Abstract
The cell‐adhesion glycoprotein PODXL is associated with an aggressive tumor phenotype in several forms of cancer. Here, we report that high PODXL expression was an independent predictor of worse overall survival of pancreatic cancer patients, and that PODXL promoted pancreatic cancer cell motility and invasion by physically binding to the cytoskeletal protein gelsolin. Suppression of PODXL or gelsolin decreased membrane protrusions with abundant peripheral actin structures, and in turn inhibited cell motility and invasion. Transfection of a PODXL‐rescue construct renewed the expression of gelsolin bound to peripheral actin structures in cell protrusions, and abrogated the decreased cell protrusions caused by the knockdown of PODXL. Furthermore, transfection of a PODXL‐rescue construct into pancreatic cancer cells in which both PODXL and gelsolin were suppressed failed to increase the formation of the protrusions. Thus, PODXL enhances motility and invasiveness through an increase in gelsolin–actin interactions in cell protrusions.
Keywords: Actin‐cytoskeleton, cell invasion, cell protrusions, pancreatic cancer, podocalyxin‐like protein
Pancreatic ductal adenocarcinoma (PDAC) is highly invasive and highly metastatic; however, the detailed mechanism by which PDAC cells invade and metastasize is still unknown. The podocalyxin homologue (also called podocalyxin‐like protein, PODXL) is a transmembrane glycoprotein with a cytoplasmic tail containing consensus phosphorylation sites for protein kinase C and casein kinase II.1 The extracellular domain is modified heavily by O‐linked glycosylation and the addition of highly charged sialic acid residues.1 PODXL is a CD34 ortholog normally expressed on hematopoietic stem cells, hemangioblasts, vascular endothelial cells, podocytes, and a subset of neural progenitors.2 Overexpression of PODXL has been found in several different forms of cancer including breast and prostate cancer, malignant brain tumors, and testicular, hepatocellular, and renal cell carcinoma.3, 4, 5, 6, 7, 8, 9, 10 PODXL expressed in ovarian cancer cells decreases its adhesivity by altering β1‐integrin levels, and PODXL expression on the cell surface is associated with poor prognosis in high‐grade serous carcinomas.11 PODXL has an important role in epithelial–mesenchymal transition, a process involved in initiating the invasive and metastatic behavior of epithelial cancer cells, by regulating and interacting with collagen type 1, E‐cadherin, and vimentin.12 PODXL interacts with ezrin, an established mediator of metastasis, in prostate cancer cells,6, 13 and induces actin recruitment and microvillus formation in breast cancer cells.14 PODXL leads to increased migration and invasion, increased MMP expression, and increased activation of phosphoinositide 3‐kinase (PI3K) in prostate and breast cancer cells.15 Thus, PODXL could play a critical role in cancer cell invasion and metastasis.
Gelsolin is an actin‐binding protein, and regulates the length of actin filaments mainly by severing and capping the fast growing (+) ends of actin filaments.16 Gelsolin release from actin filaments (uncapping) is mediated by phospholipids such as phosphatidylinositol 4,5 bisphosphate17 and lysophosphatidic acid,18 permitting actin polymerization. Gelsolin overexpression promotes tumor cell motility and invasion through modulation of several pathways, including epidermal growth factor receptor, PI3K, and Ras–PI3K–Rac1.19, 20, 21 In contrast, gelsolin suppresses epithelial–mesenchymal transition in mammary epithelial cells22 and acts as a metastasis suppressor in melanoma cells.23 The role of gelsolin differs during the course of tumor progression, and in more advanced disease it may cooperate with other oncogenic factors to accelerate progression.24
Here, we show that the overexpression of PODXL in PDAC tissue is significantly correlated with overall survival and that PODXL contributes to the formation of additional membrane protrusions through gelsolin recruitment toward filamentous actin in the protrusions, resulting in increased motility and invasiveness of PDAC cells.
Materials and Methods
Primary human PDAC samples
Patients (n = 102) who underwent surgical treatment for PDAC at the Departments of Surgery, Kochi Medical School Hospital (Nankoku, Japan) and Matsuyama Municipal Hospital (Matsuyama, Japan) between 1999 and 2014 were studied (clinicopathological findings from these 102 patients are summarized in Table S1). The follow‐up period for survivors ranged from 18 to 192 months (median, 64 months). Of these patients, 83 received adjuvant chemotherapy with gemcitabine or S‐1, or chemoradiation therapy after resection of PDAC. Tumors were classified according to the classification of pancreatic carcinoma of the Japan Pancreas Society25 and the Union for International Cancer Control (UICC) TNM classification.26 The study was approved by the ethical review board of Kochi Medical School and Matsuyama Municipal Hospital prior to patient recruitment. Informed consent was obtained from each patient.
Immunohistochemical staining
Tissue sections from normal pancreas, brain, lung, liver, and kidney were purchased from Biochain (Hayward, CA, USA). The sections were deparaffinized and autoclaved at 108°C for 15 min. After endogenous peroxidase activity was quenched by incubation for 30 min in 0.33% hydrogen peroxide diluted in methanol, the sections were incubated with FBS for blocking. Sections were then incubated with anti‐PODXL antibody at room temperature for 1 h and washed with PBS. Immunodetection was carried out with peroxidase‐labeled anti‐rabbit immunoglobulin (Dako Cytomation, Carpinteria, CA, USA). Finally, the reactants were developed with 3,3′‐diaminobenzidine (Dako), and the sections were counterstained with hematoxylin.
Evaluation of PODXL staining
The staining was evaluated by one researcher (K.T.) with two independent observers (S.N. and M.F.) who were blinded to clinical and outcome data. Immunoreactivity was scored semiquantitatively according to the estimated percentage of positive tumor cells (1, <50% reacting cells; 2, 50–80% reacting cells; 3, >80%) and intensity (1, weaker than the intensity of surface staining in the islet of Langerhans; 2, equal to the intensity of the islet of Langerhans; 3, stronger than the intensity of the islet of Langerhans). Slides on which islet of Langerhans was not significantly stained were considered to be in bad condition and were not evaluated. A total immunohistochemical score was calculated by summing the percentage score and the intensity score. The quantity of PODXL expression was classified into two groups by the total score (low group, 2–3; high group, 4–6).
Cell culture
The human PDAC cell line S2‐013, a subline of SUIT‐2, was obtained from Dr. T. Iwamura (Miyazaki Medical College, Miyazaki, Japan).27 The human PDAC cell lines PANC‐1 and BxPc‐3 were purchased from ATCC (Manassas, VA, USA). HPNE immortalized normal pancreatic epithelial cells were a kind gift from Dr. Michel Ouellette (University of Nebraska Medical Center, Omaha, NE, USA).28 All cells were grown in DMEM (Gibco‐BRL, Carlsbad, CA) supplemented with 10% heat‐inactivated FCS at 37°C in a humidified atmosphere saturated with 5% CO2.
Supplementary methods are included in Documents S1–S9.
Results
Expression of PODXL in human PDAC tissues
We examined PODXL expression in surgical specimens from 102 patients with PDAC by immunohistochemical analysis. Expression levels of PODXL were evaluable in all 102 cases, and these cases were classified into low‐expressing (70.6%, n = 72; total immunohistochemical score = 2 and 3) and high‐expressing (29.4%, n = 30; total immunohistochemical score = 4, 5, and 6) PODXL groups, as described in Materials and Methods (Table S1). PODXL localized in the cytoplasm of cell bodies (Fig. 1a); notably, some PODXL also accumulated at the cell membranes of PDAC cells (Fig. 1b). Pancreatic ducts were not obviously stained in normal pancreas, and normal brain, lung, liver, and kidney were not obviously stained with the PODXL antibody (Fig. S1).
Figure 1.
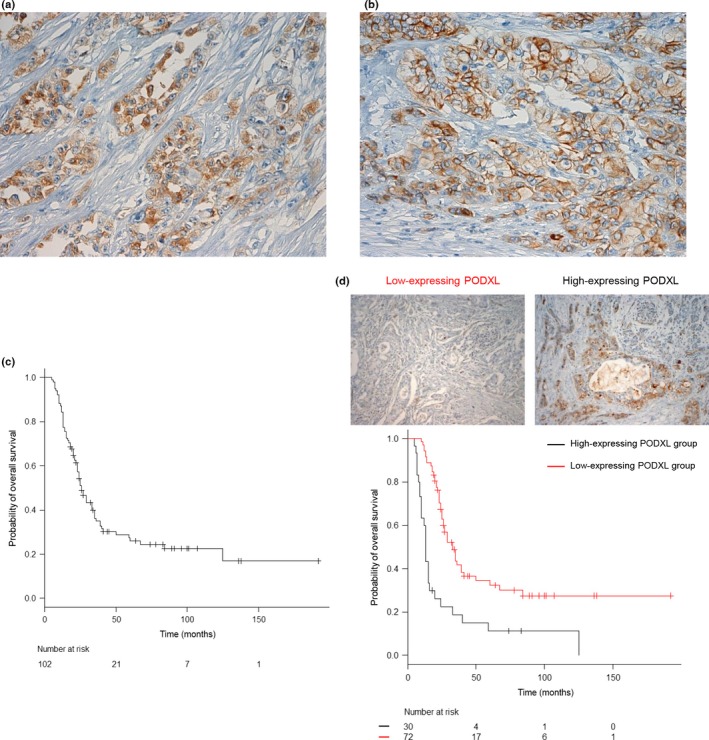
Association of high expression of podocalyxin‐like protein (PODXL) with poor outcome in patients with pancreatic ductal adenocarcinoma (PDAC). (a) Immunohistochemical staining of PDAC tissues using anti‐PODXL antibody. PODXL staining was present in the cytoplasm of tumor cells. Magnification, ×200. (b) Immunohistochemical staining of PDAC tissues using anti‐PODXL antibody. Membrane staining of PODXL was observed in tumor cells. Magnification, ×200. (c,d) Kaplan–Meier analysis of PDAC‐specific survival (c) and overall survival (d) according to PODXL expression.
Association between PODXL expression and clinicopathological characteristics and survival
We analyzed the relationship between PODXL expression and clinicopathologic features, as shown in Table 1. PODXL expression was correlated with histological grade (P = 0.048). There was no significant association between PODXL expression and other clinicopathologic features (Doc. S1).
Table 1.
Correlation between PODXL expression and clinicopathological parameters in patients with pancreatic ductal adenocarcinoma
| PODXL expression | P‐value | ||
|---|---|---|---|
| Low (%) | High (%) | ||
| Stage† | |||
| 0 | 2.7 (n = 2) | 0.0 (n = 0) | 0.710 |
| IA | 4.2 (n = 3) | 3.3 (n = 1) | |
| IB | 9.7 (n = 7) | 3.3 (n = 1) | |
| IIA | 33.3 (n = 24) | 26.7 (n = 8) | |
| IIB | 43.2 (n = 31) | 63.4 (n = 19) | |
| III | 2.7 (n = 2) | 0.0 (n = 0) | |
| IV | 4.2 (n = 3) | 3.3 (n = 1) | |
| Primary tumor† | |||
| Tis | 2.7 (n = 2) | 0.0 (n = 0) | 0.973 |
| T1 | 5.4 (n = 4) | 6.6 (n = 2) | |
| T2 | 14.1 (n = 10) | 16.7 (n = 5) | |
| T3 | 75.1 (n = 54) | 76.7 (n = 23) | |
| T4 | 2.7 (n = 2) | 0.0 (n = 0) | |
| Regional lymph nodes† | |||
| N0 | 50.0 (n = 36) | 36.7 (n = 11) | 0.277 |
| N1 | 50.0 (n = 36) | 63.3 (n = 19) | |
| Distant metastasis† | |||
| M0 | 95.8 (n = 69) | 96.7 (n = 29) | 1.000 |
| M1 | 4.2 (n = 3) | 3.3 (n = 1) | |
| Histology‡ | |||
| PanIN | 2.7 (n = 2) | 0.0 (n = 0) | 0.048 |
| Well | 33.3 (n = 24) | 20.0 (n = 6) | |
| Moderate | 59.8 (n = 43) | 60.0 (n = 18) | |
| Poor | 4.2 (n = 3) | 20.0 (n = 6) | |
| Venous invasion‡ | |||
| v0 + v1 | 90.3 (n = 65) | 76.7 (n = 23) | 0.110 |
| v2 + v3 | 9.7 (n = 7) | 23.3 (n = 7) | |
| Lymphatic invasion‡ | |||
| ly0 + ly1 | 77.8 (n = 56) | 70.0 (n = 21) | 0.452 |
| ly2 + ly3 | 22.2 (n = 16) | 30.0 (n = 9) | |
†Classified according to the International Union against Cancer. ‡Classified according to the classification of pancreatic cancer by the Japan Pancreas Society. PanIN, pancreatic intraepithelial neoplasia; Tis, carcinoma in situ.
Kaplan–Meier plots showed that there was a significant difference in overall survival rates (P < 0.001) between groups with high and low PODXL expression (Fig. 1c,d). Furthermore, univariate and multivariate analyses were used to assess the prognostic value of PODXL expression in PDAC. Stage III and IV disease (UICC) (hazard ratio [HR], 3.035; 95% confidence interval [CI], 1.301–7.081; P = 0.010) and high PODXL expression (HR, 0.345; 95% CI, 0.213–0.559; P < 0.001) were independent and significant prognostic factors for worse patient survival by univariate Cox regression analysis (Table 2). Multivariate survival analysis found that UICC stage III and IV (HR, 18.92; 95% CI, 5.085–70.42; P < 0.001) and high PODXL expression (HR, 0.296; 95% CI, 0.178–0.494; P < 0.001) proved to be independent prognostic factors for worse patient survival (Table 2). The effects of UICC stage III and IV disease and PODXL expression on overall survival were similar between univariate and multivariate analyses.
Table 2.
Univariate and multivariate analysis of prognostic factors for overall survival in patients with pancreatic ductal adenocarcinoma
| Overall survival | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Stage† | ||||
| 0 + IA + IB | 1.0 (reference) | 1.0 (reference) | ||
| IIA | 1.159 (0.714–1.881) | 0.5490 | 5.459 (1.876–15.89) | 1.847e‐03 |
| IIB | 1.356 (0.854–2.151) | 0.1960 | 4.797 (1.692–13.60) | 3.191e‐03 |
| III + IV | 3.035 (1.301–7.081) | 0.0100 | 18.92 (5.085–70.42) | 1.156e‐05 |
| Age | 1.021 (0.995–1.048) | 0.1100 | 1.020 (0.992–1.048) | 0.075 |
| Gender | 1.107 (0.696–1.761) | 0.6660 | 1.278 (0.795–2.052) | 0.451 |
| PODXL expression | 0.345 (0.213–0.559) | 0.585e‐05 | 0.296 (0.178–0.494) | 3.126e‐06 |
| Diameter of primary tumor | 1.338 (1.176–1.524) | 1.045e‐05 | ||
| Histology‡ | 2.336 (1.359–4.017) | 0.0021 | ||
| Lymphatic invasion‡ (ly0 + ly1 or ly2 + ly3) | 1.269 (0.751–2.145) | 0.3733 | ||
| Venous invasion‡ (v0 + v1 or v2 + v3) | 1.928 (1.034–3.593) | 0.0388 | ||
| Intrapancreatic nerve invasion‡ (n0 + n1 or n2 + n3) | 1.500 (0.947–2.377) | 0.0839 | ||
†Classified according to the Union for International Cancer Control. ‡Classified according to the classification of pancreatic cancer by the Japan Pancreas Society. CI, confidence interval; HR, hazard ratio.
Subcellular localization of PODXL in PDAC cells grown on fibronectin
We used immunocytochemistry to determine the subcellular localization of PODXL in a cultured PDAC cell line, S2‐013, a moderately differentiated PDAC line.27 Notably, when S2‐013 cells that are initially in suspension attach to an immobilized fibronectin substrate, nascent membrane protrusions (de novo formation of actin patches at the cell periphery) form, and as these protrusions mature, they promote cell motility.29, 30 In S2‐013 cells grown on fibronectin, PODXL was mainly present in granules in the cytoplasm of cell bodies, but some PODXL was accumulated in membrane protrusions that contained many peripheral actin structures (Fig. 2a, Docs S2,S3). Z‐stack panels showed that S2‐013 cells grown on fibronectin exhibited intracellular expression of PODXL in membrane protrusions (Fig. 2b).
Figure 2.
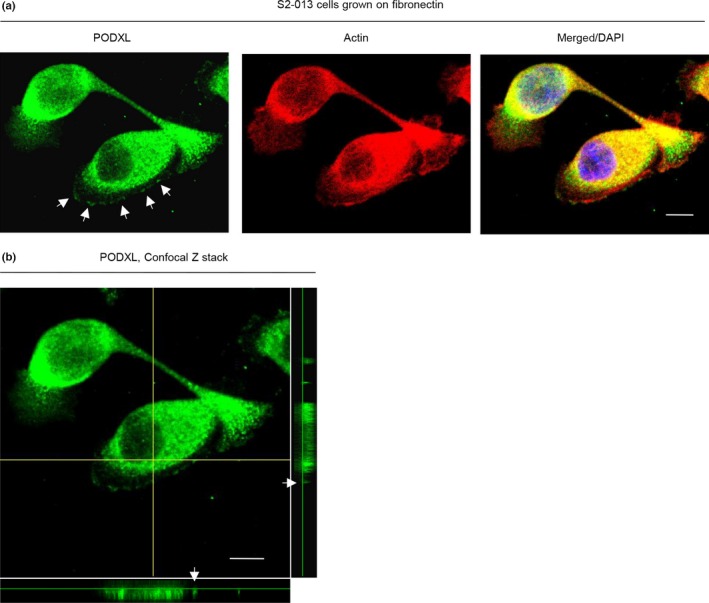
Subcellular localization of podocalyxin‐like protein (PODXL) in pancreatic ductal adenocarcinoma cells grown on fibronectin. (a) Confocal immunofluorescence microscopic images. S2‐013 cells were cultured on fibronectin and then labeled with anti‐PODXL antibody (green). Actin filaments were labeled by phalloidin (red). Arrows, PODXL localized in cell protrusions. Blue, DAPI staining. Bar = 10 μm. (b) Confocal Z stack shows abundant intracellular PODXL and the accumulation of PODXL (green) in membrane protrusions of S2‐013 cells grown on fibronectin. Arrows, PODXL localized in cell protrusions. The lower and right panels in the confocal Z stack show a vertical cross‐section (yellow lines) through the cells. Bar = 10 μm.
Effects of knockdown of PODXL on cell motility and invasion of PDAC cells
To determine whether PODXL participated in the motility and invasiveness of PDAC cells, PODXL expression in S2‐013 and PANC‐1 cells was transiently suppressed by PODXL‐specific siRNA oligonucleotides. Based on Western blot data, 48 h after transfection, expression of PODXL was markedly higher in scrambled control siRNA‐transfected S2‐013 and PANC‐1 cells than in PODXL siRNA‐transfected cells (Fig. 3a, Docs S4,S5). Suppression of PODXL in S2‐013 and PANC‐1 cells did not affect cell growth in an in vitro MTT assay (data not shown), but it did inhibit cell motility in motility assays (Fig. 3b, Doc. S6). In two‐chamber invasion assays, PODXL siRNA‐transfected cells were significantly less invasive than the control siRNA‐transfected S2‐013 and PANC‐1 cells (Fig. 3c, Doc. S7). When a PODXL‐rescue construct was transfected into PODXL siRNA‐transfected S2‐013 cells, exogenous PODXL was localized in intracellular granules both in the cytoplasm of cell bodies and in cell protrusions, similar to endogenous PODXL (Fig. 3d, Doc. S8). The transfection of a PODXL‐rescue construct abrogated the changes to cell motility and invasiveness caused by the PODXL siRNA (Fig. 3e).
Figure 3.
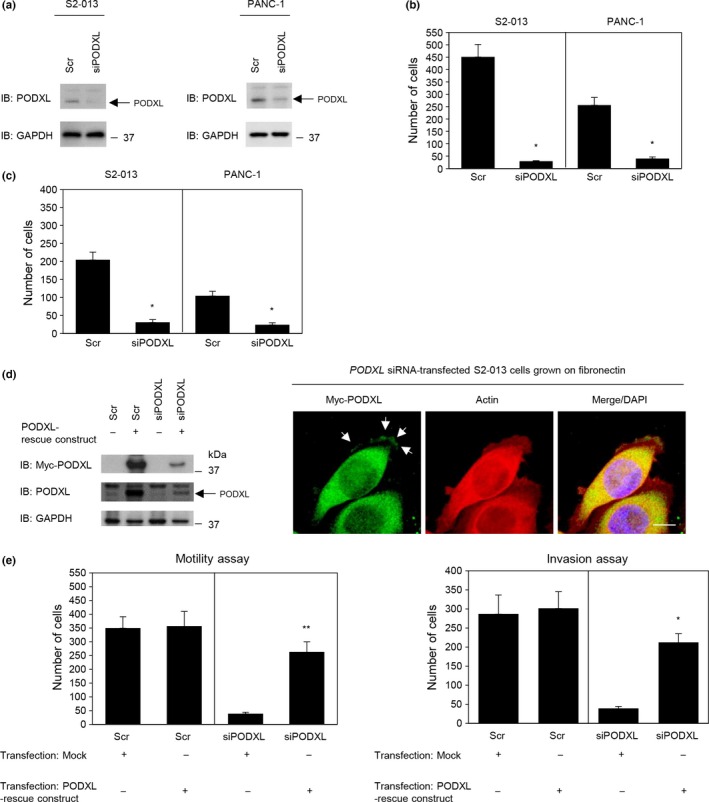
Roles of podocalyxin‐like protein (PODXL) in the motility and invasiveness of pancreatic ductal adenocarcinoma cells. (a) RNA oligonucleotides were transiently transfected into S2‐013 and PANC‐1 cells; the siRNAs targeted PODXL (siPODXL) and the negative control was a scrambled RNA (Scr). Western blotting was carried out using anti‐PODXL antibody. (b,c) Oligonucleotides targeting PODXL or Scr were transiently transfected into S2‐013 or PANC‐1 cells. Motility (b) and two‐chamber invasion assays (c) were undertaken. Migrating cells in four fields per group were scored. Data are derived from three independent experiments. Columns, mean; bars, SD. *P < 0.001 compared with Scr‐transfected control (Student's t‐test). (d) Confocal immunofluorescence microscopic images (right panels). A myc‐tagged PODXL‐rescue construct was transfected into PODXL siRNA‐transfected S2‐013 cells. Forty‐eight hours later, cells were incubated on fibronectin. Cells were stained with anti‐myc antibody (green). Actin filaments were labeled by phalloidin (red). Blue, DAPI staining. Bar = 10 μm. Western blots probed with anti‐myc and anti‐PODXL antibodies are shown in left panels. (e) Mock control vector or myc‐tagged PODXL‐rescue construct were transiently transfected into scrambled control siRNA‐transfected and PODXL siRNA‐transfected S2‐013 cells; 48 h later, motility (left panel) and two‐chamber invasion assays (right panel) were carried out. Migrating cells in four fields per group were counted. Data are derived from three independent experiments. Columns, mean; bars, SD. *P < 0.001, **P < 0.005 compared with corresponding PODXL siRNA‐transfected S2‐013 cells that were transfected with mock vector (Student's t‐test). IB, immunoblot.
Association of PODXL with gelsolin
To investigate the mechanism by which PODXL promoted cell motility and invasiveness, immunoprecipitation (IP) experiments were undertaken with lysates from S2‐013 cells grown on fibronectin; isotype control antibody or a specific anti‐PODXL antibody was used to detect multiprotein complexes that contained PODXL (Doc. S9). PODXL immunoprecipitated with anti‐PODXL, but it did not immunoprecipitate with isotype control antibody (Fig. 4a). Control and anti‐PODXL immunoprecipitates were subject to SDS‐PAGE and separated proteins were silver stained. An 80‐kDa band was evident in the anti‐PODXL sample that was not present in the isotype control sample (Fig. 4a). The band was excised, and liquid chromatography–tandem mass spectrometry was used to identify the constituent protein after in‐gel trypsin digestion; the protein was gelsolin. The peptide sequence coverage was 4% (Fig. S2). Immunoblot analysis showed that a strong gelsolin band was detected in the anti‐PODXL immunoprecipitates, but it was not detected in control immunoprecipitates from S2‐013 cells grown on fibronectin (Fig. 4b). Immunocytochemical signals from PODXL and gelsolin were present in granules in the cytoplasm of cell bodies, and a portion of PODXL and gelsolin was accumulated in cell protrusions of both S2‐013 and PANC‐1 cells grown on fibronectin (Fig. 4c).
Figure 4.
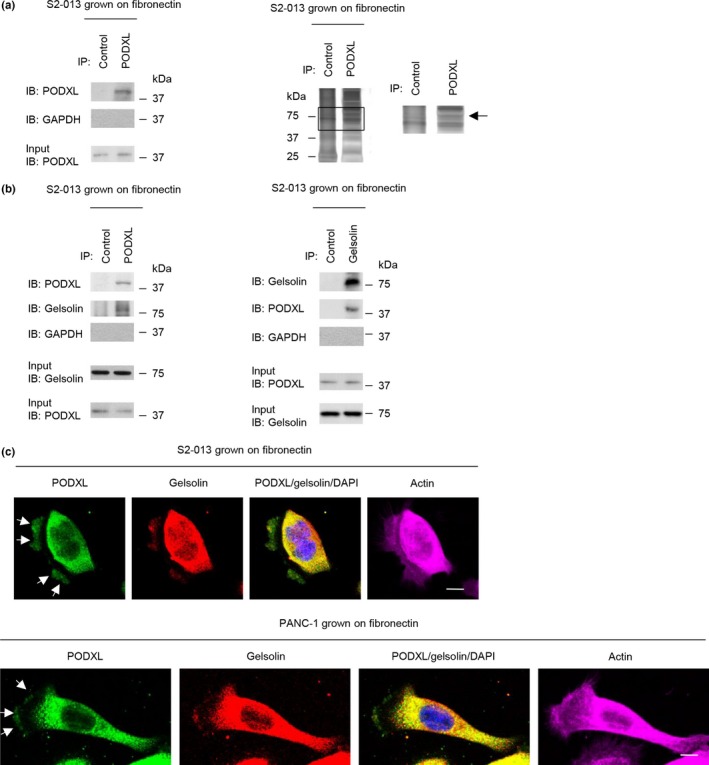
Association of podocalyxin‐like protein (PODXL) with gelsolin. (a) Immunoprecipitation of PODXL from S2‐013 pancreatic cancer cells cultured on fibronectin. Proteins within immunoprecipitates (IP) were examined on Western blots probed with anti‐PODXL antibody (left panels). Proteins in immunoprecipitates were examined by silver stain analysis (right panels). The box depicts the position of the section enlarged. Rabbit IgG isotype control antibody was used as an isotype control. Arrow indicates an 80‐kDa band. (b) Immunoprecipitation of PODXL or gelsolin from S2‐013 cells cultured on fibronectin. Proteins within immunoprecipitates were examined on Western blots probed with antibodies against PODXL and gelsolin. Rabbit IgG isotype control antibody for PODXL and mouse IgG isotype control antibody for gelsolin were used as isotype controls. (c) Confocal immunofluorescence microscopic images. S2‐013 (upper panels) and PANC‐1 (lower panels) cells were cultured on fibronectin and then labeled with anti‐PODXL (green) and anti‐gelsolin (red) antibodies. Actin filaments were labeled by phalloidin (violet). Arrows, PODXL colocalized with gelsolin in cell protrusions. Blue, DAPI staining. Bar = 10 μm. IB, immunoblot.
Roles of PODXL in colocalization of gelsolin with peripheral actin‐filaments
Gelsolin is a potent filamentous actin‐severing protein that, after filament cleavage, remains bound to the newly formed end (capping).31 Immunoprecipitation experiments were carried out with lysates from S2‐013 cells grown on fibronectin, and a specific anti‐PODXL antibody was used to detect filamentous actin in multiprotein complexes that contained PODXL. Immunoblot analysis showed a strong actin band and gelsolin band in the anti‐PODXL immunoprecipitates (Fig. 5a), indicating that actin was enriched in PODXL‐IP materials containing gelsolin. Immunocytochemistry showed that gelsolin bound to peripheral actin structures in cell protrusions was decreased in PODXL siRNA‐transfected S2‐013 cells grown on fibronectin compared to control siRNA‐transfected S2‐013 cells grown on fibronectin (Fig. 5b). Transfection of a PODXL‐rescue construct renewed the expression of gelsolin bound to peripheral actin structures in membrane protrusions of PODXL siRNA‐transfected S2‐013 cells (Fig. 5c).
Figure 5.
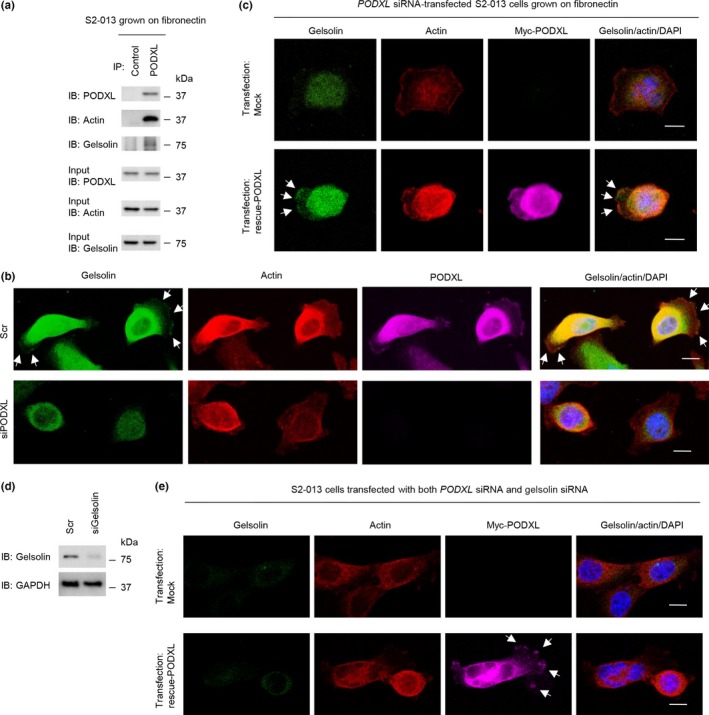
Roles of podocalyxin‐like protein (PODXL) in translocation of gelsolin to actin‐filaments in pancreatic cancer cell protrusions. (a) Immunoprecipitation of PODXL from S2‐013 cells cultured on fibronectin. Proteins within immunoprecipitates were examined on Western blots probed with antibodies against PODXL, gelsolin, and actin. Rabbit IgG isotype control antibody was used as an isotype control. (b) Confocal immunofluorescence microscopic images. Oligonucleotides (siRNAs targeting PODXL [siPODXL] or scrambled RNAs [Scr] as the negative control) were transiently transfected into S2‐013 cells. Transfected cells were incubated on fibronectin and were subsequently stained with anti‐PODXL antibody (violet), anti‐gelsolin antibody (green), and phalloidin (red). Arrows, gelsolin bound to peripheral actin structures in cell protrusions. Blue, DAPI staining. Bars = 10 μm. (c) Confocal immunofluorescence microscopic images. A myc‐tagged PODXL‐rescue construct was transfected into S2‐013 cells that had been transfected with PODXL siRNA. Forty‐eight hours later, cells were incubated on fibronectin. Cells were stained with anti‐myc antibody (violet), anti‐gelsolin antibody (green), and phalloidin (red). Arrows, exogenous PODXL localized in cell protrusions. Blue, DAPI staining. Bar = 10 μm. (d) RNA oligonucleotides were transiently transfected into S2‐013 cells; the siRNAs targeted gelsolin (siGelsolin); the negative control was a scrambled RNA (Scr). Western blot was undertaken using anti‐gelsolin antibody. (e) Confocal immunofluorescence microscopic images. A myc‐tagged PODXL‐rescue construct was transfected into S2‐013 cells that had been transfected with both PODXL siRNA and gelsolin siRNA. After 48 h, cells were incubated on fibronectin. Cells were stained with anti‐myc antibody (violet), anti‐gelsolin antibody (green), and phalloidin (red). Arrows, exogenous PODXL localized in cell protrusions. Blue, DAPI staining. Bar = 10 μm. IB, immunoblot.
Gelsolin expression in S2‐013 cells was transiently suppressed by gelsolin‐specific siRNA oligonucleotides. Based on Western blot data, 72 h after transfection, expression of gelsolin was markedly higher in scrambled control siRNA‐transfected S2‐013 cells than in gelsolin siRNA‐transfected cells (Fig. 5d). Transfection of a PODXL‐rescue construct into S2‐013 cells in which both PODXL and gelsolin had been suppressed did not increase the formation of protrusions in which peripheral actin‐filaments were assembled compared with cells without transfection of the PODXL‐rescue construct (Fig. 5e).
Roles of PODXL and gelsolin in forming cell protrusions
To determine whether PODXL participated in the induction of membrane protrusions, we analyzed peripheral actin structures in membrane ruffles of control siRNA‐transfected and PODXL siRNA‐transfected S2‐013 cells cultured on fibronectin. Peripheral actin structures in cell protrusions were less abundant in PODXL siRNA‐transfected S2‐013 cells than in control siRNA‐transfected S2‐013 cells (Fig. 6a). Similarly, suppression of gelsolin also decreased peripheral actin structures in cell protrusions compared to control siRNA transfections (Fig. 6a). Furthermore, transfection of a PODXL‐rescue construct into PODXL siRNA‐transfected S2‐013 cells reproduced cell protrusions in which peripheral actin‐filaments were assembled (Fig. 6b). In contrast, transfection of a PODXL‐rescue construct into S2‐013 cells in which both PODXL and gelsolin had been suppressed did not reproduce cell protrusions in which peripheral actin‐filaments were assembled (Fig. 6c). These results are summarized in Figure 6(d), and indicated that PODXL and gelsolin cooperatively drove rearrangement of peripheral actin to induce formation of additional membrane protrusions.
Figure 6.
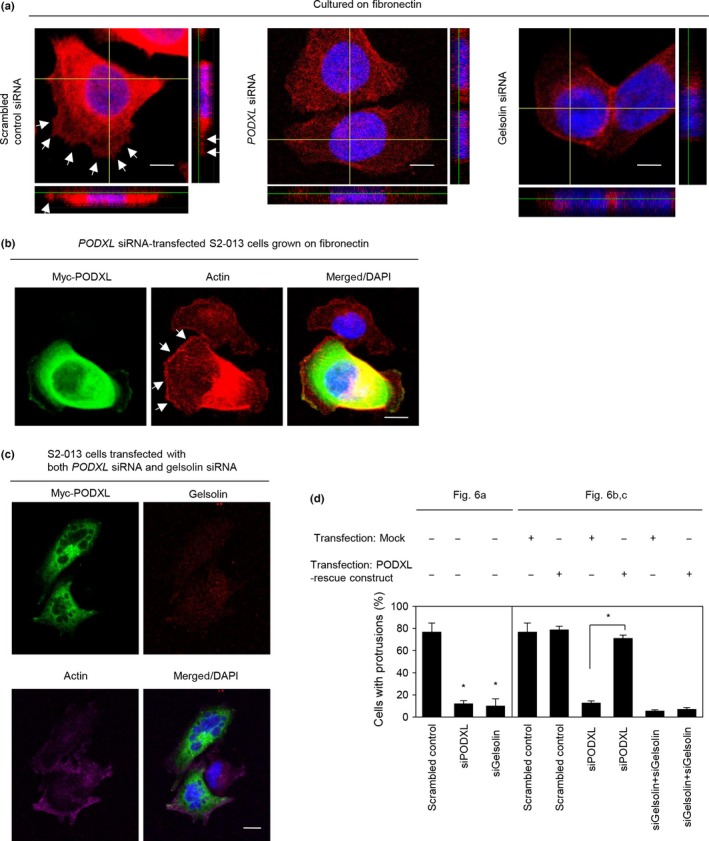
Roles of podocalyxin‐like protein (PODXL) and gelsolin in forming cell protrusions. (a) Confocal Z stack shows phalloidin‐labeled peripheral actin structures (red) and DAPI‐labeled nuclei (blue) in scrambled control siRNA‐transfected S2‐013 pancreatic ductal adenocarcinoma cells, PODXL siRNA‐transfected S2‐013 cells, or gelsolin siRNA‐transfected S2‐013 cells grown on fibronectin. Arrows, peripheral actin structures in cell protrusions. The lower and right panels in the confocal Z stack show a vertical cross‐section (yellow lines) through the cells. Bar = 10 μm. (b) Confocal immunofluorescence microscopic images. A myc‐tagged PODXL‐rescue construct was transfected into S2‐013 cells that had been transfected with PODXL siRNA. After 48 h, cells were incubated on fibronectin. Cells were stained with anti‐myc antibody (green) and phalloidin (red). Arrows, cell protrusions reproduced by exogenous PODXL in PODXL siRNA‐transfected cells. Blue, DAPI staining. Bar = 10 μm. (c) Confocal immunofluorescence microscopic images. A myc‐tagged PODXL‐rescue construct was transfected into S2‐013 cells that had been transfected with PODXL siRNA and gelsolin siRNA. After 48 h, the cells were incubated on fibronectin. The cells were stained with anti‐myc antibody (green), anti‐gelsolin antibody (red), and phalloidin (violet). Blue, DAPI staining. Bar = 10 μm. (d) Quantification of data shown in (a–c); the values represent the number of cells with fibronectin‐mediated cell protrusions in which peripheral actin structures were increased. All cells in four fields per group were scored. Data are derived from three independent experiments. Columns, mean; bars, SD. *P < 0.001 compared with corresponding PODXL siRNA‐transfected S2‐013 cells that were transfected with mock vector (Student's t‐test).
Roles of PODXL and gelsolin in cell motility and invasion of PDAC cells
Transwell motility and Matrigel invasion assays and siRNA‐mediated knockdown were used to examine the effect of gelsolin on motility and invasiveness of S2‐013 and PANC‐1 cells. In Transwell motility assays, the motility of S2‐013 and PANC‐1 cells was significantly lower in gelsolin knockdown cells than in control cells (Fig. 7a). In two‐chamber invasion assays, the invasiveness of S2‐013 and PANC‐1 cells was significantly lower in gelsolin knockdown cells than in control cells (Fig. 7b). To evaluate whether binding to gelsolin is necessary for PODXL to promote cell motility and invasion, we undertook motility and two‐chamber invasion assays in S2‐013, PANC‐1, and BxPc‐3 cell lines. Western blot analysis showed that high levels of PODXL expression were present in all of these PDAC cells when compared to normal pancreatic ductal HPNE cells (Fig. 7c). High levels of gelsolin were present in both S2‐013 and PANC‐1 cells, but only faint expression of gelsolin was seen in BxPc‐3 cells, the same level as in HPNE cells (Fig. 7c). Transfection of a PODXL‐rescue construct into S2‐013 and PANC‐1 cells in which both PODXL and gelsolin had been suppressed did not abrogate the changes to cell motility and invasiveness caused by the PODXL siRNA and gelsolin siRNA (Fig. 7d,e for S2‐013 and PANC‐1, respectively). Consistently, suppression of PODXL in BxPc‐3 cells that expressed low levels of endogenous gelsolin compared to S2‐013 and PANC‐1 cells did not significantly affect cell motility or invasion, but the transfection of a PODXL‐rescue construct abrogated the changes to cell motility and invasiveness caused by the PODXL siRNA in PANC‐1 cells (Fig. 7f). These results indicated that gelsolin was necessary for the PODXL‐associated promotion of motility and invasiveness.
Figure 7.
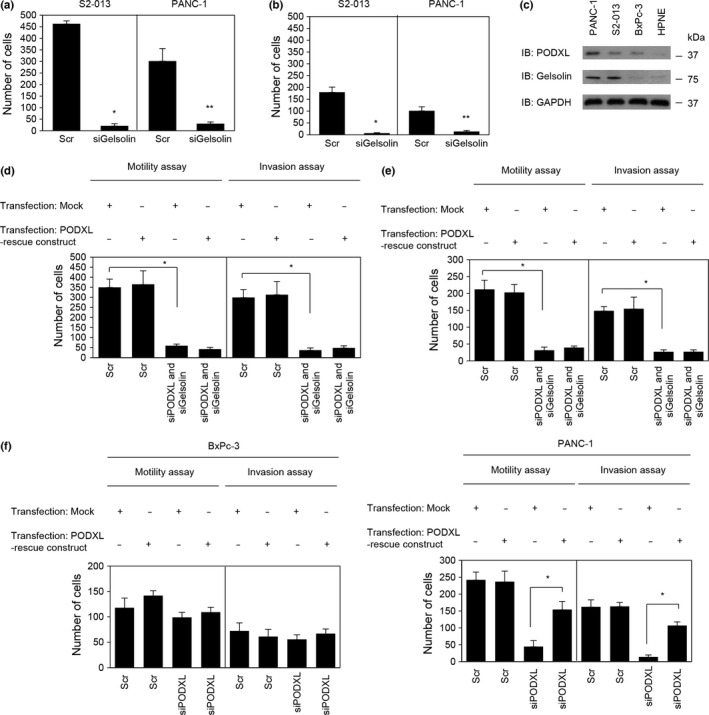
Roles of podocalyxin‐like protein (PODXL) and gelsolin in the motility and invasiveness of pancreatic ductal adenocarcinoma cells. (a,b) Oligonucleotides (siRNAs targeting gelsolin [siGelsolin] or scrambled RNAs [Scr] as the negative control) were transiently transfected into S2‐013 and PANC‐1 cells. Motility (a) and two‐chamber invasion assays (b) were carried out. Migrating cells in four fields per group were scored. Data are derived from three independent experiments. Columns, mean; bars, SD. *P < 0.004 and **P < 0.002 compared with Scr‐transfected S2‐013 cells (Student's t‐test). (c) Expression of endogenous PODXL and gelsolin in PDAC cells (PANC‐1, S2‐013, and BxPc‐3) compared with the HPNE cell line, as determined by Western blotting. (d,e) A myc‐tagged PODXL‐rescue construct was transfected into S2‐013 (d) and PANC‐1 (e) cells that had been transfected with PODXL siRNA and gelsolin siRNA; 48 h later, motility and two‐chamber invasion assays were undertaken. Migrating cells in four fields per group were counted. Data are derived from three independent experiments. Columns, mean; bars, SD. *P < 0.001 compared with corresponding PODXL siRNA and gelsolin siRNA‐transfected S2‐013 cells that were transfected with mock vector (Student's t‐test). (f) Mock control vector or myc‐tagged PODXL‐rescue construct were transiently transfected into BxPc‐3 (left panel) and PANC‐1 (right panel) cells that had been transfected with PODXL‐siRNA; 48 h later, motility and two‐chamber invasion assays were carried out. Migrating cells in four fields per group were counted. Data are derived from three independent experiments. Columns, mean; bars, SD. *P < 0.006 compared with PODXL siRNA‐transfected PANC‐1 cells that were transfected with mock vector (Student's t‐test).
Discussion
In this study, we showed that PODXL is a significant prognostic factor that predicts the overall survival of patients with PDAC. PODXL expression was correlated with histological grade, but the other individual clinicopathologic factors were not statistically significantly correlated with PODXL expression (Table 1), which may be due to the remarkably short overall survival in patients with PDAC. It is notable that patients with high PODXL expression showed significantly worse overall survival in both univariate and multivariate analyses, suggesting that it might be a novel and independent prognostic factor for PDAC (Table 2). Consistent with our results, 92.2% of PDAC specimens have been found to be positive for PODXL, and high PODXL expression associates significantly with higher risk of death from PDAC.32 High PODXL expression in PDAC associates with poor differentiation, perineural invasion, and perivascular invasion,32 but the present study showed significant association between high PODXL expression and histological grade alone. Interestingly, membranous expression of PODXL is significantly higher in the pancreatobiliary type of PDAC as with intestinal‐type periampullary adenocarcinomas, with it being an independent factor for poor prognosis in the latter.33 Moreover, we showed that PODXL was not clearly expressed in normal organs such as pancreas, brain, lung, liver, and kidney. The present study indicates that PODXL accumulated in cell protrusions of PDAC cells, as seen by immunocytochemistry. As the formation of cell protrusions is essential for cell motility and invasion, we investigated the role of PODXL in the promotion of motility and invasiveness of PDAC cells through the formation of cell protrusions. Pancreatic ductal adenocarcinoma is one of the deadliest of cancers due to its ability to extensively invade surrounding tissues and to metastasize at an early stage.34 Extensive local infiltration and metastasis are the main causes of death in PDAC.35 Consequently, PODXL is an essential marker of poor prognosis that is functionally related to cell motility and invasion through an increase in the formation of cell protrusions.
Membranous PODXL is a functional E‐ and L‐selectin ligand expressed by metastatic PDAC cells.36, 37 As E‐ and L‐selectins play a vital role in cell–cell interactions pertinent to cancer metastasis, membranous PODXL promotes metastatic spread by facilitating circulating PDAC cell binding to selectin‐expressing host cells. The cytoplasmic domain of membranous PODXL can link to the actin cytoskeleton and several intracellular proteins.38 PODXL interacts with the actin‐binding ezrin–radixin–moesin (ERM) protein ezrin through Na+/H+ exchange regulatory cofactors (NHERFs).39 Both NHERF1 and NHERF2 are scaffolding proteins that have a C‐terminal ERM‐binding domain and two tandem PDZ domains.40 Thus, the association of the cytoplasmic domain of membranous PODXL with NHERFs might lead to a wide range of downstream functions related to rearrangement of peripheral actin. It is possible that membranous PODXL contributes to metastasis of PDAC cells by interacting with P‐selectin on opposing cells and induces increased peripheral actin structures associated with motility and invasion.
Gelsolin is a cytoskeletal protein that participates in actin‐filament dynamics and promotes cell motility and plasticity.41 Gelsolin contributes to the formation of lamellipodia protrusions in migrating cells.42 Our immunocytochemical analysis showed that PODXL was necessary for the binding of gelsolin and actin‐filaments in cell protrusions of PDAC cells grown on fibronectin. The mechanism by which gelsolin is transported to actin‐filaments in cell protrusions is still unknown, but intracellular granules containing PODXL and gelsolin could function as part of the intracellular trafficking machinery for gelsolin, transporting it to cell protrusions in PDAC cells. Similar to PODXL, gelsolin knockdown also decreased cell protrusions in which peripheral actin structures were abundant and inhibited cell motility and invasion of PDAC cells. The rescued expression of PODXL increased the association of gelsolin with peripheral actin filaments in the protrusions and induced formation of the protrusions in PODXL siRNA‐transfected S2‐013 cells grown on fibronectin. Our work shows that, in addition to previous work highlighting membranous PODXL's roles in driving lamellipodia protrusion and turnover in migration,38, 42 intracellular PODXL accumulated in cell protrusions also confers motility and invasiveness through its ability to increase actin‐filaments through physically binding to the actin‐binding protein gelsolin. The molecular differences (sequence and post‐translational modifications) between intracellular PODXL and membranous PODXL are currently unknown. We investigated whether knocking down both forms (membranous PODXL and intracellular PODXL) modulated cell motility and invasion in PDAC cells. It is possible that membranous PODXL also functions to inhibit cell motility and invasion; however, the mechanism by which membranous PODXL inhibits cell motility and invasion is still unknown. As membranous PODXL was rarely expressed in migrating cancer cells (Fig. 2a), intracellular PODXL may be more important for the inhibition of cell motility and invasion than membranous PODXL. Considering the importance of PODXL localization for motility and invasion, gelsolin plays an important role in intracellular PODXL‐associated promotion of the motility and invasiveness of PDAC cells.
The data presented here indicated to us that inhibition of: (i) PODXL; (ii) binding of PODXL and gelsolin; (iii) binding of gelsolin and actin‐filaments; or (iv) some combination thereof may be effective for targeted molecular therapy, because any such therapy would inhibit the formation of cell protrusions and consequently limit the motility and invasiveness of PDAC cells.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Expression of PODXL in normal organs.
Fig. S2. Liquid chromatography–tandem mass spectrometry analysis.
Table S1. Summary of characteristics in 102 patients with pancreatic cancer.
Doc. S1. Statistical analysis.
Doc. S2. Antibodies.
Doc. S3. Confocal immunofluorescence microscopy.
Doc. S4. siRNA treatment.
Doc. S5. Immunoblot analysis of cell lysates.
Doc. S6. Transwell motility assay.
Doc. S7. Matrigel invasion assay.
Doc. S8. PODXL‐rescue construct.
Doc. S9. Immunoprecipitation and mass spectrometric analysis of PODXL.
Acknowledgments
We thank Aki Tanouchi, Hiroko Oshita, Makiko Tsuboi, and Shunichi Manabe for their excellent technical assistance. We thank Aki Tanouchi and Masahiko Sakaguchi for the statistical analysis of the data. This study was supported by a Grant‐in‐Aid for Scientific Research (KAKENHI #24591013, #15K14396) (to K.T.).
Cancer Sci 107 (2016) 1430–1442
Funding Information
Grant‐in‐Aid for Scientific Research (KAKENHI #24591013, #15K14396), Japan Society for the Promotion of Science.
References
- 1. Kershaw DB, Beck SG, Wharram BL et al Molecular cloning and characterization of human podocalyxin‐like protein: orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem 1997; 272: 15708–14. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen JS, McNagny KM. The role of podocalyxin in health and disease. J Am Soc Nephrol 2009; 20: 1669–76. [DOI] [PubMed] [Google Scholar]
- 3. Gregoire M, Schopperle WM, DeWolf WC. Distinct glycoforms of a tumor specific glycoprotein, gp200, in human testis and testicular tumors. J Urol 1995; 154: 275–7. [PubMed] [Google Scholar]
- 4. Schopperle WM, Kershaw DB, DeWolf WC. Human embryonal carcinoma tumor antigen, Gp200/GCTM‐2, is podocalyxin. Biochem Biophys Res Commun 2003; 300: 285–90. [DOI] [PubMed] [Google Scholar]
- 5. Somasiri A, Nielsen JS, Makretsov N et al Overexpression of the anti‐adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res 2004; 64: 5068–73. [DOI] [PubMed] [Google Scholar]
- 6. Casey G, Neville PJ, Liu X et al Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Hum Mol Genet 2006; 15: 735–41. [DOI] [PubMed] [Google Scholar]
- 7. Hayatsu N, Kaneko MK, Mishima K et al Podocalyxin expression in malignant astrocytic tumors. Biochem Biophys Res Commun 2008; 374: 394–8. [DOI] [PubMed] [Google Scholar]
- 8. Koch LK, Zhou H, Ellinger J, Biermann K, Höller T et al Stem cell marker expression in small cell lung carcinoma and developing lung tissue. Hum Pathol 2008; 39: 1597–605. [DOI] [PubMed] [Google Scholar]
- 9. Hsu YH, Lin WL, Hou YT et al Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol 2010; 176: 3050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung HH, Davis AJ, Lee TL et al Methylation of an intronic region regulates miR‐199a in testicular tumor malignancy. Oncogene 2011; 30: 3404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cipollone JA, Graves ML, Köbel M et al The anti‐adhesive mucin podocalyxin may help initiate the transperitoneal metastasis of high grade serous ovarian carcinoma. Clin Exp Metastasis 2012; 29: 239–52. [DOI] [PubMed] [Google Scholar]
- 12. Meng X, Ezzati P, Wilkins JA. Requirement of podocalyxin in TGFbeta induced epithelial mesenchymal transition. PLoS ONE 2011; 6: e18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmieder S, Nagai M, Orlando RA et al Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol 2004; 15: 2289–98. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen JS, Graves ML, Chelliah S et al The CD34‐related molecule podocalyxin is a potent inducer of microvillus formation. PLoS ONE 2007; 2: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sizemore S, Cicek M, Sizemore N et al Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res 2007; 67: 6183–91. [DOI] [PubMed] [Google Scholar]
- 16. Sun HQ, Yamamoto M, Mejillano M et al Gelsolin, a multifunctional actin regulatory protein. J Biol Chem 1999; 274: 33179–82. [DOI] [PubMed] [Google Scholar]
- 17. Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5‐bisphosphate. Nature 1987; 325: 362–4. [DOI] [PubMed] [Google Scholar]
- 18. Meerschaert K, De Corte V, De Ville Y et al Gelsolin and functionally similar actin‐binding proteins are regulated by lysophosphatidic acid. EMBO J 1998; 17: 5923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Corte V, Bruyneel E, Boucherie C et al Gelsolin‐induced epithelial cell invasion is dependent on Ras‐Rac signaling. EMBO J 2002; 21: 6781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen P, Murphy‐Ullrich JE, Wells A. A role for gelsolin in actuating epidermal growth factor receptor‐mediated cell motility. J Cell Biol 1996; 134: 689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lader AS, Lee JJ, Cicchetti G et al Mechanisms of gelsolin‐dependent and ‐independent EGF‐stimulated cell motility in a human lung epithelial cell line. Exp Cell Res 2005; 307: 153–63. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka H, Shirkoohi R, Nakagawa K et al siRNA gelsolin knockdown induces epithelial‐mesenchymal transition with a cadherin switch in human mammary epithelial cells. Int J Cancer 2006; 118: 1680–91. [DOI] [PubMed] [Google Scholar]
- 23. Fujita H, Okada F, Hamada J et al Gelsolin functions as a metastasis suppressor in B16‐BL6 mouse melanomacells and requirement of the carboxyl‐terminus for its effect. Int J Cancer 2001; 93: 773–80. [DOI] [PubMed] [Google Scholar]
- 24. Zhuo J, Tan EH, Yan B et al Gelsolin induces colorectal tumor cell invasion via modulation of the urokinase‐type plasminogen activator cascade. PLoS ONE 2012; 7: e43594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Japan Pancreatic Society . Classification of Pancreatic Carcinoma, 2nd English edn Tokyo: Kanehara & Co, 2003. [Google Scholar]
- 26. Sobin LH, Gospodarowicz MK, Witteknd C. TNM Classification of Malignant Tumors. 7th edn New York: Wiley‐Blackwell, 2009; 132–5. [Google Scholar]
- 27. Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT‐2) producing carcinoembryonic antigen and carbohydrate antigen 19‐9. Jpn J Cancer Res 1987; 78: 54–62. [PubMed] [Google Scholar]
- 28. Lee KM, Yasuda H, Hollingsworth MA et al Notch2‐positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest 2005; 85: 1003–12. [DOI] [PubMed] [Google Scholar]
- 29. Taniuchi K, Yokotani K, Saibara T. BART inhibits pancreatic cancer cell invasion by Rac1 inactivation through direct binding to active Rac1. Neoplasia 2012; 14: 440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taniuchi K, Furihata M, Hanazaki K et al IGF2BP3‐mediated translation in cell protrusions promotes cell invasiveness and metastasis of pancreatic cancer. Oncotarget 2014; 5: 6832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gremm D, Wegner A. Gelsolin as a calcium‐regulated actin filamentcapping protein. Eur J Biochem 2000; 267: 4339–45. [DOI] [PubMed] [Google Scholar]
- 32. Saukkonen K, Hagström J, Mustonen H et al Podocalyxin is a marker of poor prognosis in pancreatic ductal adenocarcinoma. PLoS ONE 2015; 10: e0129012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heby M, Elebro J, Nodin B et al Prognostic and predictive significance of podocalyxin‐like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin Pathol 2015; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baumgart M, Heinmöller E, Horstmann O et al The genetic basis of sporadic pancreatic cancer. Cell Oncol 2005; 27: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahrendt SA, Pitt HA. Surgical management of pancreatic cancer. Oncology 2002; 16: 725–34. [PubMed] [Google Scholar]
- 36. Dallas MR, Chen SH, Streppel MM et al Sialofucosylated podocalyxin is a functional E‐ and L‐selectin ligand expressed by metastatic pancreatic cancer cells. Am J Physiol Cell Physiol 2012; 303: C616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shea DJ, Wirtz D, Stebe KJ et al Distinct kinetic and mechanical properties govern mucin 16‐ and podocalyxin‐mediated tumor cell adhesion to E‐ and L‐selectin in shear flow. Oncotarget 2015; 6: 24842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci 2008; 121(Pt 22): 3683–92. [DOI] [PubMed] [Google Scholar]
- 39. Takeda T, McQuistan T, Orlando RA et al Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 2001; 108: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Li J, Straight SW et al PDZ domain‐mediated interaction of rabbit podocalyxin and Na(+)/H(+) exchange regulatory factor‐2. Am J Physiol Renal Physiol 2002; 282: F1129–39. [DOI] [PubMed] [Google Scholar]
- 41. Azuma T, Witke W, Stossel TP et al Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J 1998; 17: 1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chou J, Stolz DB, Burke NA et al Distribution of gelsolin and phosphoinositol 4,5‐bisphosphate in lamellipodia during EGF induced motility. Int J Biochem Cell Biol 2002; 34: 776–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of PODXL in normal organs.
Fig. S2. Liquid chromatography–tandem mass spectrometry analysis.
Table S1. Summary of characteristics in 102 patients with pancreatic cancer.
Doc. S1. Statistical analysis.
Doc. S2. Antibodies.
Doc. S3. Confocal immunofluorescence microscopy.
Doc. S4. siRNA treatment.
Doc. S5. Immunoblot analysis of cell lysates.
Doc. S6. Transwell motility assay.
Doc. S7. Matrigel invasion assay.
Doc. S8. PODXL‐rescue construct.
Doc. S9. Immunoprecipitation and mass spectrometric analysis of PODXL.


