD (DPNCheck; NeuroMetrix Inc., Waltham, MA, USA) in patients with a clinical diagnosis of CIPN of grade 1 or higher. We compared SNAP and SNCV among patients with different grades of CIPN according to the Common Terminology Criteria for Adverse Events. A total of 50 patients (22 men, 28 women; median age, 64 years; grade 1/2/3, 21/18/11) were evaluated. Anticancer drugs responsible for CIPN were cisplatin in five patients, oxaliplatin in 15, carboplatin in 5, paclitaxel in 16, docetaxel in 14, nab‐paclitaxel in 7, vincristine in 6, and bortezomib in 3. Unadjusted SNAP was 8.45 ± 3.67 μV (mean ± SD) in patients with grade 1 CIPN, 5.42 ± 2.68 μV with grade 2, and 2.45 ± 1.52 μV with grade 3. Unadjusted SNCV was 49.71 ± 4.77 m/s in patients with grade 1 CIPN, 48.78 ± 6.33 m/s with grade 2, and 44.14 ± 7.31 m/s with grade 3. The adjusted SNAP after controlling for age significantly differed between each CTCAE grade (P < 0.001, ancova). The adjusted SNCV after controlling for age and height also differed significantly (P = 0.027). Differences in the severity of CIPN could be detected objectively and quantitatively using this POCD.
Keywords: Chemotherapy, nerve conduction study, peripheral neurotoxicity, point‐of‐care, sensory neuropathy
Chemotherapy‐induced peripheral neurotoxicity (CIPN) is a common, persistent toxic effect among patients who receive cancer chemotherapy.1 For many cancer survivors, long‐term toxicity of chemotherapy has a serious impact on their quality of life.2 Chemotherapy‐induced peripheral neurotoxicity seriously impairs patients’ quality of life in a cumulative and dose‐dependent manner.3 Measures for the prevention and treatment of CIPN have yet to be established.4 Early detection is essential because dose reduction or discontinuation of chemotherapy is the only effective management for this notorious toxicity.1, 5
Assessment of CIPN usually depends on patients’ subjective evaluation of symptoms, rated according to clinical oncology grading scales such as the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE).5 The grades of symptoms according to this scale are primarily evaluated by health providers on the basis of patients’ symptoms and functional impairment. Use of the CTCAE is associated with inevitable disagreements among observers, and health providers sometimes underestimate the severity of CIPN.6, 7 Instead, several patient‐oriented questionnaires, which are based entirely on patients’ self‐evaluation, have been developed, but remain to be formally validated.5 Nerve conduction studies (NCS), the gold standard for the diagnosis of peripheral neuropathies,8 are not widely used to evaluate cancer patients in daily clinical practice because they require referral to specialized neurological laboratories.2 Moreover, NCS often causes discomfort to patients during the procedure.2 Therefore, a simple, easy‐to‐use, non‐invasive method for the objective and quantitative assessment of CIPN needs to be established.
DPNCheck (NeuroMetrix, Waltham, MA, USA) is a point‐of‐care nerve conduction device (POCD) that was originally developed for the detection and evaluation of diabetic peripheral neuropathy (DPN).9 Because DPN is primarily a length‐dependent sensory neuropathy, the sural nerve, the longest sensory nerve in humans, is generally used to assess DPN.9 DPNCheck can be easily handled by non‐technical personnel and be specifically used to evaluate sensory nerve action potential (SNAP) amplitude and sensory nerve conduction velocity (SNCV) of the sural nerve. Both SNAP and SNCV measured by DPNCheck are in good agreement with the values obtained by standard NCS.9, 10, 11 A recent study reported that as the severity of DPN worsened, SNAP and SNCV measured by POCD significantly decreased in overt diabetic patients.12 Diabetic peripheral neuropathy is caused by axonal degeneration, which is also the most widely accepted mechanism underlying CIPN.1, 2, 3 Therefore, CIPN might be able to be evaluated in the same manner as DPN by means of POCD.
In this study, we prospectively evaluated cancer patients who had been given a clinical diagnosis of CIPN to validate POCD for the objective and quantitative assessment of CIPN.
Materials and Methods
Ethics statement
This study was approved by the Institutional Review Board and Ethics Committee of Nagoya University Hospital. The study was carried out in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
Patients
Japanese cancer patients who were receiving chemotherapy at an outpatient chemotherapy center were enrolled. Eligible patients had to: (i) be 20 years of age or older; (ii) have a current or previous history of cancer chemotherapy that could cause peripheral neuropathy, such as platinum analogues (cisplatin, oxaliplatin, or carboplatin), taxanes (paclitaxel, docetaxel, or nab‐paclitaxel), vinca alkaloids (vincristine), and proteasome inhibitors (bortezomib); (iii) have a histologically confirmed diagnosis of cancer; and (iv) have a clinical diagnosis of CIPN with peripheral sensory neuropathy of grade 1 or higher according to CTCAE version 4.0 (Table 1). Toxic effects were assessed with the use of a standardized checklist by medical oncologists and well‐trained nurses at the outpatient chemotherapy center who were specialized in cancer treatment and care.
Table 1.
Peripheral sensory neuropathy according to the Common Terminology Criteria for Adverse Events version 4.0
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Peripheral sensory neuropathy | Asymptomatic; loss of deep tendon reflexes or paresthesia | Moderate symptoms; limiting instrumental ADLs† | Severe symptoms; limiting self‐care ADLs‡ |
†Preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc. ‡Bathing, dressing and undressing, using the toilet, taking medications, and not being bedridden. ADLs, activities of daily living.
Patients were excluded if they had: (i) a history of peripheral neuropathy apparently unrelated to chemotherapy; (ii) known risk factors for peripheral neuropathy, such as diabetes mellitus, severe renal failure, liver impairment, and alcoholism; (iii) brain and central nervous system metastases; or (iv) conditions such as leg amputation, leg deformities, leg infection, open ulcers, or leg injuries, which hamper the appropriate setting of the device.
Study design
Sensory nerve action potential amplitude and SNCV of the sural nerve were measured using DPNCheck as described previously.9 This hand‐held device consists of a biosensor and stimulating probes located at a fixed distance (92.2 mm) from the biosensor. These probes are attached to the lateral side of the ankle, posterior to the lateral malleolus, the area of innervation of the sural nerve during the procedure. The sural nerve is automatically stimulated 6–20 times within 15–20 s and the response of the sural nerve is recorded by a biosensor placed on the lower calf. A single measurement usually takes <1 min. Compared with conventional NCS, this POCD causes far less discomfort to the patient.
The SNAP and SNCV of the right and left legs were measured, and the mean values were calculated. Examinations were carried out by the same personnel (A.M.). Additional tests were repeated up to four times for each leg to obtain valid results.
The SNAP and SNCV values are provided as rounded‐up whole numbers; for example, both 7.5 and 8.4 μV are measured as 8 μV. Those SNAP values <1.5 μV are automatically adjusted to zero; thus, a SNAP value of 1.4 μV is measured as zero. In this study, the SNAP values measured as “zero” were analyzed as zero. If valid SNCV values were not obtained technically, these data were treated as missing.
The endpoint of this study was to validate the POCD for the objective and quantitative assessment of CIPN. Our hypothesis was that the measured value of SNAP would decrease as the CTCAE grade worsened. To confirm this hypothesis, SNAP and SNCV were compared among patients with different CTCAE grades. Correlations between the measured values and CTCAE grades were also examined.
Statistical analysis
Previous studies have reported that sural SNAP significantly depends on subject age, and SNCV depends on subject age and height.13, 14, 15, 16 The SNAP values decrease by 1 μV for every 10 years, whereas SNCV values decrease by 1.3 m/s for every 10 years and 2.0 m/s for every 10 cm of height.13 Thus, one‐way ancova was used to eliminate the confounding effects of age and height. Because homogeneity of variances was violated (P = 0.002, by Levene's test), the measured value of SNAP was transformed into the square root of the values (SNAPsqrt). The independent variable was the CTCAE grade, and the dependent variables were SNAPsqrt and SNCV. The covariate was subject age for SNAPsqrt, and subject age and height for SNCV. As a post hoc analysis, adjusted means of SNAPsqrt and SNCV were compared between each CTCAE grade using t‐tests with a Bonferroni correction. Correlations between the measured values of SNAP or SNCV and the CTCAE grade were also examined using Spearman's rank‐order correlation coefficient. All calculations were carried out using the spss software package, version 23 (SPSS, Chicago, IL, USA) and two‐sided P‐values <0.05 were considered to indicate statistical significance.
Results
From February 2015 through June 2015, a total of 52 patients were initially enrolled. Two patients were excluded from the analysis; one declined the examination because of discomfort after a single measurement, and the other could not provide valid data after four consecutive errors. Eventually, 50 Japanese patients, 22 men and 28 women with a median age of 64.0 years (range, 34–85 years) and a mean height of 160.3 ± 8.6 cm, were evaluated (Table 2). Anticancer drugs responsible for CIPN were cisplatin in five patients, oxaliplatin in 15, carboplatin in 5, paclitaxel in 16, docetaxel in 14, nab‐paclitaxel in 7, vincristine in 6, and bortezomib in 3. The median interval from the last dose of drugs responsible for CIPN was 21 days (interquartile range, 14–28 days; range, 3–1530 days), and 34% (17/50) of the patients had received multiple anticancer drugs responsible for CIPN. Medications for the treatment of neuropathy included pregabalin in 10 patients, Goshajinkigan (Kampo medicine) in 8, vitamin B12 in 6, and duloxetine in 3. The mean unadjusted SNAP and SNCV were 6.04 ± 3.74 μV and 48.50 ± 6.0 m/s, respectively (Table 3, Figs 1 and 2).
Table 2.
Characteristics of 50 patients with chemotherapy‐induced peripheral neurotoxicity (CIPN) who underwent bilateral sural nerve conduction testing by a point‐of‐care nerve conduction device
| Sex, male/female | n | 22/28 |
|---|---|---|
| Age, years | Median (range) | 64.0 (34–85) |
| Height, cm | Mean ± SD | 160.3 ± 8.6 |
| Interval from the last dose of drugs responsible for CIPN, days |
Median [IQR] (range) |
21 [14–28] (3–1530) |
| Cancer origin | n | |
| Colon | 13 | |
| Breast | 8 | |
| Gastric | 5 | |
| Pancreas | 5 | |
| Hematology | 7 | |
| Gynecology | 5 | |
| Other | 7 | |
| Responsible drug/cumulative dose, mg | n/median [IQR] | |
| Cisplatin | 5/300 [260–650] | |
| Oxaliplatin | 15/1000 [850–1390] | |
| Carboplatin | 5/6100 [4600–10 000] | |
| Paclitaxel | 16/3100 [1700–3900] | |
| Docetaxel | 14/520 [310–730] | |
| Nab‐paclitaxel | 7/1400 [1200–3200] | |
| Vincristine | 6/8 [5.8–9.7] | |
| Bortezomib | 3/170 [100–210] | |
| CTCAE (grade 1/2/3) | n | 21/18/11 |
CTCAE, Common Terminology Criteria for Adverse Events; IQR, interquartile range.
Table 3.
Sensory nerve action potential (SNAP) and sensory nerve conduction velocity (SNCV) values measured by point‐of‐care nerve conduction device according to Common Terminology Criteria for Adverse Events
| Mean ± SD | Median [IQR] | Range | n | |
|---|---|---|---|---|
| SNAP, μV | ||||
| Grade 1 | 8.45 ± 3.67 | 8.0 [6–11] | 4–17 | 21 |
| Grade 2 | 5.42 ± 2.68 | 5.5 [4–8] | 1–10 | 18 |
| Grade 3 | 2.45 ± 1.52 | 3.0 [1.5–4] | 0–4 | 11 |
| Total | 6.04 ± 3.74 | 5.5 [4–8.5] | 0–17 | 50 |
| SNCV, m/s | ||||
| Grade 1 | 49.71 ± 4.77 | 49.0 [47–53] | 40–61 | 21 |
| Grade 2 | 48.78 ± 6.33 | 49.5 [43.5–54] | 39–59 | 18 |
| Grade 3 | 44.14 ± 7.31 | 44.5 [41.5–50] | 31–52 | 7 |
| Total | 48.50 ± 6.00 | 49.0 [45–53] | 31–61 | 46 |
IQR, interquartile range.
Figure 1.

Box plots of the measured value of sensory nerve action potential (SNAP) in 50 patients with chemotherapy‐induced peripheral neurotoxicity, according to Common Terminology Criteria for Adverse Events. Each rectangle represents the lower quartile (25th percentile) and the upper quartile (75th percentile). The horizontal lines inside the rectangles indicate the median value. The vertical lines on either side of the rectangle indicate the lowest and the highest values.
Figure 2.
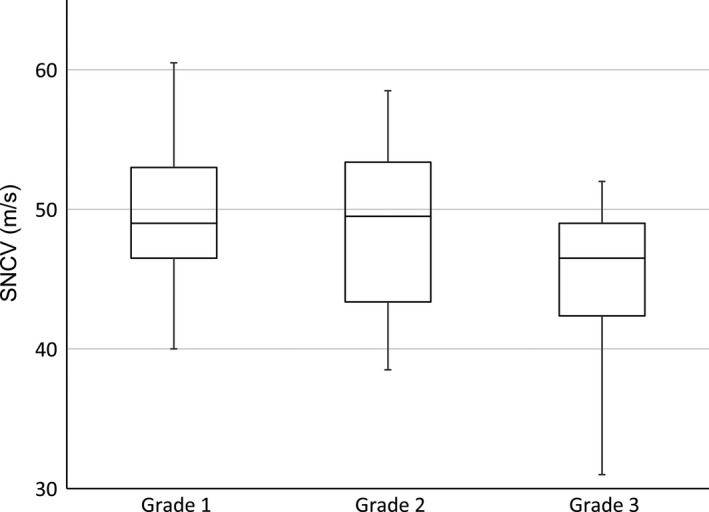
Box plots of the measured value of sensory nerve conduction velocity (SNCV) in 50 patients with chemotherapy‐induced peripheral neurotoxicity, according to Common Terminology Criteria for Adverse Events. Each rectangle represents the lower quartile (25th percentile) and the upper quartile (75th percentile). The horizontal lines inside the rectangles indicate the median value. The vertical lines on either side of the rectangle indicate the lowest and the highest values.
After adjusting for age, there was a significant difference in SNAPsqrt between CTCAE grades, F(2,46) = 20.08, P < 0.001, partial η2 = 0.47 (ancova). The adjusted means of SNAPsqrt for each CTCAE grade significantly differed from each other (Table 4). After adjusting for age and height, there was also a significant difference in SNCV between CTCAE grades, F(2,41) = 3.94, P = 0.027, partial η2 = 0.16 (ancova). The adjusted mean of SNCV was significantly lower in the patients with grade 3 than in those with grade 1 (Table 5). Differences in other pairwise comparisons did not reach statistical significance.
Table 4.
Analysis of covariance adjusted means and multiple comparisons for sensory nerve action potential transformed into the square root of the values (SNAPsqrt) according to Common Terminology Criteria for Adverse Events (CTCAE)
| CTCAE | SNAPsqrt | ||||
| Unadjusted | Adjusted | n | |||
| Mean | SD | Mean | SE | ||
| Grade 1 | 2.85 | 0.61 | 2.88 | 0.13 | 21 |
| Grade 2 | 2.25 | 0.60 | 2.13 | 0.15 | 18 |
| Grade 3 | 1.39 | 0.75 | 1.52 | 0.19 | 11 |
| Comparison | Mean difference | SE |
Bonferroni adjusted 95% CI |
P‐value | |
| Grade 1 vs grade 2 | 0.75* | 0.20 | 0.26, 1.24 | 0.001* | |
| Grade 1 vs grade 3 | 1.36* | 0.22 | 0.80, 1.20 | <0.001* | |
| Grade 2 vs grade 3 | 0.61* | 0.24 | 0.01, 1.22 | 0.047* | |
*P < 0.05, where P‐values are adjusted using the Bonferroni correction. CI, confidence interval. R 2 = 0.53, adjusted R 2 = 0.50, adjustments based on age = 62.08. Homogeneity of regression tested and not significant: F = 0.83, P > 0.05. Age regression coefficient = −0.022, P = 0.006.
Table 5.
Analysis of covariance adjusted means and multiple comparisons for sensory nerve conduction velocity (SNCV) in patients with chemotherapy‐induced peripheral neurotoxicity according to Common Terminology Criteria for Adverse Events (CTCAE)
| CTCAE | SNCV | ||||
| Unadjusted | Adjusted | n | |||
| Mean | SD | Mean | SE | ||
| Grade 1 | 49.71 | 4.77 | 49.64 | 1.15 | 21 |
| Grade 2 | 48.78 | 6.33 | 49.18 | 1.28 | 18 |
| Grade 3 | 44.14 | 7.31 | 43.33 | 2.02 | 7 |
| Comparison | Mean difference | SE |
Bonferroni adjusted 95% CI |
P‐value | |
| Grade 1 vs grade 2 | 0.46 | 1.76 | −3.93, 4.85 | 1.000 | |
| Grade 1 vs grade 3 | 6.31* | 2.29 | 0.59, 12.03 | 0.026* | |
| Grade 2 vs grade 3 | 5.85 | 2.46 | −0.29, 11.98 | 0.066 | |
*P < 0.05, where P‐values are adjusted using the Bonferroni correction. CI, confidence interval. R 2 = 0.32, adjusted R 2 = 0.25, adjustments based on age = 61.28, height = 159.96. Homogeneity of regression tested and not significant: F = 0.29 (age), F = 1.19 (height), P > 0.05. Age regression coefficient = −0.13, P = 0.066. Height regression coefficient = −0.35, P = 0.001. Although age was not significantly related to the SNCV, we used age as covariate in this model according to previous reports.13, 14, 15, 16
There was a strong negative correlation between SNAP and the CTCAE grade (n = 50, ρ = −0.69, P < 0.001), whereas SNCV did not correlate with the CTCAE grade (n = 46, ρ = −0.21, P = 0.16) (Spearman's correlation coefficient).
Discussion
To our knowledge, this is the first study to validate DPNCheck for the assessment of CIPN. Differences in the severity of CIPN could be detected objectively and quantitatively using this POCD. Progression of CIPN was associated with a significant decrease in SNAP with relative preservation of SNCV, which confirms axonal degeneration.
A previous study failed to establish the utility of this POCD in a similar patient group: neither SNAP nor SNCV differed significantly between 24 patients with CIPN and 24 age‐matched healthy volunteers.17 Moreover, SNAP and SNCV did not correlate with the severity of CIPN. However, that study had crucial differences in methods and patients’ characteristics from the present study. First, they used a patient‐oriented questionnaire to grade the severity of CIPN. There is a discrepancy between patients’ self‐reported severity of symptoms and health providers’ assessments.18 Patients tend to report a significantly higher severity of symptoms than that assessed by health providers. Indeed, the decrease in the measured value of SNAP was less in their study than in our study (10.13 ± 3.12 μV vs 6.04 ± 3.74 μV). This means that the severity of CIPN in the previous study was not as high as that in our study. Therefore, the previous study could probably not detect small differences in the severity of CIPN. Second, the patients in the previous study had terminated chemotherapy at least 12 months before the evaluation. The axonal degeneration might have recovered during the chemotherapy‐free period with no apparent improvement in patients’ symptoms.
The decrease in SNAP was more remarkable than that in SNCV in our study. The most widely accepted mechanism of CIPN is axonal degeneration rather than demyelination, which is caused by injury to the dorsal root ganglia, dysfunction of microtubules within axons, interference in mitochondrial energy production, and direct axonal damage at distal terminals.1, 2, 3 Sensory nerve action potential reflects the number of axons conducting impulses, whereas SNCV reflects the degree of myelination in the axons.19 In patients with CIPN, the decrease in SNAP generally precedes that in SNCV, reflecting dominant axonopathy.1, 2, 3 In fact, the adjusted mean of SNCV was significantly lower in the patients with grade 3 CIPN than in those with grade 1.
It has been reported that an early decline in SNAP before patients recognize their symptoms or functional impairment might predict the subsequent development of CIPN.20, 21, 22, 23 However, because of its limited availability, conventional NCS has not been widely used in daily practice. In contrast, DPNCheck is simple to use by non‐technical personnel at the bedside. Future studies should evaluate its clinical value for the early detection of CIPN. Moreover, this device would most likely be useful in clinical trials designed to develop improved procedures for the clinical management of CIPN.
Our study had several limitations. First, we did not compare SNAP and SNCV measured by DPNCheck with the values obtained by standard NCS, the gold standard for the objective assessment of peripheral neuropathies. Instead, we used the CTCAE grade, derived from the scale most widely used in clinical practice, as a reference. Although previous studies have shown strong correlations between results obtained with this POCD and conventional NCS in both healthy subjects and DPN patients,9, 10, 11 an additional validation study in patients with CIPN would provide a better understanding of the effective management of CIPN. Second, we did not evaluate the inter‐rater or intra‐rater reliability of the measurements with the POCD. Because excellent reliability was reported in patients with DPN,9 high reliability is also expected for the assessment of CIPN. Third, we did not evaluate the sensitivity or specificity for the diagnosis of CIPN, because the aim of this study was to validate the POCD for the quantitative assessment of CIPN, rather than for diagnosis.
In conclusion, the severity of CIPN could be evaluated objectively and quantitatively by POCD, especially by using the measured value of SNAP. A decrease in sural SNAP with relative preservation of SNCV confirms axonal degeneration. Future studies should evaluate the clinical value of POCD for the early detection of CIPN. Moreover, this device would most likely be useful in clinical trials designed to develop procedures for the clinical management of CIPN.
Disclosure Statement
The POCD equipment was provided by Omron Healthcare Co., Ltd, distributor of DPNCheck in Japan. The company had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. Hitoshi Kiyoi received research funding from Bristol‐Myers Squibb. Yasuhiro Kodera received honoraria from Taiho Pharmaceutical Co., Ltd, and research funding from Bristol‐Myers Squibb, Pfizer Japan Inc., Sanofi K.K., Yakult Honsha Co., Ltd, and Taiho Pharmaceutical Co., Ltd. Hidemi Goto received research funding from Bristol‐Myers Squibb and Taiho Pharmaceutical Co., Ltd. Yuichi Ando received honoraria from Yakult Honsha Co., Ltd and Taiho Pharmaceutical Co., Ltd, and research funding from Yakult Honsha Co., Ltd. The other authors have no conflict of interests.
Acknowledgments
We would like to thank Masahiko Ando for his expert assistance in performing the statistical analysis. This work was supported in part by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Training Program for Oncology Professionals).
Cancer Sci 107 (2016) 1453–1457
Funding Information Ministry of Education, Culture, Sports, Science and Technology of Japan.
Clinical trial registration no. UMIN000016505.
References
- 1. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy‐induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res 2014; 6: 135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park SB, Goldstein D, Krishnan AV et al Chemotherapy‐induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013; 63: 419–37. [DOI] [PubMed] [Google Scholar]
- 3. Fehrenbacher JC. Chemotherapy‐induced peripheral neuropathy. Prog Mol Biol Transl Sci 2015; 131: 471–508. [DOI] [PubMed] [Google Scholar]
- 4. Hershman DL, Lacchetti C, Dworkin RH et al Prevention and management of chemotherapy‐induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014; 32: 1941–67. [DOI] [PubMed] [Google Scholar]
- 5. Cavaletti G, Marmiroli P. Chemotherapy‐induced peripheral neurotoxicity. Nat Rev Neurol 2010; 6: 657–66. [DOI] [PubMed] [Google Scholar]
- 6. Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy‐induced peripheral neuropathy. Ann Oncol 1998; 9: 739–44. [DOI] [PubMed] [Google Scholar]
- 7. Postma TJ, Heimans JJ. Grading of chemotherapy‐induced peripheral neuropathy. Ann Oncol 2000; 11: 509–13. [DOI] [PubMed] [Google Scholar]
- 8. England JD, Gronseth GS, Franklin G et al Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005; 64: 199–207. [DOI] [PubMed] [Google Scholar]
- 9. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point‐of‐care sural nerve conduction device for identification of diabetic neuropathy. PLoS One 2014; 9: e86515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perkins BA, Orszag A, Grewal J, Ng E, Ngo M, Bril V. Multi‐site testing with a point‐of‐care nerve conduction device can be used in an algorithm to diagnose diabetic sensorimotor polyneuropathy. Diabetes Care 2008; 31: 522–4. [DOI] [PubMed] [Google Scholar]
- 11. Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point‐of‐care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care 2006; 29: 2023–7. [DOI] [PubMed] [Google Scholar]
- 12. Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point‐of‐care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol 2015; 9: 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neurometirx . Nc‐Stat Dpncheck Normative Database: Collection, Analysis and Recommended Normal Limits. 2013. [Cited 20 April 2016.] Available from URL: http://www.dpncheck.com/resources/Resources/nc-stat_dpncheck_normative_data_monograph_for_software_version_2_0_pn2203866_rev_a.pdf. [Google Scholar]
- 14. Benatar M, Wuu J, Peng L. Reference data for commonly used sensory and motor nerve conduction studies. Muscle Nerve 2009; 40: 772–94. [DOI] [PubMed] [Google Scholar]
- 15. Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve 2001; 24: 1134–41. [DOI] [PubMed] [Google Scholar]
- 16. Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropometric factors on nerve conduction measures. Muscle Nerve 1992; 15: 1095–104. [DOI] [PubMed] [Google Scholar]
- 17. Sharma S, Venkitaraman R, Vas PR, Rayman G. Assessment of chemotherapy‐induced peripheral neuropathy using the LDIFLARE technique: a novel technique to detect neural small fiber dysfunction. Brain Behav 2015; 5: e00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cavaletti G, Frigeni B, Lanzani F et al Chemotherapy‐Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer 2010; 46: 479–94. [DOI] [PubMed] [Google Scholar]
- 19. Wilbourn AJ. Sensory nerve conduction studies. J Clin Neurophysiol 1994; 11: 584–601. [DOI] [PubMed] [Google Scholar]
- 20. Velasco R, Bruna J, Briani C et al Early predictors of oxaliplatin‐induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 2014; 85: 392–8. [DOI] [PubMed] [Google Scholar]
- 21. Argyriou AA, Polychronopoulos P, Koutras A et al Peripheral neuropathy induced by administration of cisplatin‐ and paclitaxel‐based chemotherapy. Could it be predicted? Support Care Cancer 2005; 13: 647–51. [DOI] [PubMed] [Google Scholar]
- 22. Park SB, Lin CS, Krishnan AV, Friedlander ML, Lewis CR, Kiernan MC. Early, progressive, and sustained dysfunction of sensory axons underlies paclitaxel‐induced neuropathy. Muscle Nerve 2011; 43: 367–74. [DOI] [PubMed] [Google Scholar]
- 23. McHugh JC, Tryfonopoulos D, Fennelly D, Crown J, Connolly S. Electroclinical biomarkers of early peripheral neurotoxicity from oxaliplatin. Eur J Cancer Care 2012; 21: 782–9. [DOI] [PubMed] [Google Scholar]


