Abstract
In order to prevent central nervous system (CNS) involvement and improve the prognosis of primary intraocular lymphoma (PIOL), we prospectively evaluated the efficacy of combined therapy using intravitreal methotrexate (MTX) and systemic high‐dose MTX on treatment‐naïve PIOL. Patients with newly diagnosed PIOL whose lymphoma was limited to the eyes were enrolled. The patients were treated with weekly intravitreal MTX until the ocular lesions were resolved, followed by five cycles of systemic high‐dose MTX (3.5 g/m2) every other week. Ten patients were enrolled in this study and completed the treatment. All patients achieved complete response for their ocular lesions with rapid decrease of intravitreal interleukin‐10 concentration. Adverse events of intravitreal and systemic high‐dose MTX were mild and tolerable. With a median follow‐up of 29.5 months, four patients (40%) experienced the CNS disease development and the mean CNS lymphoma‐free survival (CLFS) time was 51.1 months. Two‐year CLFS, which was the primary end‐point of the study, was 58.3% (95% confidence interval, 23.0–82.1%). In contrast, eight patients were treated with intravitreal MTX alone in our institute, and their 2‐year CLFS was 37.5% (95% confidence interval, 8.7–67.4%). In conclusion, systemic high‐dose MTX following intravitreal MTX is feasible and might be effective in preventing CNS involvement of PIOL. Further arrangements are worth considering in order to improve the effects. This study was registered with UMIN Clinical Trials Registry (UMIN000003921).
Keywords: Central nervous system disease, interleukin‐10, intraocular lymphoma, methotrexate, uveitis
Intraocular lymphoma (IOL) is a non‐Hodgkin's lymphoma whose initial lesions are located in the intraocular tissues: the vitreous and the retina. It is a rare form of extranodal lymphoma, accounting for less than 1% of all non‐Hodgkin's lymphomas.1 Its morphological and genetic findings show that most cases are diffuse large B‐cell lymphoma.2 Some IOL present as ocular involvement of previously diagnosed or simultaneous central nervous system (CNS) lymphoma, and others present as isolated ocular lymphoma without evidence of CNS disease, termed primary intraocular lymphoma (PIOL).
The diagnosis of PIOL is often challenging. The clinical presentations of PIOL mimic chronic uveitis and pathological confirmation is indispensable for the diagnosis of PIOL.3 However, it is hard to obtain sufficient biopsy material from the retina due to the irreversible damage to the visual acuity. Diagnosis is usually made by cytology of the vitreous fluid, but the detection rate is low (20–44.5%).4, 5 Recently, adjunctive diagnostic examinations of the vitreous fluid have been developed, including PCR analysis, flow cytometry, and cytokine profiling. A large cohort study in Japan showed the detection rate of IGH gene rearrangements by PCR was 80.6%.5 In addition, diagnosis can be supported by the interleukin (IL)‐10/IL‐6 cytokine ratio in the vitreous fluid. We previously reported that 86% of patients with an IL‐10/IL‐6 ratio >1.0 had IOL, whereas the majority of patients with benign uveitis had the ratio of <1.0.4
Despite the progression of the diagnostic procedures, the optimal treatment strategy for IOL still remains undetermined, especially for isolated PIOL without CNS involvement. Radiotherapy (RT) (30–45 Gy) alone yielded initial ocular remission in 87–100% of cases.6, 7, 8 However, the rate of local recurrence was 7–56%,7, 9 and the treatment is not repeatable due to the risk of cataract, retinopathy, or optic neuropathy.
In the late 1990s, intravitreal injection of methotrexate (MTX) was suggested and has been used as an alternative local therapy for PIOL.10 It is also efficacious in obtaining ocular remission without serious adverse events in most (91%) patients,11 and is repeatable for recurrent cases. However, despite good response to the initial therapies, the prognosis for PIOL has been generally poor, because approximately 60–85% of patients eventually develop CNS disease within 29 months.3 The high incidence of CNS progression suggests the existence of concurrent microscopic CNS involvement in PIOL at diagnosis. Thus, the optimal treatment of PIOL should be designed not only to eradicate the ocular disease but also to prevent subsequent CNS progression.
Systemic high‐dose MTX offers the possibility of preventing CNS progression of PIOL. Batchelor and colleagues undertook a prospective study in order to evaluate the efficacy of high‐dose MTX (8 g/m2/day for 11 cycles) against IOL with or without CNS involvement.12 They reported that high‐dose MTX achieved complete response (CR) in all CNS lesions (7/7). However, in terms of ocular lesions, two of nine (22%) patients had refractory disease and three of seven (42%) who had initial response experienced recurrent IOL.
These reports indicated that intravitreal MTX could not prevent CNS involvement and high‐dose MTX alone was insufficient for the treatment of ocular lesions. Hence, we hypothesized that combining intravitreal MTX with high‐dose MTX could be efficacious in both obtaining local control and preventing CNS involvement, thus improving the prognosis of PIOL. To confirm this hypothesis, we prospectively evaluated the efficacy of intravitreal MTX followed by systemic high‐dose MTX on treatment‐naive PIOL. As there has been no report of the treatment outcome of intravitreal MTX alone against PIOL in terms of CNS prophylaxis, we compared the CNS lymphoma‐free survival (CLFS) time of the patients to that of PIOL patients who did not participate in the study and were treated with intravitreal MTX therapy alone.
Patients and Methods
Diagnosis of PIOL
Primary intraocular lymphoma was defined as IOL localized to the eyes. Intraocular lymphoma was diagnosed according to a previous report2 using the following criteria: (i) typical eye involvement, a cloudy vitreous body and/or subretinal proliferative lesions; (ii) presence of lymphoma cells in the vitreous fluid cytology; and (iii) clonality of the infiltrating lymphoma cells in the vitreous fluid using either PCR analysis of IGH or T‐cell receptor gene rearrangements, or flow cytometry analysis. Patients who had (i) accompanied by either (ii) or (iii) were diagnosed with IOL. All patients underwent systemic computed tomography (CT) with or without PET, brain MRI, bone marrow biopsy, and cerebrospinal fluid (CSF) analysis to confirm that the lymphoma was localized to the eyes.
Primary intraocular lymphomas with lesions restricted to the vitreous body or retina were defined as vitreous type and retinal type, respectively. Disease with both lesions was defined as mixed type.
Flow cytometry
The infiltrating cells were isolated from the vitreous fluid and obtained for flow cytometry. We examined the surface expression of B‐cell markers (CD19 and CD20), T‐cell markers (CD3, CD4, CD5, and CD8), κ light chains, and λ light chains. We defined a monoclonal κ population as one in which the κ/λ ratio was 3:1 or greater, and monoclonal λ population as one that had a λ/κ ratio in excess of 2:1, according to the criteria suggested by Levy et al.13
Patient eligibility
Patients with any of the following were excluded: lymphoma involving extraocular lesion, age under 20 years, pregnant women, history of asthma or other allergic diathesis, and those who cannot tolerate high‐dose MTX treatment due to severe drug hypersensitivity, impaired liver, kidney, or heart function, complication of pleural effusion or ascites, or any other reason that participating physicians considered unsuitable.
Study design and procedures
This was a single‐arm prospective study. Patients were diagnosed with PIOL at Tokyo Medical and Dental University (TMDU; Tokyo, Japan) and treated at TMDU and/or Flowers and Forest Tokyo Hospital (Tokyo, Japan). They were initially treated with weekly intravitreal MTX (400 μg/100 μL) injection in the affected eyes. This treatment was repeated until the lesions were resolved. We determined the resolution of the lesion as clarification of the vitreous fluid and/or disappearance of the subretinal proliferative lesions determined by indirect ophthalmoscopy, color fundus photograph, and optical coherence tomography. According to Fishburne et al. intravitreal injection of MTX (400 μg) was first reported by de Smet et al. in 1995.10 In 2008, Frenkel et al. reported the results of the treatment.11 In the study, injections were given twice a week for 4 weeks, then weekly for 4 weeks, and finally monthly for 1 year. The researchers reported that 95% of the eyes needed 13 injections or less for the malignant cells to become absent. These findings indicated that intravitreal injection of MTX was effective for PIOL; therefore, we first treated PIOL using this protocol. However, this treatment was discontinued due to keratitis ≥ grade 2, which occurred in more than 50% of the patients (unpublished data). We reduced the treatment to weekly in the study of intravitreal MTX alone for IOL (UMIN000021943). In the present study, we followed this protocol. Ocular irradiation was acceptable if the lesions persisted after intravitreal MTX. Systemic high‐dose MTX was given following the local therapies. We used 3.5 g/m2 MTX because this dose was capable of penetrating the blood–brain barrier and yielded sustained therapeutic CSF levels lasting longer than 24 h.14 High‐dose MTX was repeated every other week for a total of five cycles. Each MTX treatment was given with adequate hydration followed by i.v. leucovorin rescue (15 mg every 6 h for 3 days) started 24 h after the completion of MTX injection. The leucovorin dose was increased to 51 mg if high serum MTX concentration was observed 24, 48, or 72 h after the initiation of MTX injections. Patients were then followed up and screened for ocular, CNS, or any other lymphoma appearance with physical examination including ophthalmoscopy at every visit and brain CT or MRI every 6 months until CNS disease development or death.
This study was carried out in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants prior to inclusion. The study protocol was approved by the institutional ethical committee of each participating institution. This study was registered with UMIN Clinical Trials Registry (UMIN000003921).
Response criteria and end‐points
Clarification of the vitreous fluid and/or disappearance of the subretinal proliferative lesions were defined as complete response of ocular lesion. Findings were determined by indirect ophthalmoscopy, color fundus photograph, and optical coherence tomography.
The primary end‐point was the 2‐year CLFS rate, defined as the rate of development of CNS lymphoma 2 years after the treatment. The CLFS time was defined as the time from diagnosis of PIOL to CNS lymphoma appearance. The secondary end‐points were safety and ocular or any other lymphoma relapse rate. Adverse events were graded using Common Terminology Criteria for Adverse Events, version 4.0.
Measurement of intravitreal cytokine concentrations
To determine the concentrations of IL‐6 or IL‐10 in the vitreous fluids, 50 μL vitreous supernatant from each patient was used for ELISA according to the manufacturer's instructions (Invitrogen, Camarillo, CA, USA). Interleukin‐10 concentration was measured serially at the time of intravitreal MTX treatment.
Primary intraocular lymphoma patients treated with intravitreal MTX alone
Intravitreal MTX for IOL was approved by the institutional review board of TMDU independently in 2007 (UMIN Clinical Trials Register: UMIN000021943). Intraocular lymphomas diagnosed by the criteria described above were treated with weekly intravitreal MTX (400 μg/100 μL) injection in the affected eyes until the lesions were resolved. We selected PIOL from the patients and analyzed their clinical courses. The data were retrospectively collected from their charts.
Statistical analysis
Central nervous system lymphoma‐free survival was estimated using the Kaplan–Meier method. The Mann–Whitney U‐test was used to determine the statistical significance between the study group and the patients treated with intravitreal MTX alone. P‐values ≤0.05 were considered statistically significant. Statistical analyses were undertaken with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R software (The R Foundation for Statistical Computing, Vienna, Austria).15
Results
Patients
Patients with newly diagnosed PIOL were subsequently recruited at TMDU between January 2007 and December 2013. Ten patients were enrolled in this study, including four men and six women, with a median age of 68.5 years. Seven patients (70.0%) had bilateral lesions at diagnosis. The median duration from the onset to the diagnosis was 8 months (Table 1).
Table 1.
Baseline characteristics of patients with primary intraocular lymphoma treated with intravitreal methotrexate (MTX) and systemic high‐dose MTX
| Intravitreal and systemic high‐dose MTX (n = 10) | |
|---|---|
| Age, years | |
| Median (range) | 68.5 (46–78) |
| Sex, n (%) | |
| Male | 4 (40) |
| Female | 6 (60) |
| Laterality, n (%) | |
| Unilateral | 3 (30) |
| Bilateral | 7 (70) |
| Initial symptoms, n (%)† | |
| Blurred vision | 8 (80) |
| Visual loss | 3 (30) |
| Floaters | 2 (20) |
| Photopsia | 1 (10) |
| Time to diagnosis, months | |
| Median (range) | 8 (4–18) |
| Disease type, n (%) | |
| Vitreous type | 7 (70) |
| Subretinal type | 0 (0) |
| Mixed type | 3 (30) |
| Cytology, n (%)‡ | |
| Positive | 7 (70) |
| Flow cytometry, n (%)‡ | |
| B‐cell monoclonality (+) | 8 (80) |
| IGH rearrangement, n (%)‡ | |
| Positive | 6 (60) |
| Cytokine analysis | |
| IL‐10, pg/mL, median (range) | 1348 (0–3544) |
| IL‐10/IL‐6 > 1, n (%)‡ | 9 (90) |
†Some patients had >1 initial symptom. ‡Ratio = number of positive cases/total number of cases examined. IGH, immunoglobulin heavy chain; IL, interleukin.
Effects of treatment
All patients enrolled in the study completed the treatment and were included in the analysis. Their outcomes are summarized in Table 2. Ocular lesions resolved soon after the intravitreal MTX injections were initiated. All patients achieved CR and no‐one received additional radiation therapy. The median number of MTX injections was five (range, 2–8). Following intravitreal MTX, systemic high‐dose MTX was initiated. During the chemotherapy, one patient was temporarily suspended from the treatment due to influenza virus infection, but the others completed the treatment as scheduled. With a median follow‐up of 29.5 months (range, 20–75 months), two patients (20%) experienced ocular relapse, but no contralateral relapse was observed. Four patients (40%) experienced CNS involvement. Median CLFS of the study group was not reached, and mean CLFS was 51.1 months. Two‐year CLFS was 58.3% (95% confidence interval, 23.0–82.1%) (Fig. 1). Three of the patients who had CNS involvement were treated with multi‐agent chemotherapy including high‐dose MTX. They obtained partial or complete remission of the CNS lesions but all of them experienced exacerbation within a few months. One patient with CNS involvement was treated with gamma knife therapy and maintained the second CR for more than 1 year.
Table 2.
Summary of clinical features and treatment outcomes of patients with primary intraocular lymphoma treated with intravitreal and systemic high‐dose methotrexate (MTX)
| No. | Age, years | Sex | Disease type | Time to diagnosis, months | Involved eye | Cytology | FCM | PCR | IL‐10/IL‐6 | Times of IV‐MTX | Ocular relapse (months) | CNS progression (months) | Outcomes | Follow‐up period, months | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravitreal and systemic high‐dose MTX | 1 | 69 | F | Mixed | 4 | R | − | − | + | >1 | 6 | − | − | Alive in CR | 75+ |
| 2 | 72 | F | Vitreous | 4 | R | + | N/A | N/A | >1 | 4 | + (14) | − | Died from unknown cause while in 2nd ocular CR | 35 | |
| L | − | + | + | >1 | 4 | ||||||||||
| 3 | 68 | M | Vitreous | 9 | R | + | N/A | N/A | >1 | 4 | − | + (22) | Died from CNS disease 9 months after initial CNS progression | 31 | |
| L | + | + | + | >1 | 4 | ||||||||||
| 4 | 53 | M | Vitreous | 5 | L | − | − | + | ≤1 | 2 | − | − | Alive in CR | 38 | |
| 5 | 72 | M | Mixed | 18 | R | + | + | − | >1 | 5 | − | − | Alive in CR | 37+ | |
| L | + | − | − | >1 | 5 | ||||||||||
| 6 | 78 | F | Mixed | 7 | R | − | + | − | >1 | 5 | − | + (19) | Lost to follow‐up in 2nd PR (CNS) with salvage HD‐MTX regimen | 21 | |
| L | + | + | − | >1 | 8 | ||||||||||
| 7 | 46 | F | Vitreous | 11 | R | + | + | − | >1 | 4 | − | − | Alive in CR | 28+ | |
| 8 | 63 | M | Vitreous | 13 | R | − | + | − | >1 | 5 | − | + (18) | Alive in 2nd PR (CNS) with salvage HD‐MTX regimen | 24+ | |
| L | N/A | N/A | N/A | N/A | 5 | ||||||||||
| 9 | 61 | F | Vitreous | 16 | R | + | + | + | >1 | 5 | + (15) | − | Alive in CR | 21+ | |
| L | + | + | + | >1 | 6 | ||||||||||
| 10 | 78 | F | Vitreous | 4 | R | + | + | + | >1 | 5 | − | + (11) | Alive in 2nd CR (CNS) with salvage radiation therapy | 20+ | |
| L | N/A | N/A | N/A | N/A | 5 |
No contralateral relapse was observed. CNS, central nervous system; CR, complete remission; F, female; FCM, flow cytometry; HD‐MTX, high‐dose methotrexate; IV‐MTX, intravitreal methotorexate; L, left; M, male; N/A, not available; PR, partial response; R, right.
Figure 1.
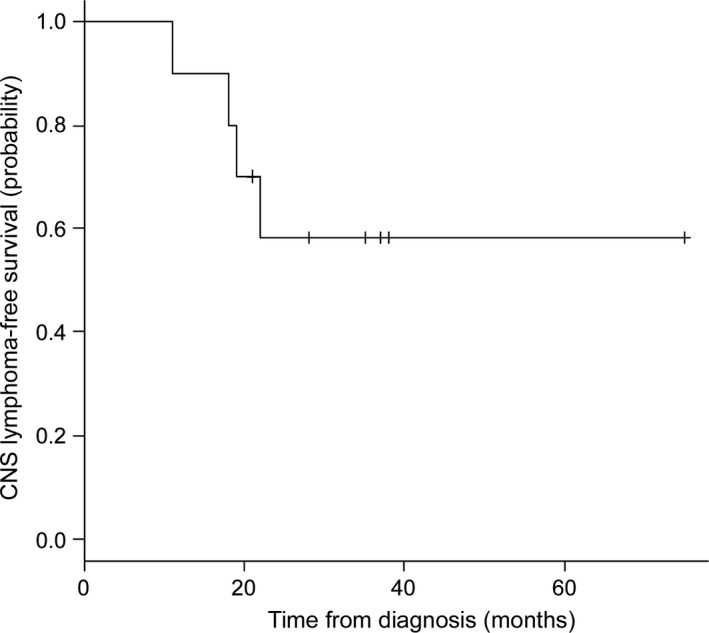
Kaplan–Meier estimates of central nervous system (CNS) lymphoma‐free survival of patients treated with intravitreal and systemic high‐dose methotrexate (n = 10).
Adverse events
The adverse events were mild and tolerable, as shown in Table 3. During treatment with intravitreal MTX injection, three patients experienced grade 2 keratitis that resolved after completion of the injection.
Table 3.
Adverse events (AEs) of intravitreal methotrexate (MTX) and systemic high‐dose MTX in patients with primary intraocular lymphoma
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| AE of intravitreal MTX | |||
| Keratitis | 0 | 3 | 0 |
| AEs of systemic high‐dose MTX | |||
| Nausea | 1 | 3 | 0 |
| Anorexia | 3 | 1 | 1 |
| Constipation | 0 | 2 | 0 |
| Hypokalemia | 1 | 0 | 0 |
| Elevated LDH | 1 | 0 | 0 |
| Elevated AST/ALT | 2 | 0 | 0 |
| Elevated serum creatinin | 3 | 0 | 0 |
| Anemia | 0 | 0 | 1 |
| Viral infection | 0 | 1 | 0 |
| Neoplasms | 0 | 0 | 1 |
ALT, alanine transaminase; AST, aspartate transaminase; LDH, lactate dehydrogenase.
Regarding systemic high‐dose MTX, severe adverse events ≥grade 3 were observed in three patients. Grade 3 anorexia occurred in case 7, requiring i.v. fluid therapy for 5 days during the first cycle of systemic high‐dose MTX. It was manageable with additional use of antiemetics from the following cycles. In case 10, grade 3 anemia occurred during systemic MTX, and it was sustained for 3 months after completion of the treatment. Neither gastrointestinal bleeding nor hematopoietic disorder was detected by systemic examination including bone marrow aspiration. The anemia resolved spontaneously without treatment. Anemia developed in case 3 2 years after the completion of systemic MTX. Local colon cancer of the transverse colon was detected, but their correlation cannot be confirmed. Curative resection by laparoscopy was carried out. Other adverse events more serious than grade 2 were not detected. Neurotoxicity such as cognitive dysfunction or leukoencephalopathy was not observed during the follow‐up except for the patients who had CNS involvement.
Interleukin‐10 and IL‐6 levels in vitreous fluid
In all but one patient, in 14 of 15 examined eyes, the IL‐10/IL‐6 ratio was greater than 1.0 at diagnosis. Median concentration of IL‐10 at diagnosis in the affected vitreous was 1348 pg/m. Interleukin‐10 decreased below the detection limit after starting intravitreal MTX in all patients (Fig. 2).
Figure 2.
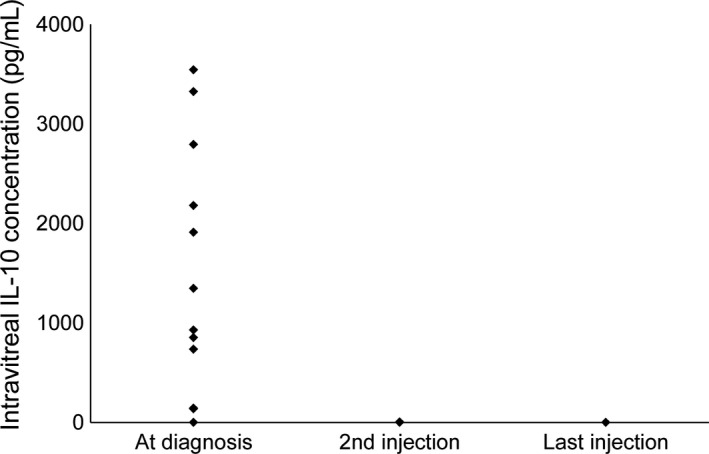
Intravitreal cytokine concentrations of interleukin‐10 (IL‐10) at the time of diagnosis of intraocular lymphoma and indicated intravitreal methotrexate injections.
Clinical course of patients treated with intravitreal MTX alone
Eight patients were treated with intravitreal MTX alone from January 2008 to April 2012. Their clinical features and treatment outcomes are summarized in Tables S1 and S2, respectively. They were treated during the same period and did not receive systemic chemotherapy because of lack of eligibility, such as renal impairment or patient refusal. The number of intravitreal MTX injections varied between cases, with the median number being 14 (range, 4–35). All patients obtained CR without receiving additional radiotherapy. Some patients were treated with intravitreal MTX maintenance therapy over subsequent years. The median follow‐up was 40 months (range, 4–71 months); two patients (25.0%) experienced ocular relapse and seven patients (87.5%) experienced CNS involvement (Table S2). Median CLFS of patients in the intravitreal MTX only group was 19 months, and the mean CLFS was 25.8 months. The 2‐year CLFS was 37.5% (95% confidence interval, 8.7–67.4%) and shorter than that of the present study, although we could not rationally compare them simply by statistical methods.
Discussion
To the best of our knowledge, this is the first prospective trial to evaluate the effectiveness of intravitreal MTX combined with systemic high‐dose MTX against PIOL without CNS involvement. Weekly intravitreal MTX injections produced complete response of the ocular lesions in all patients. Systemic high‐dose MTX was given safely without serious adverse events. The 2‐year CLFS was 58.3%, whereas that of the patients treated with intravitreal MTX alone was 37.5%. We could not simply compare the CLFS of the present study with that of the patients treated with intravitreal MTX alone, because their backgrounds, including the median ages, were different. However, there was a trend for patients who received intravitreal and systemic high‐dose MTX to have a longer CLFS than those treated with intravitreal MTX alone.
There has been no report of large, prospective, randomized controlled trials addressing the treatment strategy for PIOL, which is difficult to undertake due to the disease rarity. The International Primary Central Nervous System Lymphoma Collaborative Group retrospectively reviewed 83 PIOL patients without CNS involvement (11% had positive CSF cytology) from 16 institutions.16 According to the report, there was no difference in CNS relapse or survival between local therapy and extensive therapy including systemic chemotherapy and/or whole brain RT. Riemens et al.17 recently reported an European multicenter retrospective cohort study of 78 PIOL patients without CNS involvement. No difference in CNS relapse or 5‐year survival was observed between local, systemic, and combined therapy groups in this study as well. These two relatively large series suggested that local therapy was reasonable to control PIOL with minimal toxicity. However, their retrospective nature limits their ability to draw a definitive conclusion. In addition, these multicenter studies included heterogeneous treatment regimens. In both studies, “systemic chemotherapy” included not only high‐dose MTX but also other regimens that would not penetrate the blood–brain barrier. This might lead to the underestimation of the efficacy of high‐dose MTX in terms of CNS prophylaxis. Our study, despite being relatively small in size, overcomes these drawbacks by its prospective design with uniform diagnostic criteria and a single treatment protocol.
Recently, Hashida et al.18 retrospectively studied 26 PIOL patients treated with various regimens including high‐dose MTX, intrathecal MTX, and the local therapy. Although the difference in CNS relapse rate was not observed, time to developing CNS involvement was significantly prolonged with high‐dose MTX compared to local therapy (48.0 vs 10.2 months). Several other case reports supported the efficacy of systemic chemotherapies including high‐dose MTX on PIOL with good therapeutic outcomes. Stefanovic et al.19 reported a prospectively followed data of PIOL patients treated with a uniform therapy including high‐dose MTX and ocular RT. Five out of six patients maintained CR for a median of 40 months without significant adverse event. Taoka et al.20 treated five PIOL patients with a regimen including high‐dose MTX, whole brain RT, and ocular RT. With a median follow‐up of 32 months, all patients were alive without relapse. In addition to these reports, our study provides good evidence for the effectiveness of CNS prophylaxis with systemic high‐dose MTX against PIOL.
Another noteworthy finding in our study is that intravitreal IL‐10 concentrations rapidly decreased along with improvement of the ocular lesions. Interleukin‐10 became undetectable within one or two intravitreal MTX injections. There have been few case series that monitored the response in IL‐10 concentrations to intravitreal chemotherapies.21, 22 Along with those reports, our study confirmed that the detection of IL‐10 could reflect the status of the disease in the vitreous. We previously reported the correlation between gain of the IL‐10 gene and intravitreal IL‐10 concentration.2 These findings indicated that IL‐10 was secreted to the vitreous from the infiltrating lymphoma cells.
The present study, however, has several points that demand further consideration. Ocular relapse was detected in 20% and 25% of the study group and the control group, respectively. Frenkel and colleagues reported that no ocular relapse was detected in patients who received intravitreal MTX twice a week.11 The rate of the local relapse might be high in the present study under the weekly MTX regimen. The optimal administration should be evaluated in the future. Small case series have demonstrated the activity of intravitreal rituximab treatment for PIOL.22, 23 Although CD20 is actually difficult to detect on PIOL tumor cells, this strategy is worth investigating. Even with systemic high‐dose MTX, four out of 10 patients developed CNS involvement within 2 years in the present study. However, five patients maintained complete remission for more than 2 years. Our results suggest that PIOL is a heterogeneous disease, with some patients carrying high risk for early CNS progression, and others having a favorable prognosis without CNS involvement for a long period of time. Due to the small sample size, we could not determine the clinical difference between relapsed and non‐relapsed cases. To elucidate predictors of early CNS progression, further analysis with larger numbers of patients is necessary. Also, we are planning to undertake a genetic study, such as expression profiling and cytokine array of the vitreous fluid, to determine the risk factors of CNS progression and to understand the molecular pathogenesis of the disease. In addition, novel approaches are required to improve the outcomes of patients with high risk for CNS progression. In order to intensify the treatment, concurrent intravitreal and systemic MTX could be suggested. We previously treated three patients with IOL and CNS involvement using simultaneous intravitreal and systemic MTX.24 The treatment achieved CR in all of them, with one grade 3 adverse event, leukocytopenia, in one patient. This treatment strategy needs to be further evaluated.
As seen in the local therapy group of this study, a number of patients were considered unsuitable to receive high‐dose MTX because of their age or organ impairments, which resulted in an extremely high incidence of CNS progression. Some sort of CNS prophylaxis is necessary for these comorbid patients as well. Reduced dose of systemic MTX might be one of the options that should be evaluated.
In conclusion, systemic high‐dose MTX following intravitreal MTX is feasible and might be effective in preventing CNS involvement of PIOL. Some modification, however, is required to improve the results of the strategy.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Baseline characteristics of patients with intraocular lymphoma treated with intravitreal methotrexate alone.
Table S2. Summary of clinical features and treatment outcomes of patients with intraocular lymphoma treated with intravitreal methotrexate alone.
Acknowledgments
This study was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15K09468).
Cancer Sci 107 (2016) 1458–1464
Funding Information
Ministry of Education, Culture, Sports, Science, and Technology of Japan (15K09468).
References
- 1. Bardenstein DS. Intraocular lymphoma. Cancer Control 1998; 5: 317–25. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Sato‐Otsubo A, Sugita S et al High‐resolution genomic copy number profiling of primary intraocular lymphoma by single nucleotide polymorphism microarrays. Cancer Sci 2014; 105: 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol 2004; 242: 901–13. [DOI] [PubMed] [Google Scholar]
- 4. Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol 2009; 53: 209–14. [DOI] [PubMed] [Google Scholar]
- 5. Kimura K, Usui Y, Goto H, Group JILS . Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol 2012; 56: 383–9. [DOI] [PubMed] [Google Scholar]
- 6. Margolis L, Fraser R, Lichter A, Char DH. The role of radiation therapy in the management of ocular reticulum cell sarcoma. Cancer 1980; 45: 688–92. [DOI] [PubMed] [Google Scholar]
- 7. Isobe K, Ejima Y, Tokumaru S et al Treatment of primary intraocular lymphoma with radiation therapy: a multi‐institutional survey in Japan. Leuk Lymphoma 2006; 47: 1800–5. [DOI] [PubMed] [Google Scholar]
- 8. Berenbom A, Davila RM, Lin HS, Harbour JW. Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye (Lond) 2007; 21: 1198–201. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman PM, McKelvie P, Hall AJ, Stawell RJ, Santamaria JD. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye (Lond) 2003; 17: 513–21. [DOI] [PubMed] [Google Scholar]
- 10. Fishburne BC, Wilson DJ, Rosenbaum JT, Neuwelt EA. Intravitreal methotrexate as an adjunctive treatment of intraocular lymphoma. Arch Ophthalmol 1997; 115: 1152–6. [DOI] [PubMed] [Google Scholar]
- 11. Frenkel S, Hendler K, Siegal T, Shalom E, Pe'er J. Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol 2008; 92: 383–8. [DOI] [PubMed] [Google Scholar]
- 12. Batchelor TT, Kolak G, Ciordia R, Foster CS, Henson JW. High‐dose methotrexate for intraocular lymphoma. Clin Cancer Res 2003; 9: 711–5. [PubMed] [Google Scholar]
- 13. Levy N, Nelson J, Meyer P et al Reactive Lymphoid Hyperplasia with Single Class (Monoclonal) Surface Immunoglobulin. Am J Clin Pathol 1983; 80: 300‐8. [DOI] [PubMed] [Google Scholar]
- 14. Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol 2000; 18: 3144–50. [DOI] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimm SA, Pulido JS, Jahnke K et al Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol 2007; 18: 1851–5. [DOI] [PubMed] [Google Scholar]
- 17. Riemens A, Bromberg J, Touitou V et al Treatment strategies in primary vitreoretinal lymphoma: a 17‐center European collaborative study. JAMA Ophthalmol 2015; 133: 191–7. [DOI] [PubMed] [Google Scholar]
- 18. Hashida N, Nakai K, Saitoh N, Nishida K. Association between ocular findings and preventive therapy with onset of central nervous system involvement in patients with primary vitreoretinal lymphoma. Graefes Arch Clin Exp Ophthalmol 2014; 252: 687–93. [DOI] [PubMed] [Google Scholar]
- 19. Stefanovic A, Davis J, Murray T, Markoe A, Lossos IS. Treatment of isolated primary intraocular lymphoma with high‐dose methotrexate‐based chemotherapy and binocular radiation therapy: a single‐institution experience. Br J Haematol 2010; 151: 103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taoka K, Yamamoto G, Kaburaki T, Takahashi T, Araie M, Kurokawa M. Treatment of primary intraocular lymphoma with rituximab, high dose methotrexate, procarbazine, and vincristine chemotherapy, reduced whole‐brain radiotherapy, and local ocular therapy. Br J Haematol 2012; 157: 252–4. [DOI] [PubMed] [Google Scholar]
- 21. Sou R, Ohguro N, Maeda T, Saishin Y, Tano Y. Treatment of primary intraocular lymphoma with intravitreal methotrexate. Jpn J Ophthalmol 2008; 52: 167–74. [DOI] [PubMed] [Google Scholar]
- 22. Raja H, Snyder MR, Johnston PB et al Effect of intravitreal methotrexate and rituximab on interleukin‐10 levels in aqueous humor of treated eyes with vitreoretinal lymphoma. PLoS One 2013; 8: e65627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Itty S, Pulido JS. Rituximab for intraocular lymphoma. Retina 2009; 29: 129–32. [DOI] [PubMed] [Google Scholar]
- 24. Nakauchi Y, Takase H, Sugita S et al Concurrent administration of intravenous systemic and intravitreal methotrexate for intraocular lymphoma with central nervous system involvement. Int J Hematol 2010; 92: 179–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of patients with intraocular lymphoma treated with intravitreal methotrexate alone.
Table S2. Summary of clinical features and treatment outcomes of patients with intraocular lymphoma treated with intravitreal methotrexate alone.


