Abstract
System l amino acid transporter 1 (LAT1) is highly expressed in various types of human cancer, and contributes to cancer growth and survival. Recently, we have shown that LAT1 expression is closely related to the growth and aggressiveness of esophageal cancer, and is an independent marker of poor prognosis. However, it remains unclear whether LAT1 inhibition could suppress esophageal cancer growth. In this study, we investigated the tumor‐suppressive effects of the inhibition of LAT1. Both LAT1 and CD98, which covalently associates to LAT1 on the membrane, were expressed in human esophageal cancer cell lines KYSE30 and KYSE150. Quantitative PCR analysis showed that the expression of LAT1 was much higher than other subtypes of LAT. A selective inhibitor of LAT, 2‐aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH), suppressed cellular uptake of l‐14C‐leucine and cell proliferation in a dose‐dependent manner. It also suppressed phosphorylation of mammalian target of rapamycin, 4E‐BP1, and p70S6K protein, and induced cell cycle arrest at G1 phase. These results suggest that suppression of both mammalian target of rapamycin signaling and cell cycle progression is involved in BCH‐induced growth inhibition. In tumor‐bearing mice, daily treatment with BCH significantly delayed tumor growth and decreased glucose metabolism, indicating that LAT1 inhibition potentially suppresses esophageal cancer growth in vivo. Thus, our results suggest that LAT1 inhibition could be a promising molecular target for the esophageal cancer therapy.
Keywords: 2‐Aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid; esophageal cancer; system l amino acid transporter 1; molecular target; mammalian target of rapamycin
Esophageal cancer is one of the most common cancers and is highly lethal. In 2008 data reported by Jemal et al.,1 approximately 480 000 new cases and 400 000 deaths occurred worldwide. Esophageal cancer is classified in cervical, thoracic, and gastroesophageal cancer; the major type in Asia is squamous cell carcinoma (SCC). Although the curative rate is improved by novel therapeutic approaches, 5‐year survival of patients is still low.2 Early detection of esophageal cancer is difficult because patients rarely experience subjective symptoms in the early stages of the cancer. In addition, as esophageal cancer easily metastasizes, patients often have advanced or metastatic disease on admission. Patients with unresectable disease are usually treated with chemotherapy such as a combination of cisplatin (CDDP) and 5‐fluorouracil (5‐FU). However, common chemotherapeutic agents are not curative, and then the prognosis after treatment remains dismal. Therefore, it is important to establish novel molecular targets for improvement of therapeutic efficacy in esophageal cancer.
Amino acid transporters are essential for not only normal cells but also growth and survival of cancer. System l amino acid transporter 1 (LAT1) is highly expressed in various primary human cancers and tumor cell lines.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 It couples with CD98 on the plasma membrane for its functional expression, and sodium‐independently transports neutral amino acid that has large side‐chain.14 LAT1 has significant roles for cancer growth and survival through efficient supply of amino acids and activation of mammalian target of rapamycin (mTOR) signaling.3, 15 The high expression of LAT1 is correlated with various biomarkers of malignancy, such as p53, Ki‐67, and CD34, and could be an independent prognostic marker in prostate, breast, pancreatic, lung, tongue, and biliary tract cancer.7, 8, 9, 10, 11, 12, 13 Therefore, the possibility of molecular‐targeted therapy focused on LAT1 inhibition has been investigated in many cancers. A competitive inhibitor of LAT, 2‐aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH), or other inhibitors of LAT1 significantly inhibit cellular uptake of amino acids and mTOR phosphorylation, leading to cell cycle arrest and apoptosis in vitro, and delays the growth of tumor in vivo.6, 12, 16, 17, 18, 19
Recently we have found that LAT1 is closely related to the expression of CD98, growth, angiogenesis, and glycolysis in surgically resected human esophageal cancer, and expression of LAT1 could be an independent prognostic marker of esophageal cancer.20 However, the therapeutic efficacy of LAT1 inhibition against esophageal cancer remains unclear. In this study, we undertook both in vitro and in vivo studies to investigate the suppressive effects of LAT1 inhibition on the growth of esophageal cancer.
Materials and Methods
Cell culture
Human esophageal cancer cell lines, KYSE30 (JCRB0188) and KYSE150 (JCRB1095) were purchased from the Health Science Research Resources Bank (Osaka, Japan),21 and routinely maintained in DMEM (Wako Pure Chemical Industries, Osaka, Japan) containing 10% heat‐inactivated FBS (AusGeneX, Loganholme, Australia), penicillin (100 units/mL), streptomycin (100 μg/mL), and l‐glutamine (2 mM) at 37°C in 5% CO2, 95% air. HEK293‐mock and HEK293‐hLAT1 established by Khunweeraphong et al.22 were routinely maintained in Eagle's minimum essential medium (Wako Pure Chemical Industries) containing 10% heat‐inactivated FBS (AusGeneX), non‐essential amino acids (Wako Pure Chemical Industries), penicillin (100 units/mL), streptomycin (100 μg/mL), and l‐glutamine (2 mM) at 37°C in 5% CO2, 95% air.
Quantification of mRNA
Quantitative PCR (qPCR) analysis was carried out to quantify the expression of LAT1, LAT2, LAT3, and LAT4 mRNA in KYSE30 and KYSE150. Total RNA was isolated from cells using a NucleoSpin RNA II kit (Macherey‐Nagel, Düren, Germany). The first‐strand cDNA was synthesized from 0.5 μg total RNA with PrimeScript Reverse Transcriptase (Takara Bio, Shiga, Japan). Full‐length DNA of LAT1, LAT2, LAT3, and LAT4 was synthesized from cDNA of KYSE150 with KOD‐Plus‐Neo (Toyobo, Osaka, Japan) under the following conditions: 98°C for 60 s; five cycles of 98°C for 10 s, 74°C for 60 s; five cycles of 98°C for 10 s, 72°C for 60 s; five cycles of 98°C for 10 s, 70°C for 60 s; 20 cycles of 98°C for 10 s, 68°C for 60 s; and a final extension at 72°C for 10 min. After purification with High Pure PCR Product Purification Kit (Roche Applied Science, Indianapolis, IN, USA), PCR products were analyzed using agarose gel electrophoresis and single band of each full‐length DNA of LATs was detected. Concentration of the full‐length DNA of LATs was determined with BioPhotometer (Eppendorf, Hamburg, Germany). The qPCR carried out using serial dilutions of known concentrations of full‐length DNA of LATs as templates and generated standard curves. The quantity of LAT1, LAT2, LAT3, and LAT4 mRNA in KYSE30 or KYSE150 cells was determined from the standard curves. The qPCR condition was as follow: after incubating each cDNA sample with the primers (0.5 μM each) and Thunderbird SYBR qPCR Mix (Toyobo), amplification was carried out for 40 cycles (95°C for 15 s, 60°C for 30 s) with a Piko‐Real thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA). The sequences of specific primers for analysis are shown in Table S1.
Suppression of amino acid uptake into cells with LAT1 inhibition
Inhibition of amino acid transport by BCH (NARD Institute, Hyogo, Japan) was examined according to our previous report.12 Briefly, cells (1.0 × 105 cells/well) were plated in 24‐well plates and incubated in growth medium for 24 h. After the incubation, the cells were washed three times with Na+‐free HBSS. The cells were incubated in Na+‐free HBSS for 10 min at 37°C, then the supernatant was replaced by Na+‐free HBSS containing 1 μM l‐14C‐leucine (PerkinElmer Life Sciences, Boston, MA, USA), and various concentrations of BCH (1, 3, 10, 30, 100, 300, 1000, or 3000 μM). At 1 min after treatment with l‐14C‐leucine, uptake was terminated by removing the uptake solution followed by washing three times with ice‐cold Na+‐free HBSS. Cells were solubilized with 0.1 N NaOH, and radioactivity was measured by liquid scintillation spectrometry (AccuFLEX LSC‐7200; Hitachi Aloka Medical, Tokyo, Japan). The l‐14C‐leucine uptake was shown as %dose, which was calculated from the following formula: %dose = radioactivity of the solubilized cells/radioactivity of added l‐14C‐leucine × 100.
Suppression of cell proliferation with LAT1 inhibition
Cells were plated at a concentration of 1 × 103 cells/well in 96‐well plates and incubated in the growth medium for 24 h. At first, in order to determine the effect of LAT1 inhibition on esophageal cancer, cells were treated with BCH (1, 3, 5, 10, 20, 30, 40, 50, or 100 mM) and incubated for 3 days. Next, the effect of LAT1 inhibition on the antitumor activity of CDDP (Eli Lilly, Indianapolis, IN, USA) or 5‐FU (Kyowa Hakko Kirin, Shizuoka, Japan) was evaluated. Cells were incubated for 3 days with CDDP (0.3, 1, 0.5, 1, or 10 μM) or 5‐FU (1, 10, 100, or 1000 μM) in the presence or absence of 30 mM BCH. Cells were then incubated with 0.5 mg/mL MTT for 4 h at 37°C. The resulting formazan was solubilized, and the absorbance was read at 590 nm with a microtiter plate reader (V max; Molecular Devices, Sunnyvale, CA, USA).
Detection of lactate dehydrogenase release
Cells (1.0 × 104 cells/well) were incubated for 24 h in a 96‐well culture plate and treated with BCH (1, 3, 5, 10, 20, 30, 40, 50, or 100 mM) and incubated for 24 h. At the end of incubation, supernatants were collected and the lactate dehydrogenase (LDH) content was measured using a Cytotoxicity Detection Kit (Roche Applied Sciences, Laval, Canada). Lactate dehydrogenase release is expressed as a percentage of total content, which was determined by lysing an equal amount of cells with 1% Triton X‐100.
Cell cycle analysis
Cells were plated at a concentration of 2 × 105 cells in 100 mm dishes and incubated in the growth medium for 24 h. After incubation, cells were treated with growth medium or BCH (30 mM) for 24 and 48 h. After incubation, cells were harvested from the dish, washed with PBS, and fixed with 70% ethanol at −20°C for 24 h. Cells were then washed three times with PBS and stained with 1 mg/mL propidium iodide solution containing 1 mg/mL RNase. Cell cycle was analyzed using a flow cytometer (EC800; Sony, Tokyo, Japan).
Immunoblotting
Cells were dissolved in sample buffer (25% glycerin, 1% SDS, 62.5 mM Tris‐Cl, 10 mM DTT) and incubated at 65°C (LAT1) or 95°C (CD98, β‐actin, mTOR, p‐mTOR, 4E‐BP‐1, p‐4E‐BP‐1, p70S6K, and p‐p70S6K) for 15 min. Aliquots of samples containing 40 μg protein were analyzed by 10% SDS‐PAGE and transferred onto a PVDF membrane. Blots were incubated at 4°C overnight in 10 mM Tris–HCl, 100 mM NaCl, 0.1% Tween‐20, pH 7.5 (TBST), with 5% skim milk (LAT1, CD98, and β‐actin) or 1% BSA (mTOR, p‐mTOR, 4E‐BP‐1, p‐4E‐BP‐1, p70S6K, and p‐p70S6K). Blots were then incubated with antibodies at 4°C overnight. Details of antibodies are described in Table S2.23 After washing with TBST, the blots were incubated with HRP‐conjugated anti‐rabbit IgG antibody for 1.5 h at room temperature. The blots were further washed with TBST, and specific proteins were visualized by using ECL Western blotting detection reagents (GE Healthcare, Piscataway, NJ).
Antitumor effect of LAT1 inhibition in tumor‐xenograft model
The animals were cared for and treated in accordance with the guidelines of the animal care and experimentation committee at our facility. Antitumor effect of LAT1 inhibition was examined as previously described.12 Briefly, KYSE150 cells (1 × 107 cells) were s.c. inoculated into the flank of 5‐week‐old male BALB⁄c nude mice (CLEA Japan, Tokyo, Japan). After tumor volumes had reached approximately 50 mm3, the mice were divided into a control group and a treatment group (n = 10). Saline or BCH (200 mg/kg) was given i.v. once daily from the day of grouping (day 0) for 14 days to control group and treated group, respectively. Tumor volume and body weight were measured two or three times a week for 42 days. [18F]fluoro‐2‐deoxyglucose (18F‐FDG)‐PET was carried out with an animal PET scanner (Inveon; Siemens, Knoxville, TN, USA) to assess the therapeutic effect of LAT1 inhibition, as 18F‐FDG‐PET has been clinically used for therapeutic monitoring with regard to tumor metabolism. The 18F‐FDG was synthesized in our facility. Randomly selected mice were fasted for 12 h with free access to water before 18F‐FDG‐PET. 18F‐FDG (10 MBq) was given i.v. to mice followed by 10 min of data acquisition at 2 h after the administration. Mice were maintained under isoflurane anesthesia through the administration of 18F‐FDG to PET scan. For analysis of the image, a region of interest was drawn around the edge of the tumor uptake of 18F‐FDG, and standardized uptake value (SUV) was calculated. The maximum value of SUV (SUVmax) in the region of interest was compared between BCH‐treated mice and control mice.
Statistical analysis
Results are expressed as mean ± SEM. The statistical significance of differences between two groups was calculated using the unpaired Student's t‐test. The statistical significance of differences between the control and other groups was calculated using Dunnett's test. The criterion of significance was P < 0.05, as determined with GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA).
Results
Expression profile of LAT and CD98 in human esophageal cancer cell lines
The expression of LAT1 mRNA was higher than other subtypes of LAT, and the expression of LAT2 mRNA was particularly low in both cell lines (Fig. 1a). The amount of LAT1 mRNA in KYSE150 cells was approximately 26‐fold higher than that in KYSE30 cells. As shown in Figure 1(b), both LAT1 and CD98 protein were expressed in human esophageal cancer cells. Similar to mRNA expression, high expression of LAT1 protein was observed in KYSE150 cells. However, differences in CD98 expression were not observed.
Figure 1.
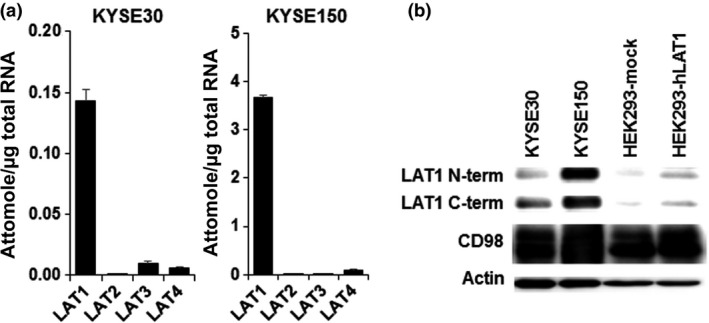
Expression of system l amino acid transporters (LATs) and CD98 in KYSE30 and KYSE150 esophageal cancer cells. (a) Expression of LAT1, LAT2, LAT3, and LAT4 mRNA quantified by quantitative PCR (n = 4). Each quantity of LAT mRNA was calibrated by total RNA. (b) Protein expression of LAT1 and CD98 in KYSE30, KYSE150, HEK293‐mock, and HEK293‐hLAT1 cells. Actin was detected as the internal control. Representative images from three independent experiments were shown. C‐term, C‐terminal; N‐term, N‐terminal.
l‐leucine uptake and cell proliferation suppressed by BCH in esophageal cancer cells
The uptake of l‐14C‐leucine, one of the substrates of LAT1,24 was inhibited by treatment with BCH in a dose‐dependent manner in both KYSE30 and KYSE150 cells (Fig. 2a). l‐14C‐leucine uptake was higher in KYSE150 than in KYSE30 cells. The difference was consistent with the difference in the expression of LAT1. Cell proliferation was also suppressed by treatment with BCH in a dose‐dependent manner (Fig. 2b). In contrast, the LDH level in culture medium was not different at 24 h after treatment with BCH, indicating that cell death was not induced by BCH treatment in esophageal cancer cells.
Figure 2.
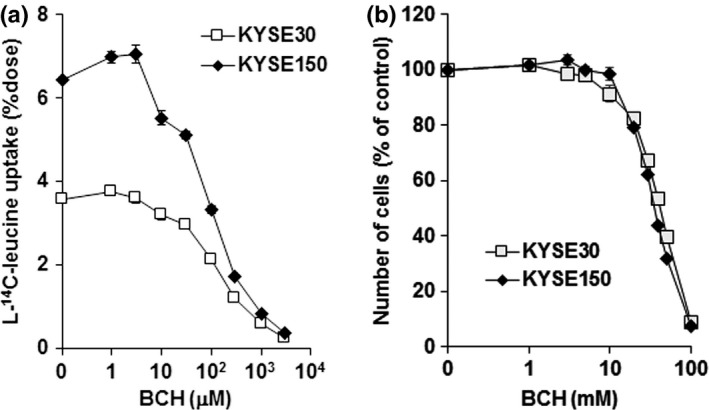
2‐Aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH) inhibits leucine uptake and cellular growth. (a) BCH inhibits l‐14C‐leucine uptake concentration‐dependently in KYSE30 and KYSE150 esophageal cancer cells (n = 4). Ordinate shows a percentage of applied dose of l‐14C‐leucine. (b) BCH inhibits the growth of KYSE30 and KYSE150 cells concentration‐dependently (n = 4). Ordinate shows number of cells in a percentage of control (without BCH).
Cell cycle arrest and suppression of mTOR signaling induced by BCH
The mechanism of BCH‐mediated growth inhibitory effect on the cell cycle and mTOR signaling were examined. The cell population in the G0/G1 phase was significantly increased at 24 and 48 h after treatment with 30 mM BCH in both KYSE30 and KYSE150 cells, indicating that BCH induced cell cycle arrest at G1 phase (Table 1). Phosphorylation of mTOR was decreased at 30 min after treatment with BCH, but restored at the control level by 24 h in both KYSE30 and KYSE150 cells (Fig. 3). Phosphorylation of 4E‐BP1 and p70S6K was decreased at 30 min and the decrease was continued for 24 h after treatment with BCH. The amount of mTOR, 4E‐BP1, and p70S6K proteins was slightly decreased.
Table 1.
Cell cycle profile in KYSE30 and KYSE150 esophageal carcinoma cells after treatment with 2‐aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH) (n = 3)
| KYSE30 | G0/G1 ratio, % | S ratio, % | G2/M ratio, % |
|---|---|---|---|
| 24 h | |||
| Control | 28.82 ± 4.12 | 32.71 ± 1.88 | 34.36 ± 1.13 |
| BCH (30 mM) | 39.90 ± 3.52* | 27.56 ± 4.48 | 28.52 ± 3.86 |
| 48 h | |||
| Control | 45.42 ± 2.00 | 25.80 ± 1.27 | 23.71 ± 0.85 |
| BCH (30 mM) | 55.18 ± 2.70** | 18.90 ± 0.21*** | 19.53 ± 1.45* |
| KYSE150 | G0/G1 ratio, % | S ratio, % | G2/M ratio, % |
| 24 h | |||
| Control | 42.21 ± 2.67 | 35.68 ± 2.00 | 23.06 ± 1.09 |
| BCH (30 mM) | 64.05 ± 1.31*** | 21.39 ± 0.84*** | 13.24 ± 0.77*** |
| 48 h | |||
| Control | 45.05 ± 0.97 | 30.30 ± 1.35 | 23.66 ± 0.63 |
| BCH (30 mM) | 62.82 ± 1.97*** | 21.62 ± 1.18** | 13.64 ± 1.79*** |
*P < 0.05, **P < 0.01, ***P < 0.001, control versus BCH (30 mM).
Figure 3.
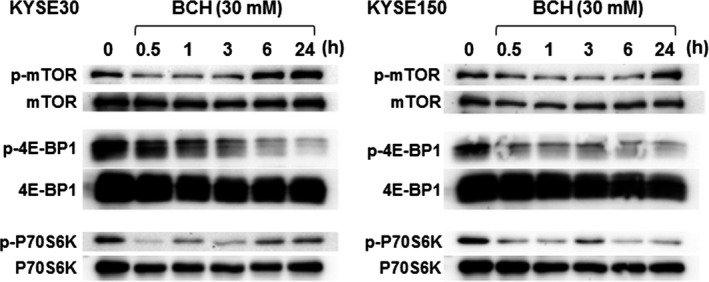
2‐Aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH) blocked the phosphorylation (p) of mammalian target of rapamycin (mTOR), p70S6K, and 4E‐BP1 in KYSE30 and KYSE150 esophageal cancer cells. Whole proteins of mTOR, p70S6K, and 4E‐BP1 were detected as control. Representatives from three independent experiments were shown.
Antitumor activity of chemotherapeutic agents enhanced by BCH
The combined effect of BCH and chemotherapeutic agents on cell proliferation was examined. In this study, 5‐FU and CDDP were used because they are standard chemotherapeutic agents for esophageal cancer. As shown in Figure 4, BCH decreased the survival fraction of both KYSE30 and KYSE150 cells treated with 5‐FU and CDDP. These results indicate that BCH additively enhanced growth inhibition of these chemotherapeutic agents.
Figure 4.
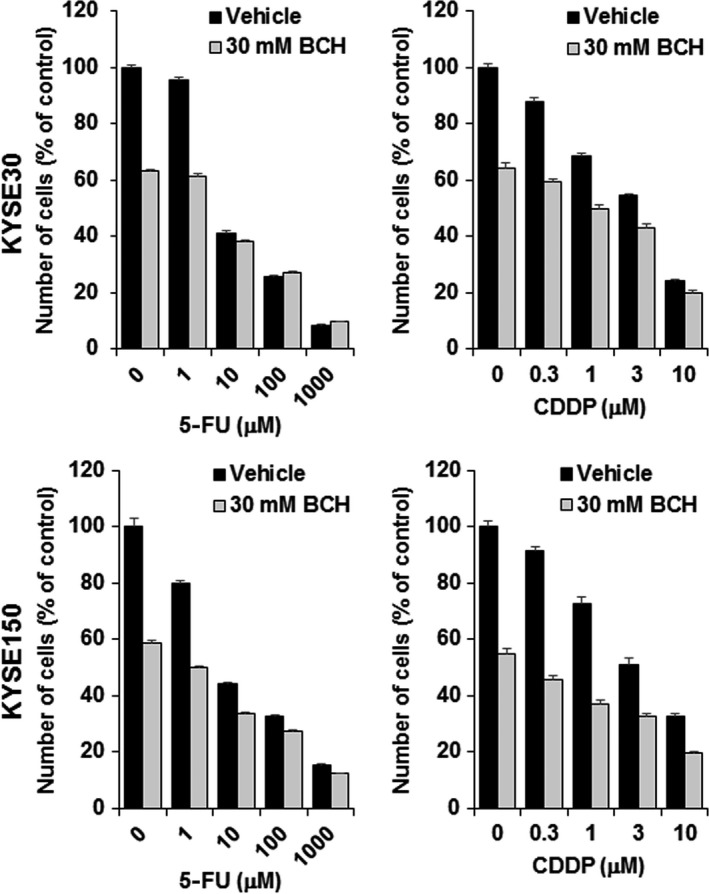
2‐Aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH) treatment enhances antitumor effect of 5‐fluorouracil (5‐FU) and cisplatin (CDDP) on KYSE30 and KYSE150 esophageal carcinoma cells. Ordinate shows number of cells in a percentage of control (n = 4).
Systemic administration of BCH suppresses tumor growth in tumor‐xenograft model
To investigate whether LAT1 inhibition could suppress tumor growth in vivo, BCH (200 mg/kg) was given i.v. to KYSE150‐bearing mice. KYSE30 cells were inoculated into nude mice in the same manner as KYSE150 cells, but KYSE30‐bearing mice could not be established because of excessive necrosis. As shown in Figure 5(a), daily treatment with BCH for 14 days significantly delayed tumor growth. Significant decrease in the body weight as an indicator of toxicity was not observed in the BCH‐treated mice (Fig. 5b). Body weight was decreased on day 2 and day 15 in both groups, which would be caused by 12 h of fasting for 18F‐FDG‐PET. No animal died in this experiment. The SUVmax on 18F‐FDG‐PET images on day 14 was increased in the control group, but decreased in the BCH‐treated group, indicating that glucose metabolism of the tumor was decreased by treatment with BCH (Fig. 5c,d).
Figure 5.
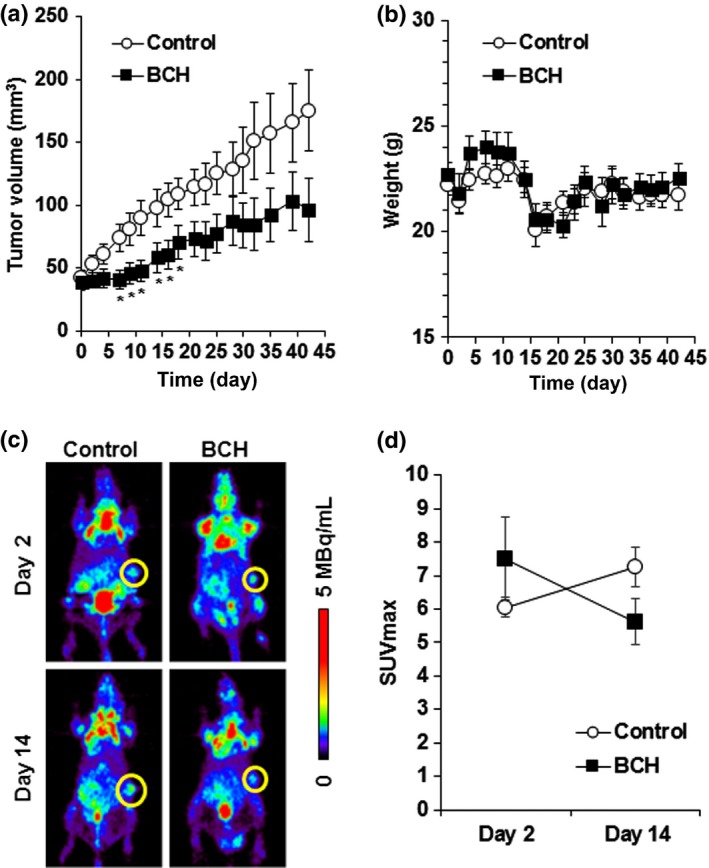
Antitumor effect of 2‐aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid (BCH) on KYSE150 tumor‐xenograft model. (a) Growth curves of KYSE150 tumor after treatment with saline or BCH (n = 10). *Statistically significant difference from control (P < 0.05). (b) Body weight of KYSE150 tumor‐bearing mice after treatment with saline or BCH (n = 10). (c) Representative coronal section of 18F‐FDG‐PET images of KYSE150‐bearing mice at 2 h after 18F‐FDG injection. PET imaging was carried out at indicated days after the day of grouping. Yellow circle shows the tumor. The calibration bar is shown to the right of images. (d) Maximum standardized uptake value (SUVmax) of 18F‐FDG‐PET images at day 2 and day 14 after the day of grouping (n = 4).
Discussion
The present study showed that LAT1 inhibition could induce antitumor effects in esophageal cancer. Selective LAT inhibitor BCH inhibited l‐14C‐leucine uptake by the KYSE30 and KYSE150 cells, and then suppressed growth of esophageal cancer. It competitively inhibits the amino acid uptake by LAT, and has been commonly used as a LAT1 inhibitor in previous reports. The activity of amino acid transport of LAT1 is higher than in other subtypes of LAT,3, 25, 26 and our results showed extremely high expression of LAT1 in both KYSE30 and KYSE150 cells. Thus, these results indicate that BCH could inhibit LAT1‐dependent amino acid uptake in these esophageal cancer cells. It is clear that LAT1 is significantly related to survival and growth of cancer through amino acid supply and signal regulation. Antitumor efficacy of LAT1 inhibition has been determined in various types of cancer.6, 12, 16, 17, 18, 19 Therefore, LAT1 is an attractive target for molecular targeting therapy.
The amounts of LAT1 protein in KYSE30 and KYSE150 cells were remarkably different, whereas the amount of CD98 protein was similar. The expression of LAT1 shown by immunoblotting was the amount of LAT1 protein in whole cell lysate. As LAT1 is expressed on the cell membrane after heterodimerization with CD98, the amounts of functional LAT1 are regulated by the expression of CD98.14 The expression of CD98 and LAT1 in KYSE cells was semiquantified by densitometry, and the ratios of each protein (KYSE150/KYSE30) were 1.68 ± 0.42 (CD98, n = 3), 5.22 ± 1.04 (LAT1 N‐terminal, n = 3), and 6.00 ± 2.30 (LAT1 C‐terminal, n = 3). The ratio of l‐14C‐leucine uptake between KYSE30 and KYSE150 cells was approximately 1.81 (KYSE150/KYSE30), which represents the ratio of functional activity of LAT1 on the cell membrane. The ratio of l‐14C‐leucine uptake was close to the ratio of CD98. These results strongly support the formation of heterodimerization of LAT1 with CD98 in esophageal cancer cells, and indicate that the amounts of LAT1 on cell membrane was close between KYSE30 and KYSE150. Therefore, remarkable differences would not be observed in growth inhibition or mTOR signaling.
The cellular nutrient condition critically affects the activity of mTOR. In amino acid‐rich conditions, rapamycin and nutrient‐sensitive complex 1 (mTORC1) is recruited to the surface of lysosomes and activated by the vacuolar H+‐ATPase‐Regulator‐Rag complex.27, 28 Activated mTORC1 phosphorylates its downstream molecules, p70S6K and 4E‐BP1, which are deeply associated with protein synthesis.15 However, amino acid deprivation inactivates mTORC1. The present study revealed that BCH suppressed phosphorylation of mTOR, 4E‐BP1, and p70S6K in esophageal cancer cells. As LAT1 mediates transport of essential amino acids, such as l‐leucine, l‐phenylalanine, and l‐tryptophan,3 LAT1 inhibition with BCH would lead to deprivation of intracellular essential amino acids, followed by suppression of mTOR signaling. Inhibition of LAT1 with BCH might be involved in the slight decrease of mTOR, 4E‐BP1, and p70S6K proteins, which would also contribute to the suppression of phosphorylation of these proteins. In amino acid‐deprived conditions, cells degrade intracellular components and maintain intracellular amino acid levels for essential cellular functions; this process is called autophagy.29 In our study, phosphorylation of these proteins had been resumed by 24 h after BCH treatment. This might be caused by recycling of amino acids induced by autophagy.30 The mTOR signal also has critical roles in cell cycle progression. It has been reported that suppression of mTOR leads to G1 arrest and both p70S6K and 4E‐BP1 independently mediate mTOR‐dependent G1 phase progression.31 Therefore, it is suggested that LAT1 inhibition with BCH suppressed mTOR signaling, followed by G1 cell cycle arrest and suppression of cell proliferation in esophageal cancer. Kim et al.16 reported that LAT1 inhibition with BCH induced apoptosis together with cell cycle arrest in oral cancer cells. In this study, BCH significantly inhibited cell growth but did not induce cell death, suggesting that LAT1 inhibition induces a cytostatic effect in esophageal cancer cells. Inhibitory effects of LAT1 inhibition would differ between the types of cancer.
Growth inhibition with conventional chemotherapeutic agents has been enhanced by LAT1 inhibition in several types of cancer.12, 19, 32, 33 In our study, BCH additively enhanced the growth‐inhibitory effect of 5‐FU and CDDP in esophageal cancer cells. Both 5‐FU and CDDP induce DNA damage, and BCH inhibits amino acids supply and mTOR signaling. Simultaneous inhibition of different growth processes would cause additive antitumor efficacy. In tumor‐bearing mice, daily injection of BCH significantly delayed tumor growth. The decrease of 18F‐FDG accumulation in tumor also supports the growth suppression induced by BCH treatment in vivo. Antitumor effects of LAT1 inhibition have been well investigated in vitro, but there have been few reports showing antitumor effects with LAT1 inhibition in vivo. Thus, our results strongly suggest that LAT1 inhibition could be a novel molecular target for esophageal cancer therapy. As the therapeutic effect of molecular‐targeting drugs, such as cetuximab and bevacizumab, to esophageal cancer have not been observed in the present clinical studies, LAT1 inhibition would have a great impact as a novel therapeutic target. Although side‐effects were not observed after BCH treatment, it would be difficult to use BCH itself as an antitumor agent in esophageal cancer therapy, because BCH is not a selective inhibitor of LAT1 and its antitumor effect was limited. Oda et al.17 developed the LAT1‐selective inhibitor, KYT‐0353, and reported its remarkable antitumor activity in colon cancer‐bearing mice. Furthermore, chemical conformation for more potent and selective inhibition to LAT1 has recently been reported.34 Selective inhibitor of LAT1, like KYT‐0353, would increase efficacy and decrease adverse events in patients with esophageal cancer.
In this study, two types of esophageal cancer cell line were used; KYSE30 is a well‐differentiated SCC, and KYSE150 is a poorly differentiated SCC.21 Among them, the expression level of LAT1 was markedly higher in KYSE150 cells. Many reports have shown that the high expression of LAT1 could be related to poor prognosis in various types of cancer.7, 8, 9, 10, 11, 12, 13 Also, the prognosis of patients with poorly differentiated cancer is generally dismal. It has been shown that the expression of LAT1 was higher in well‐differentiated cancer compared to poorly differentiated cancer.35, 36 Although the relationship between LAT1 expression and cancer differentiation is still unclear, there might be a novel clinical implication of LAT1 inhibition therapy and cancer differentiation.
In conclusion, LAT1 inhibition with BCH suppressed leucine uptake, mTOR signaling, cell cycle progression, and cell proliferation in esophageal cancer cells and significantly delayed tumor growth in tumor‐bearing mice. Furthermore, LAT1 inhibition could also enhance the antitumor effects of conventional chemotherapeutic drugs in esophageal cancer. Therefore, LAT1 could be a novel promising target for esophageal cancer therapy.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Primers for the synthesis of full‐length system l amino acid transporters (LATs) and quantitative PCR.
Table S2. Antibodies used in immunoblotting.
Acknowledgments
We are grateful to Mr. Takashi Ogasawara for operation of the biomedical cyclotron. We also would like to thank the staff of the Department of Radiation‐Applied Biology Research in National Institutes for Quantum and Radiological Science and Technology as well as the Departments of Diagnostic Radiology and Nuclear Medicine at Gunma University Graduate School of Medicine for their cooperation and helpful input. This work was supported in part by a Grant‐in‐Aid for Young Scientists (B) (16K21603) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Cancer Sci 107 (2016) 1499–1505
Funding Information
This work was supported in part by a Grant‐in‐Aid for Young Scientists (B) (16K21603) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- 3. Yanagida O, Kanai Y, Chairoungdua A et al Human L‐type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 2001; 1514: 291–302. [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi H, Ishii Y, Takayama T. Expression of L‐type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol 2005; 90: 233–8. [DOI] [PubMed] [Google Scholar]
- 5. Ichinoe M, Mikami T, Yoshida T et al High expression of L‐type amino‐acid transporter 1 (LAT1) in gastric carcinomas: comparison with non‐cancerous lesions. Pathol Int 2011; 61: 281–9. [DOI] [PubMed] [Google Scholar]
- 6. Nawashiro H, Otani N, Shinomiya N et al L‐type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer 2006; 119: 484–92. [DOI] [PubMed] [Google Scholar]
- 7. Sakata T, Ferdous G, Tsuruta T et al L‐type amino‐acid transporter 1 as a novel biomarker for high‐grade malignancy in prostate cancer. Pathol Int 2009; 59: 7–18. [DOI] [PubMed] [Google Scholar]
- 8. Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L‐type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci 2012; 103: 382–9. [DOI] [PubMed] [Google Scholar]
- 9. Kaira K, Oriuchi N, Imai H et al Prognostic significance of L‐type amino acid transporter 1 expression in resectable stage I‐III nonsmall cell lung cancer. Br J Cancer 2008; 98: 742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakanishi K, Ogata S, Matsuo H et al Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch 2007; 451: 681–90. [DOI] [PubMed] [Google Scholar]
- 11. Kaira K, Sunose Y, Arakawa K et al Prognostic significance of L‐type amino‐acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer 2012; 107: 632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaira K, Sunose Y, Ohshima Y et al Clinical significance of L‐type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer 2013; 13: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyoda M, Kaira K, Ohshima Y et al Prognostic significance of amino‐acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer 2014; 110: 2506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanai Y, Segawa H, Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 1998; 273: 23629–32. [DOI] [PubMed] [Google Scholar]
- 15. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124: 471–84. [DOI] [PubMed] [Google Scholar]
- 16. Kim CS, Cho SH, Chun HS et al BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol Pharm Bull 2008; 31: 1096–100. [DOI] [PubMed] [Google Scholar]
- 17. Oda K, Hosoda N, Endo H et al L‐type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci 2010; 101: 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim CS, Moon IS, Park JH et al Inhibition of L‐type amino acid transporter modulates the expression of cell cycle regulatory factors in KB oral cancer cells. Biol Pharm Bull 2010; 33: 1117–21. [DOI] [PubMed] [Google Scholar]
- 19. Imai H, Kaira K, Oriuchi N et al Inhibition of L‐type amino acid transporter 1 has antitumor activity in non‐small cell lung cancer. Anticancer Res 2010; 30: 4819–28. [PubMed] [Google Scholar]
- 20. Suzuki S, Kaira K, Ohshima Y et al Biological significance of fluorine‐18‐α‐methyltyrosine (FAMT) uptake on PET in patients with oesophageal cancer. Br J Cancer 2014; 110: 1985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer 1992; 69: 277–84. [DOI] [PubMed] [Google Scholar]
- 22. Khunweeraphong N, Nagamori S, Wiriyasermkul P et al Establishment of stable cell lines with high expression of heterodimers of human 4F2hc and human amino acid transporter LAT1 or LAT2 and delineation of their differential interaction with α‐alkyl moieties. J Pharmacol Sci 2012; 119: 368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morimoto E, Kanai Y, Kim do K et al Establishment and characterization of mammalian cell lines stably expressing human L‐type amino acid transporters. J Pharmacol Sci 2008; 108: 505–16. [DOI] [PubMed] [Google Scholar]
- 24. Kim DK, Kanai Y, Choi HW et al Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta 2002; 1565: 112–21. [DOI] [PubMed] [Google Scholar]
- 25. Rajan DP, Kekuda R, Huang W et al Cloning and functional characterization of a Na(+)‐independent, broad‐specific neutral amino acid transporter from mammalian intestine. Biochim Biophys Acta 2000; 1463: 6–14. [DOI] [PubMed] [Google Scholar]
- 26. Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008; 88: 249–86. [DOI] [PubMed] [Google Scholar]
- 27. Sancak Y, Bar‐Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator‐Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010; 141: 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zoncu R, Bar‐Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside‐out mechanism that requires the vacuolar H(+)‐ATPase. Science 2011; 334: 678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011; 147: 728–41. [DOI] [PubMed] [Google Scholar]
- 30. Saiki S, Sasazawa Y, Imamichi Y et al Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 2011; 7: 176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E‐BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 2004; 24: 200–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamauchi K, Sakurai H, Kimura T et al System L amino acid transporter inhibitor enhances anti‐tumor activity of cisplatin in a head and neck squamous cell carcinoma cell line. Cancer Lett 2009; 276: 95–101. [DOI] [PubMed] [Google Scholar]
- 33. Fukumoto S, Hanazono K, Fu DR et al A new treatment for human malignant melanoma targeting L‐type amino acid transporter 1 (LAT1): a pilot study in a canine model. Biochem Biophys Res Commun 2013; 439: 103–8. [DOI] [PubMed] [Google Scholar]
- 34. Geier EG, Schlessinger A, Fan H et al Structure‐based ligand discovery for the Large‐neutral Amino Acid Transporter 1, LAT‐1. Proc Natl Acad Sci USA 2013; 110: 5480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe J, Yokoyama Y, Futagami M et al L‐type amino acid transporter 1 expression increases in well‐differentiated but decreases in poorly differentiated endometrial endometrioid adenocarcinoma and shows an inverse correlation with p53 expression. Int J Gynecol Cancer 2014; 24: 659–63. [DOI] [PubMed] [Google Scholar]
- 36. Kim DK, Ahn SG, Park JC, Kanai Y, Endou H, Yoon JH. Expression of L‐type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in oral squamous cell carcinoma and its precusor lesions. Anticancer Res 2004; 24: 1671–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers for the synthesis of full‐length system l amino acid transporters (LATs) and quantitative PCR.
Table S2. Antibodies used in immunoblotting.


