Abstract
YAP1, the main Hippo pathway effector, is a potent oncogene and is overexpressed in non‐small‐cell lung cancer (NSCLC); however, the YAP1 expression pattern in small‐cell lung cancer (SCLC) has not yet been elucidated in detail. We report that the loss of YAP1 is a special feature of high‐grade neuroendocrine lung tumors. A hierarchical cluster analysis of 15 high‐grade neuroendocrine tumor cell lines containing 14 SCLC cell lines that depended on the genes of Hippo pathway molecules and neuroendocrine markers clearly classified these lines into two groups: the YAP1‐negative and neuroendocrine marker‐positive group (n = 11), and the YAP1‐positive and neuroendocrine marker‐negative group (n = 4). Among the 41 NSCLC cell lines examined, the loss of YAP1 was only observed in one cell line showing the strong expression of neuroendocrine markers. Immunostaining for YAP1, using the sections of 189 NSCLC, 41 SCLC, and 30 large cell neuroendocrine carcinoma (LCNEC) cases, revealed that the loss of YAP1 was common in SCLC (40/41, 98%) and LCNEC (18/30, 60%), but was rare in NSCLC (6/189, 3%). Among the SCLC and LCNEC cases tested, the loss of YAP1 correlated with the expression of neuroendocrine markers, and a survival analysis revealed that YAP1‐negative cases were more chemosensitive than YAP1‐positive cases. Chemosensitivity test for cisplatin using YAP1‐positive/YAP1‐negative SCLC cell lines also showed compatible results. YAP1‐sh‐mediated knockdown induced the neuroendocrine marker RAB3a, which suggested the possible involvement of YAP1 in the regulation of neuroendocrine differentiation. Thus, we showed that the loss of YAP1 has potential as a clinical marker for predicting neuroendocrine features and chemosensitivity.
Keywords: Chemosensitivity, Hippo pathway, neuroendocrine differentiation, small‐cell lung cancer, YAP1
In the 2015 WHO classification, SCLC and LCNEC have been categorized together as high‐grade neuroendocrine tumors,1 which are highly aggressive tumors with a poor prognosis. Although high‐grade neuroendocrine tumors respond to platinum‐based chemotherapy,2, 3, 4, 5, 6, 7 a cure is sometimes difficult to achieve because these tumors are likely to be widely disseminated by the time of diagnosis. In spite of the many challenges associated with identifying potential targeted therapies, no targeted agents had been approved for use in the treatment of SCLC and LCNEC cases until very recently. Therefore, the mechanisms underlying carcinogenesis in high‐grade neuroendocrine tumors need to be elucidated in more detail, and improvements in the therapies for these tumors are desired.
It has become increasingly apparent that abnormalities in the upstream and downstream members of the Hippo pathway play important roles in the tumorigenesis of various human cancers.8 The Hippo pathway has been implicated in the cell contact inhibition of proliferation as well as organ size control.9 As the main downstream effector of the Hippo pathway, YAP1 promotes cell growth as a transcription cofactor and may be inactivated through its cytoplasmic retention and phosphorylation by LATS1/2.10, 11 The YAP1 gene was previously reported to be amplified and overexpressed in several tumor types.12, 13, 14, 15, 16 The overexpression of YAP1 has also frequently been observed in NSCLC, and is a poor prognostic factor.14 Few studies have focused on YAP1 in SCLC; Wu et al.'s17 study identified some single nucleotide polymorphisms within the promoter region of YAP1 that were associated with the survival of SCLC patients, and Nishikawa et al.18 reported the inhibition of YAP1 by ASCL1 through the activation of mir375. These findings suggest the importance of YAP1 in high‐grade neuroendocrine tumors; however, its role in neuroendocrine differentiation currently remains unknown, and an immunohistochemical analysis of YAP1 using SCLC and LCNEC tissue sections has not yet been undertaken.
In the present study, we report the potential of the loss of YAP1 as a clinical marker to predict neuroendocrine features and chemosensitivity. As far as we know, this study is the first to reveal the roles of YAP1 not only in cisplatin resistance but also in determination of neuroendocrine features of high‐grade neuroendocrine tumors.
Materials and Methods
Cell lines and medium
Fourteen SCLC cell lines (SBC3, SBC5, LCMA, Lu135, N417, H2081, H146, Lu139, Lu130, H69, H446, H526, H889, and H510A), seven adenocarcinoma cell lines (A549, ABC1, LC‐2/ad, VMRC‐LCD, H292, H441, and H1651), one adenosquamous cell carcinoma cell line (H596), and one large‐cell carcinoma (H460) were used for Western blot and/or gene expression analyses per mRNA‐Seq. H460, derived as a large‐cell carcinoma, has been regarded as a LCNEC cell line in some studies.19, 20 The 14 SCLC cell lines and H460 cell line are described as 15 high‐grade neuroendocrine tumor cell lines in the present study for convenience. All cell lines were maintained in RPMI‐1640 supplemented with 10% FCS, glutamine, and antibiotics in a humidified atmosphere with 5% CO2 and 95% air. The sources and histological types of these cell lines are detailed in Table 1.
Table 1.
Histological types and sources of 24 lung carcinomas
| Cell line | Histological type | Cell type | Source |
|---|---|---|---|
| H69 | Small‐cell lung cancer | Floating | ATCC (Manassas, VA, USA) |
| H146 | Small‐cell lung cancer | Floating | ATCC |
| H510A | Small‐cell lung cancer | Floating | ATCC |
| H889 | Small‐cell lung cancer | Floating | ATCC |
| N417 | Small‐cell lung cancer | Floating | ATCC |
| H2081 | Small‐cell lung cancer | Floating | ATCC |
| H446 | Small‐cell lung cancer | Floating | ATCC |
| H526 | Small‐cell lung cancer | Floating | ATCC |
| Lu130 | Small‐cell lung cancer | Floating | Japanese Cancer Research Resources Bank (Osaka, Japan) |
| Lu135 | Small‐cell lung cancer | Floating | Japanese Cancer Research Resources Bank |
| Lu139 | Small‐cell lung cancer | Floating | Japanese Cancer Research Resources Bank |
| SBC3 | Small‐cell lung cancer | Adherent | Japanese Cancer Research Resources Bank |
| SBC5 | Small‐cell lung cancer | Adherent | Japanese Cancer Research Resources Bank |
| RERF‐LC‐MA | Small‐cell lung cancer | Adherent | Japanese Cancer Research Resources Bank |
| H460 | Large‐cell carcinoma | Adherent | ATCC |
| A549 | Adenocarcinoma | Adherent | Japanese Cancer Research Resources Bank |
| ABC‐1 | Adenocarcinoma | Adherent | Japanese Cancer Research Resources Bank |
| LC‐2/ad | Adenocarcinoma | Adherent | RIKEN Cell Bank (Tsukuba, Japan) |
| VMRC‐LCD | Adenocarcinoma | Adherent | Japanese Cancer Research Resources Bank |
| H292 | Adenocarcinoma | Adherent | ATCC |
| H441 | Adenocarcinoma | Adherent | ATCC |
| H1651 | Adenocarcinoma | Adherent | ATCC |
| H596 | Adenosquamous cell carcinoma | Adherent | ATCC |
Gene expression analysis per transcriptome sequencing
Gene expression analysis of the 15 high‐grade neuroendocrine tumor cell lines was carried out per mRNA‐Seq using an Illumina GAIIx sequencer (Illumina, San Diego, CA, USA). Details are shown in Appendix S1.
Oligonucleotide array analysis data
We used the gene expression data of the oligonucleotide array analysis on 41 NSCLC cell lines including H460 obtained in our previous studies.21, 22, 23 The sources and histological types of these cell lines are detailed in our previous study.22
Western blot analysis
Western blot analysis was carried out as previously described.24 Briefly, cell lysates were prepared from lung cancer cell lines using a lysis buffer containing a protease inhibitor mixture (200 μM 4‐(2‐aminoethyl) benzenesulfonyl fluoride, 10 μM leupeptin, and 1 μM pepstatin A). Equal amounts of total protein (20 μg) were fractionated in 7.5% SDS‐PAGE, transferred to a PVDF membrane (Millipore, Bedford, MA, USA), and incubated with an appropriate antibody. The binding of the primary antibody was detected with the ECL Western Blotting Detection Reagent (GE Healthcare, Chalfont St. Giles, UK) using a peroxidase‐conjugated secondary antibody (GE Healthcare). The sources of the antibodies used in this study are summarized in Table 2.
Table 2.
Antibodies used for Western blot analysis of lung cancer cell lines
| Antibody | Source |
|---|---|
| YAP (D8H1X) | Cell Signaling Technology, (Danvers, MA, USA) |
| AMOTL2 (N‐14) | Santa Cruz Biotechnology |
| AJUBA | Cell Signaling Technology |
| NCAM (H‐300) | Santa Cruz Biotechnology |
| Chromogranin A (LK2H10) | Abcam, (Cambridge, UK) |
| Synaptophysin (ab53166) | Abcam |
| RAB3A (K‐15) | Santa Cruz Biotechnology |
| α‐Tubulin (TU‐02) | Santa Cruz Biotechnology |
| GAPDH (V‐18) | Santa Cruz Biotechnology |
Tissue microarray sections
We used TMAs that were produced to accommodate primary lung cancer tissue core sections collected from patients (n = 201) who had undergone surgical resection at the University of Tokyo Hospital (Tokyo, Japan) between 2005 and 2008. Of the 201 core sections examined, 142 were adenocarcinomas, 40 were squamous cell carcinomas, 7 were pleomorphic carcinomas, 6 were SCLCs, and 6 were LCNECs. Informed consent was obtained from all patients, and the study was approved by the Institutional Ethics Review Committee.
Whole sections of high‐grade pulmonary neuroendocrine tumors
Tumor specimens were obtained from 71 patients (41 SCLCs and 30 LCNECs) who underwent lung cancer surgery at the Jichi Medical University Hospital (Tochigi, Japan), the Jichi Medical University Saitama Medical Center (Saitama, Japan), and the University of Tokyo Hospital. Among 71 cases, 7 were treated with platinum‐based neo‐adjuvant chemotherapy (CDDP + GEM [n = 2], CDDP + VP16 [n = 3], CDDP + VNR [n = 1], and CDDP + docetaxel [n = 1]), 63 cases were not treated with neo‐adjuvant chemotherapy, and one case was unknown. Among the 63 cases not treated with neo‐adjuvant chemotherapy, 33 cases were treated with platinum‐based adjuvant chemotherapy (CBDCA + CPT11 [n = 2], CBDCA + GEM [n = 1], CBDCA + VNR [n = 1], CBDCA + VP16 [n = 17], CDDP + VNR [n = 1], CDDP + CBDCA + vindesine [n = 1], CDDP + CBDCA + VP16 [n = 1], CDDP + CPT11 [n = 1], CDDP + picibanil [n = 1], CDDP + CPT11 + VP16 [n = 1], and CDDP + VP16 [n = 6]), only one case was treated with CAV chemotherapy, 28 cases were not treated with platinum‐based or CAV chemotherapy, and one case was unknown. Details are shown in Appendix S1. Informed consent was obtained from all patients, and the study was approved by the Institutional Ethics Review Committee.
Xenograft tumors of SCLC/NSCLC cell lines
We established xenograft tumors of SCLC/NSCLC cell lines by injecting cell suspensions (1 × 107) into the flanks of 6‐week‐old female nude mice (BALB/c nu/nu).
Immunohistochemistry and evaluation
Formalin‐fixed, paraffin‐embedded tumor specimens were analyzed by immunohistochemistry using antibodies to YAP1, synaptophysin, chromogranin A, NCAM, and ASCL1. The sources of antibodies, staining procedures, and evaluation methods are given in Appendix S1. In brief, the expression of each neuroendocrine marker antibody in a tumor was defined as positive when 10% of the tumor cells or greater were stained, and negative when less than 10% were stained. The expression of the YAP1 antibody in a tumor was defined as positive when more than 0% were stained, and negative when the tumor cells showed complete negative staining.
Generation of YAP1‐deficient cell lines
In order to achieve the stable knockdown of the YAP1 gene, SCLC cell lines (SBC3, SBC5, and LCMA) were infected on 12‐well plates with lentiviral particles expressing three distinct target‐specific shRNA or non‐targeting shRNA (sc‐38637‐V and sc‐108080) (Santa Cruz Biotechnology, Dallas, TX, USA) in the presence of 5 μg/mL polybrene (Santa Cruz Biotechnology). Stably infected cells were selected with 2 μg/mL puromycin for 2 days and 4 μg/mL puromycin for an additional 2 days.
Evaluation of transcriptional activity of YAP1 by luciferase assay
A PGLIII/TEAD2‐Luciferase plasmid was constructed by inserting four tandem repeat sequences containing a TEAD‐binding GTIIC (GGAATG) site and its flanking sequences into an XhoI‐EcoRI site upstream of a firefly luciferase reporter gene in a pGL3‐Basic Vector (Promega, Fitchburg, WI, USA). A luciferase assay was undertaken in order to monitor the transcriptional activity of YAP1 using the PGLIII/TEAD2‐Luciferase plasmid and the Renilla luciferase plasmid pRL‐TK as an internal control.
Drug sensitivity
The SBC3, SBC5, LCMA, N417, H146, Lu139, Lu130, H69, H889, H510A, the SBC3‐shControl cell lines, SBC3‐shYAP1 cell line, SBC5‐shControl cell line, SBC5‐shYAP1 cell line, LCMA‐shControl cell line, and LCMA‐shYAP1 cell line were used in triplicate. Cells (1 × 104 cells) were plated on the wells of 96‐well microtiter plates. After 24 h, cisplatin was added to the wells at the following final concentrations: 10, 3.3, 1, and 0.1 μM. The cells were then incubated for another 3 days at 37°C. Cells were removed by treatment with trypsin/EDTA solutions, and then counted using the Countess automated cell counter (Invitrogen, Waltham, MA, USA). Results were plotted as cell viability versus log10 (concentration of reagents) and the IC50 value was calculated using the software GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
Bioinformatic analyses
We used the cluster program (http://rana.lbl.gov/EisenSoftware.htm, accessed March 19, 2008) for a cluster analysis of the gene expression data of cell lines. In brief, we carried out average linkage hierarchical clustering of the 15 cell lines using the mean centering and normalization of genes. We then displayed the results obtained with the aid of TreeView software (http://rana.lbl.gov/EisenSoftware.htm, accessed March 21, 2008). The image used a color code to represent relative expression levels. Red represents expression levels greater than the mean for a given gene across all samples. Green represents expression levels less than the mean across samples.
Statistical analysis
Fisher's exact test was used to evaluate clinicopathological relationships. Calculations were carried out with StatView (Abacus Concepts, Berkeley, CA, USA). P‐values less than 0.05 were considered significant. Survival curves were generated using the Kaplan–Meier method and differences in survival were analyzed by the Wilcoxon method.
Results
Hierarchical cluster analysis of cell lines derived from high‐grade neuroendocrine tumors
We undertook a hierarchical cluster analysis of the 15 high‐grade neuroendocrine tumor cell lines (14 SCLC cell lines and H460 cell line) based on the gene expression of Hippo pathway‐correlated molecules and neuroendocrine differentiation‐correlated molecules containing neuroendocrine markers and myc family genes (gene data shown in Table S1). The results obtained are shown in Figure 1(a). Fifteen cell lines were divided into two distinctive groups: the YAP1‐negative floating cell type (n = 11) and the YAP1‐positive adherent cell type (n = 4) (Fig. 1a). The YAP1‐negative floating cell type also showed loss of expression of the WTIP, LATS2, and JUB genes (AJUBA), which are regulators of YAP1 activity, as well as the loss of the gene expression of TEAD2, a transcriptional factor that interacts with YAP1. The expression of WWTR1 (TAZ), a YAP1 paralogue, was also lost in all of the YAP1‐negative floating cell types, however, SBC5, one of the YAP1‐postive adherent cell types, also showed loss of expression of WWTR1. The cell lines showing strong gene expression of neuroendocrine markers belonged to the YAP1‐negative floating cell type, whereas all of the cell lines in the YAP1‐positive adherent cell type showed weak gene expression of neuroendocrine markers (Fig. 1a,b). We then compared the protein expression level of YAP1 and neuroendocrine markers (chromogranin A, synaptophysin, and NCAM1) among the 15 cell lines tested by Western blotting, and also carried out immunohistochemistry for YAP1 and neuroendocrine markers (chromogranin A, synaptophysin, NCAM1, and ASCL1), using the xenograft tumors of 8 of the 15 cell lines. The results of the Western blot analysis and immunohistochemistry are shown in Figure 2 and Table 3, respectively. We confirmed that cell lines with the loss of YAP1 were positive for neuroendocrine markers, whereas YAP1‐positive cell lines were negative for neuroendocrine markers at the protein level. The typical expression patterns of the neuroendocrine markers of the YAP1‐positive SCLC cell line SBC5 and YAP1‐negative SCLC cell line H69 are shown in Figure 1(c). These results suggest that the loss of YAP1 correlates with the expression of neuroendocrine markers or the floating phenotype. Our immunohistochemical analysis of the xenograft tumors also revealed that it was difficult to determine whether H460 was an LCNEC cell line, because H460 was negative for all of the neuroendocrine markers.
Figure 1.
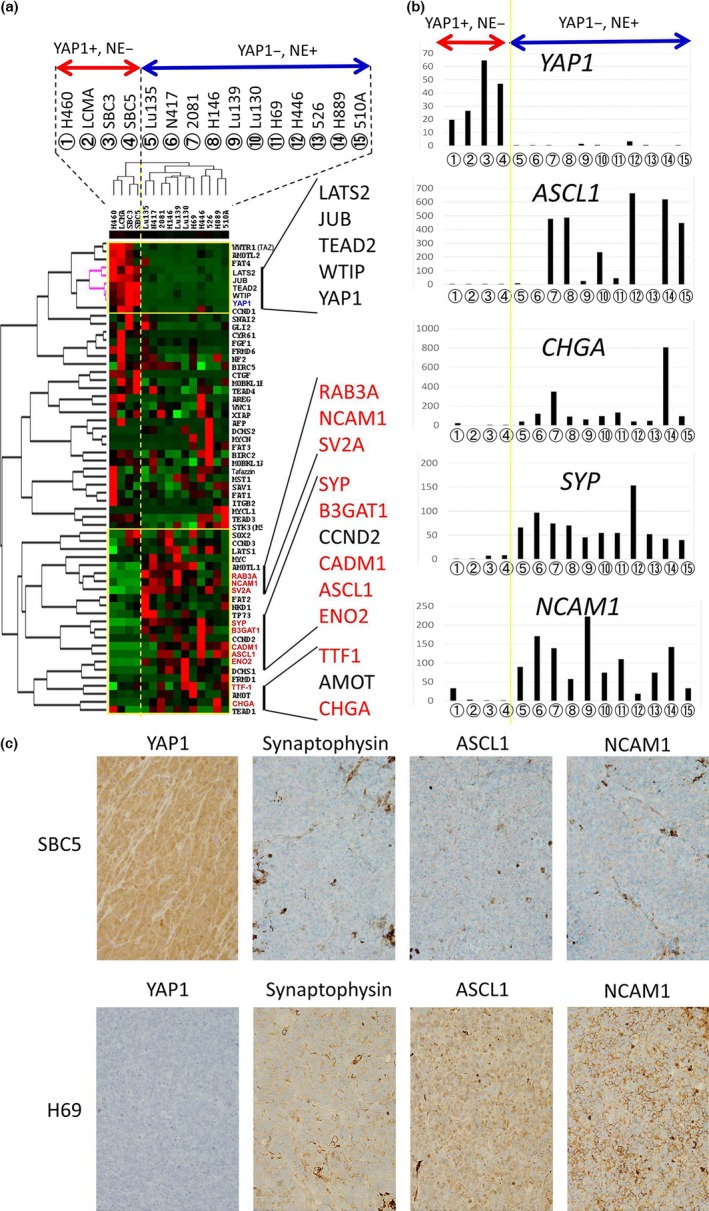
(a) Hierarchical cluster analysis of 15 high‐grade neuroendocrine lung tumor cell lines using the gene expression of Hippo pathway‐correlated molecules and neuroendocrine differentiation‐correlated molecules including neuroendocrine markers and the myc family. The genes of neuroendocrine markers are shown in red text. The red bar indicates YAP1‐positive and neuroendocrine marker‐negative cell lines (n = 4); blue bars indicate YAP1‐negative and neuroendocrine marker‐positive cell lines (n = 11). (b) Gene expression levels of neuroendocrine markers (YAP1,ASCL1,CHGA,SYP, and NCAM1) in 15 high‐grade neuroendocrine lung tumor cell lines. The numbers enclosed in circles correspond to the cell lines shown in (a). (c) Immunohistochemical expression patterns of YAP1, synaptophysin, NCAM1, and ASCL1 of a representative YAP1‐positive SCLC cell line (SBC5) and representative YAP1‐negative SCLC cell line (H69) under a high‐power view field (×400).
Figure 2.
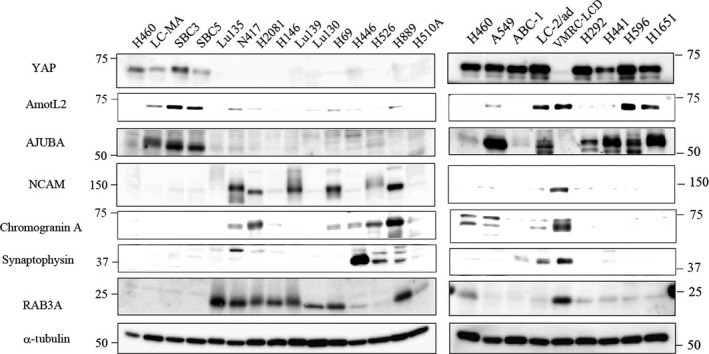
Western blot analysis of gene products selectively expressed in YAP1‐positive and YAP1‐negative groups using 15 high‐grade neuroendocrine lung tumor cell lines (left panel) and 9 non‐small‐cell lung cancer cell lines (right panel). YAP1, AMOTL2, and AJUBA are Hippo pathway‐correlated molecules and synaptophysin, ASCL1, chromogranin A, and RAB3A are neuroendocrine markers.
Table 3.
Immunohistochemical positivity for YAP1, chromogranin A, synaptophysin, NCAM1, and ASCL1 of xenograft tumors of eight lung cancer cell lines
| YAP1 | ASCL1 | Synaptophysin | Chromogranin A | NCAM1 | |
|---|---|---|---|---|---|
| H460† | + | − | − | − | − |
| SBC5† | + | − | − | − | − |
| N417‡ | − | − | + | − | + |
| 2081‡ | − | + | + | − | + |
| H69‡ | − | + | + | − | + |
| 510A‡ | − | + | + | − | + |
| Lu135‡ | − | + | − | − | + |
| H146‡ | − | + | + | − | + |
†YAP1‐positive cell lines. ‡YAP1‐negative cell lines.
Expression pattern of YAP1 in NSCLC cell lines
We extracted and compared the gene expression of YAP1, WWTR1, LATS2, WTIP, TEAD2, and neuroendocrine markers from the DNA array data of 41 NSCLC cell lines including H460, as described in our previous studies.21, 22, 23 Gene expression data is shown in Table S2. All NSCLC cell lines were positive for YAP1, except for VMRC‐LCD (Fig. 3a). VMRC‐LCD was not a floating cell line, but an adherent cell line established as a primary lung adenocarcinoma cell line. However, VMRC‐LCD showed the strongest gene expression of ASCL1, SYP, and CHGA among the 41 NSCLC cell lines examined (Fig. 3a). VMRC‐LCD showed the loss of YAP1 and stronger expression of neuroendocrine markers at the protein level than NSCLC cell lines in the Western blot analysis (Fig. 2). In xenograft tumors, histologically, VMRC‐LCD cells were found to proliferate to form solid nests with extensive necrosis in the low‐power view field (Fig. 3b). In the high‐power view field, VMRC‐LCD cells, having large nuclei with prominent nucleoli and a more abundant cytoplasm than SCLC, showed solid growth patterns with peripheral palisading (Fig. 3b). Although these tumors sometimes showed trabecular growth patterns (Fig. 2d), no mucin was detected by Alcian blue staining (not shown). VMRC‐LCD cells showed high mitotic activity; more than 100 mitotic figures per 10 high‐power fields (×400). The results of the immunohistochemical analysis revealed that VMRC‐LCD cells were completely negative for YAP1, diffusely positive for ASCL1, and also partially positive for chromogranin A and synaptophysin (Fig. 3b, Table 3). We examined xenograft tumors under an electron microscope, and found dense‐core granules in VMRC‐LCD cells (Fig. S1). Based on these results, we concluded that VMRC‐LCD was an LCNEC cell line, and that the loss of YAP1 correlated with neuroendocrine features, but not with the floating phenotype. The loss of WWTR1, LATS2, and JUB was also only observed in VMRC‐LCD cells, whereas the loss of WTIP or TEAD2 was sometimes observed among the other NSCLC cell lines tested (Table S2).
Figure 3.
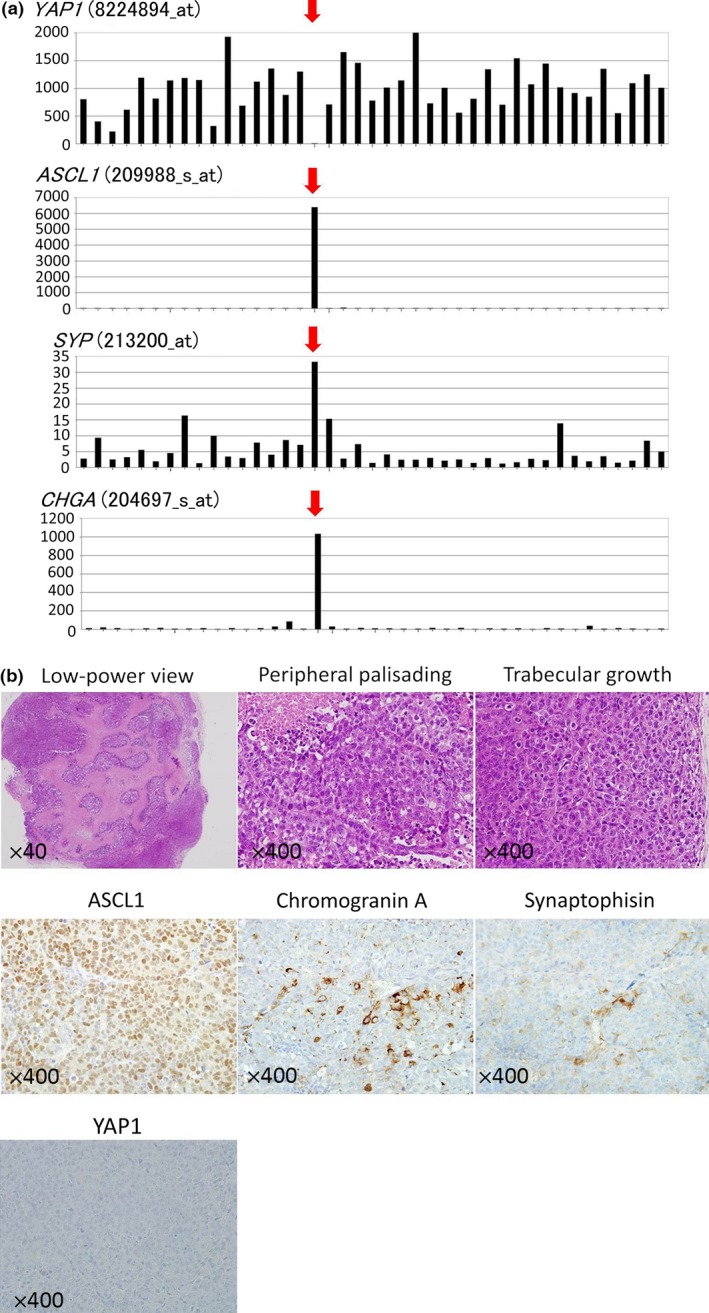
(a) YAP1 gene expression levels (8224894_at), ASCL1 gene expression levels (209988_s_at), SYP gene expression levels (213200_at), and CHGA gene expression levels (204697_s_at) in 41 NSCLC cell lines including VMRC‐LCD (red arrows). (b) Histopathology of xenograft tumors of the VMRC‐LCD cell line. Top left panel: HE section in a low‐power view field (×40) showing that VMRC‐LCD cells proliferate to form solid nests with extensive necrosis. Top center and top right panels: HE sections in a high‐power view field (×400). VMRC‐LCD cells, having large nuclei with prominent nucleoli and a more abundant cytoplasm than SCLC, showed solid growth patterns with peripheral palisading (top middle panel) as well as trabecular growth patterns (top right panel). Middle left, middle center, middle right, and bottom left panels show the immunohistochemical expression patterns of ASCL1, chromogranin A, synaptophysin, and YAP1 of xenograft tumors of the VMRC‐LCD cell line, respectively, in a high‐power view field (×400). ASCL1 was diffusely positive, chromogranin A and synaptophysin were partially positive, and YAP1 was completely negative.
Expression pattern of YAP1 in TMA sections of primary lung tumors
We carried out immunohistochemistry for YAP1 using TMA sections of 201 primary lung cancers (142 adenocarcinomas, 40 squamous cell carcinomas, 7 pleomorphic carcinomas, 6 SCLCs, and 6 LCNECs). The results obtained are shown in Table 4. The loss of YAP1 was rarer in NSCLCs, except for LCNECs (6/189, 3%), than in SCLCs (5/6, 83%) and LCNECs (3/6, 50%).
Table 4.
Positivity for YAP1 in each histological subtype of lung cancer using tissue microarray sections
| Histology | YAP1‐positive | YAP1‐negative | Total |
|---|---|---|---|
| SCLC | 1 | 5 | 6 |
| LCNEC | 3 | 3 | 6 |
| NSCLC, except for LCNEC | 183 | 6 | 189 |
| Total | 187 | 14 | 201 |
Small‐cell lung cancer (SCLC) versus non‐small‐cell lung cancer (NSCLC), except for large‐cell neuroendocrine carcinoma (LCNEC), P < 0.0001 (Fisher's exact test). LCNEC versus NSCLC, except for LCNEC, P = 0.0013 (Fisher's exact test).
Expression patterns of YAP1 and neuroendocrine markers in whole sections of high‐grade neuroendocrine lung tumors
We collected whole sections of high‐grade neuroendocrine lung tumors (41 SCLCs and 30 LCNECs), and carried out immunohistochemistry for YAP1 and neuroendocrine markers. The results obtained are shown in Table 5. Most SCLCs (40/41, 98%) were completely negative for YAP1, except for one case that was combined SCLC with squamous cell carcinoma components. Eighteen of 30 LCNECs (60%) were also completely negative for YAP1, whereas 12 LCNECs showed strong positivity for YAP1. The loss of YAP1 was significantly more frequent in SCLCs than in LCNECs (P < 0.0001) (Table 5). Combined SCLC/LCNEC with adenocarcinoma or squamous cell carcinoma components frequently showed the loss of YAP1 in the components of SCLC/LCNEC, and strong positivity in adenocarcinoma or squamous cell carcinoma components. Among high‐grade neuroendocrine lung tumors, the expression of YAP1 inversely correlated with that of synaptophysin, chromogranin A, and ASCL1 (P = 0.0010, 0.0030, and 0.0264, respectively), and there were also no positive cases for synaptophysin or chromogranin A among YAP1‐positive cases (Table 5). Figure 4 shows the typical expression patterns of YAP1 and neuroendocrine markers of combined SCLC with adenocarcinoma components (top panels), YAP1‐negative LCNEC (middle panels), and YAP1‐positive LCNEC (bottom panels). We speculated that the loss of YAP1 occurs at a relatively early stage of neuroendocrine differentiation, at least before the expression of synaptophysin and chromogranin A, but after the expression of ASCL1 because 5 out of 13 YAP1‐positive cases were positive for ASCL1 (Table 5). The relationships between YAP1 expression and clinicopathological factors are shown in Table 6. No clinicopathological factor correlated with the expression of YAP1, except for sex.
Table 5.
Comparison of YAP1 expression levels with histological subtypes and expression levels of neuroendocrine markers using whole sections of high‐grade neuroendocrine tumors
| YAP1 expression | P‐value | ||
|---|---|---|---|
| Positive | Negative | ||
| Histology | |||
| SCLC | 1 | 40 | <0.0001 |
| LCNEC | 12 | 18 | |
| Synaptophysin | |||
| Positive | 0 | 28 | 0.0010 |
| Negative | 13 | 30 | |
| Chromogranin A | |||
| Positive | 0 | 24 | 0.0030 |
| Negative | 13 | 34 | |
| ASCL1 | |||
| Positive | 5 | 42 | 0.0264 |
| Negative | 8 | 16 | |
| NCAM | |||
| Positive | 7 | 38 | 0.5280 |
| Negative | 6 | 20 | |
LCNEC, large‐cell neuroendocrine carcinoma; SLCL, small‐cell lung cancer. Underlined P‐values are considered significant (P < 0.05).
Figure 4.
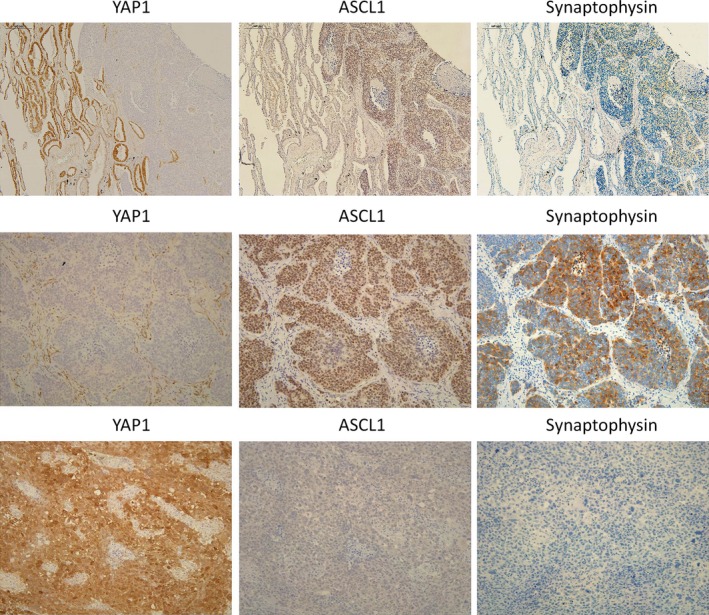
Top row: panels show YAP1 (left panel), ASCL1 (center panel), and synaptophysin (right panel) stained sections of combined small‐cell lung cancer with adenocarcinoma components using serial sections (×100). Adenocarcinoma components (left) were positive for YAP1 and negative for ASCL1 and synaptophysin, whereas small‐cell lung cancer components (right) were negative for YAP1 and positive for ASCL1 and synaptophysin. Middle and bottom panels: YAP1 (left), ASCL1 (center), and synaptophysin (right) stained sections of YAP1‐negative and YAP1‐positive large cell neuroendocrine carcinoma (LCNEC) cases, respectively, using serial sections (×200). The YAP1‐negative LCNEC case was positive for ASCL1 and synaptophysin (middle panels), whereas the YAP1‐positive LCNEC case was negative for ASCL1 and synaptophysin (bottom panels).
Table 6.
Relationships between YAP1 expression levels and clinicopathological factors in 71 high‐grade neuroendocrine tumors
| YAP1 expression | P‐value | ||
|---|---|---|---|
| Positive | Negative | ||
| Pathological stage† | |||
| Stage I | 7 | 25 | >0.9999 |
| Stages II–IV | 5 | 21 | |
| T‐stage‡ | |||
| T1 | 5 | 22 | >0.9999 |
| T2, T3, T4 | 8 | 35 | |
| Nodal involvement§ | |||
| Positive | 2 | 19 | 0.1817 |
| Negative | 10 | 28 | |
| Lymphatic invasion¶ | |||
| Positive | 2 | 31 | 0.1239 |
| Negative | 6 | 21 | |
| Vessel invasion†† | |||
| Positive | 7 | 40 | >0.9999 |
| Negative | 1 | 11 | |
| Pleural invasion‡‡ | |||
| Positive | 10 | 26 | 0.0658 |
| Negative | 3 | 30 | |
| Tumor size, cm§§ | |||
| <3 | 7 | 36 | 0.5351 |
| ≥3 | 6 | 20 | |
| Pulmonary metastasis¶¶ | |||
| Positive | 1 | 3 | 0.5751 |
| Negative | 12 | 53 | |
| Age, years | |||
| <60 | 1 | 8 | >0.9999 |
| ≥60 | 12 | 50 | |
| Sex | |||
| Male | 9 | 55 | 0.0183 |
| Female | 4 | 3 | |
†Stages (I or II–IV) of 13 cases were unknown. ‡Pathological T‐stage of one case was unknown. §Presence or absence of nodal involvement in 12 cases was unknown. ¶Presence or absence of lymphatic invasion in 11 cases was unknown. ††Presence or absence of vessel invasion in 11 cases was unknown. ‡‡Presence or absence of pleural invasion in two cases was unknown. §§Tumor sizes of two cases were unknown. ¶¶Presence or absence of pulmonary metastasis in two cases was unknown. Underlined P‐values are considered significant (P < 0.05).
Recently, Hamanaka et al.25 reported that SCLC cases with low neuroendocrine marker expression showed good prognosis. In our study, some YAP1‐negative SCLC cases were also negative for chromogranin A, synaptophysin, and NCAM1, and the survival analysis revealed that neuroendocrine marker‐negative cases showed much better prognosis than neuroendocrine marker‐positive cases among YAP1‐negative cases (Fig. S2).
Loss of YAP1 correlated with chemosensitivity in high‐grade neuroendocrine tumors
Figure 5(a) shows the results of the survival analysis of 60 high‐grade neuroendocrine tumor cases without neoadjuvant chemotherapy. Cases with adjuvant chemotherapy (n = 34) had slightly better prognoses than those without (n = 26, P = 0.1559). However, among YAP1‐negative cases (n = 48), cases with adjuvant chemotherapy (n = 28) showed significantly better prognoses than those without (n = 20, P = 0.0112) (Fig. 5a). Among cases with adjuvant chemotherapy (n = 34), YAP1‐negative cases (n = 28) showed significantly better prognoses than YAP1‐positive cases (n = 6, P = 0.0477) (Fig. 5a), whereas, among cases without adjuvant chemotherapy (n = 26), YAP1‐negative cases (n = 20) showed slightly worse prognoses than YAP1‐positive cases (n = 6, P = 0.1094) (Fig. 5a). These results suggest that, among high‐grade neuroendocrine tumors, YAP1‐negative cases were more chemosensitive than YAP1‐positive cases.
Figure 5.
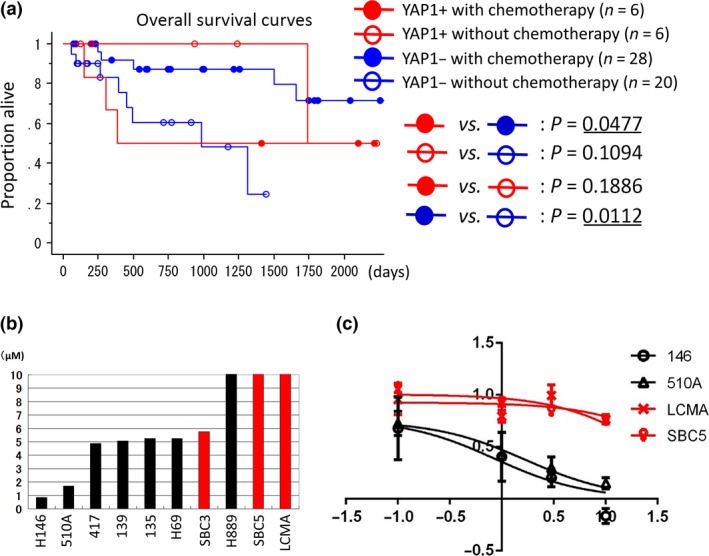
(a) Patient survival according to YAP1 expression levels. Patients were separated into the following four groups: YAP1‐positive cases with adjuvant chemotherapy (YAP1+ with chemotherapy), YAP1‐positive cases without adjuvant chemotherapy (YAP1+ without chemotherapy), YAP1‐negative cases with adjuvant chemotherapy (YAP1− with chemotherapy), and YAP1‐negative cases without adjuvant chemotherapy (YAP1− without chemotherapy). Underlined P‐values are considered significant (P < 0.05). (b) Comparisons of IC 50 values for cisplatin. The upper‐limit values of graphs are set to be 10 μmol/L. Red bars indicate YAP1‐positive cell lines; black bars indicate YAP1‐negative cell lines. (c) Dose–response curves of four cell lines: H146, H510A, LCMA, and SBC5. H146 and H510A (black lines) were YAP1‐negative small‐cell lung cancer cell lines, and LCMA and SBC5 (red lines) were YAP1‐positive small‐cell lung cancer cell lines. The x‐axis indicates the log10 (concentration of cisplatin) and the y‐axis indicates cell viability = (mean absorbance in test wells)/(mean absorbance in control wells). H146 and H510A were sensitive to cisplatin (IC 50 = 0.8573 and 1.698, respectively), while LCMA and SBC5 were resistant (IC 50 = 58.69 and 25.48, respectively).
Positivity for YAP1 correlated with resistance to cisplatin in 10 SCLC cell lines
We examined sensitivity to cisplatin in a panel of 10 SCLC cell lines classified into the YAP1‐negative group (n = 7) and YAP1‐positive group (n = 3). In a comparison of IC50 values for cisplatin between the YAP1‐positive and YAP1‐negative groups, we found that YAP1‐positive group cell lines were significantly more resistant to cisplatin than YAP1‐negative group cell lines (P = 0.0304, Mann–Whitney U‐test) (Fig. 5b). Figure 5(c) shows the dose–response curves of two YAP1‐negative cell lines (H146 and H510A) and two YAP1‐positive cell lines (SBC5 and LCMA).
Knockdown of YAP1 induces neuroendocrine marker RAB3A
In order to investigate the effects of the loss of function of YAP1, YAP1‐positive SCLC cell lines (SBC3, SBC5, and LCMA) were infected with lentiviral shYAP1 and shControl. We confirmed that the protein expression level and transcriptional activity of YAP1 were reduced more by shYAP1 than by shControl with Western blotting and luciferase assay, respectively (Fig. 6a,b). We examined sensitivity to cisplatin using SBC3‐, SBC5‐, and LCMA‐shControl and shYAP1 cell lines in order to investigate the impact of YAP1 on drug sensitivity; however, reductions in the expression of YAP1 did not improve sensitivity to cisplatin in SBC3, SBC5, or LCMA (Fig. 6c). We then undertook a gene expression analysis by mRNA‐Seq using the SBC3‐, SBC5‐, and LCMA‐shControl and shYAP1 cell lines, extracted genes with signals greater than or equal to 1 (gene data shown in Table S3), and selected genes upregulated or downregulated by shYAP1 among all three YAP1‐positive SCLC cell lines (SBC3, SBC5, and LCMA). Table 7 shows the top 20 genes upregulated or downregulated by shYAP1 depending on the average quotient of shYAP1/shControl. We carried out a cluster analysis of the 15 high‐grade neuroendocrine tumor cell lines using the genes listed in Table 7, except for the YAP1 gene. The cell lines were clearly classified into a neuroendocrine marker‐positive group (n = 11) and neuroendocrine marker‐negative group (n = 4) (Table S4, Fig. S3). These results suggest that the downregulation of YAP1 affects the genes characterizing the neuroendocrine phenotype. We extracted the genes selectively expressed as the neuroendocrine marker‐positive group or neuroendocrine marker‐negative group (NE+ group/NE− group >3 or 1 < 3) showing positive or negative correlations with the expression of YAP1 (correlation co‐efficient >0.3 or <−0.3). The results obtained are shown in Table 8. We found that RAB3A was upregulated by shYAP1. RAB3A is a synaptic vesicle‐specific protein, similar to synaptophysin, is specifically expressed in normal neuroendocrine cells and malignant neuroendocrine tumors, and was previously reported to be a useful neuroendocrine marker.26 In this report, we did not show the immunohistochemical expression pattern of RAB3A in high‐grade neuroendocrine tumor cases, but instead we confirmed by Western blotting that RAB3A was strongly expressed in YAP1‐negative cell lines with high expression levels of neuroendocrine markers (Fig. 2). We speculated that the loss of YAP1 is involved in the neuroendocrine differentiation of lung tumors through the upregulation of RAB3A. We noted that ShYAP1 also downregulated the expression of AMTOL2, JUB (AJUBA), and WTIP, which are regulators of YAP1. Using the antibodies available for Western blotting, we confirmed that the protein expression levels of AJUBA and AMOTL2 were downregulated by shYAP1 (Fig. 6d). This may have been caused by a negative‐feedback mechanism. We also confirmed that the expression of AJUBA was completely lost in the YAP1‐negative cell lines strongly expressing neuroendocrine markers (Fig. 2).
Figure 6.
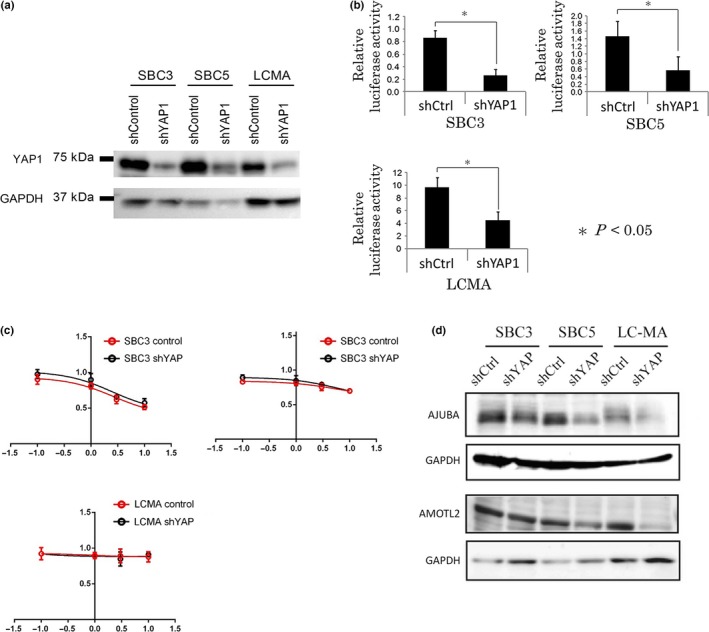
(a) SBC3, SBC5, and LCMA cell lines were infected with a lentivirus encoding the indicated shYAP1 or the control. Drug‐selected cells were examined for the expression of YAP1 and GAPDH by immunoblotting. (b) Luciferase assay to monitor the transcriptional activity of YAP1 using a PGLIII/TEAD2‐Luciferase plasmid and the Renilla luciferase plasmid pRL‐TK as an internal control. The transcriptional activity of YAP1 was downregulated more by shYAP1 than by shControl. (c) Dose–response curves of SBC3, SBC5, and LCMA cell lines infected with a lentivirus encoding the indicated shYAP1 (black line) or control (red line). The x‐axis indicates the log10 (concentration of cisplatin), and the y‐axis indicates cell viability = (mean absorbance in test wells)/(mean absorbance in control wells). (d) Western blot analysis of protein expression levels of AMOTL2 and AJUBA using SBC3‐shControl, SBC3‐shYAP1, SBC5‐shControl, SBC5‐shYAP1, LCMA‐shControl, and LCMA‐shYAP1 cell lines.
Table 7.
Top 20 genes upregulated or downregulated by shYAP1 in YAP1‐positive small‐cell lung cancer cell lines LCMA, SBC3, and SBC5
| Upregulated genes | Downregulated genes | ||
|---|---|---|---|
| Genes | shYAP1/shControl | Genes | shYAP1/shControl |
| FOS | 1.772226 | YAP1 | 0.271919 |
| EGR1 | 1.537839 | MYL9 | 0.521909 |
| C11orf71 | 1.504796 | NPIP | 0.534952 |
| RCC1 | 1.411171 | AMOTL2 | 0.565497 |
| RAB3A | 1.399324 | SORL1 | 0.57968 |
| MLLT11 | 1.390264 | MEGF6 | 0.581002 |
| ID1 | 1.389716 | WTIP | 0.613103 |
| C1orf53 | 1.389643 | LRP1 | 0.621807 |
| TUBB3 | 1.377206 | CLU | 0.629044 |
| SMAD9 | 1.374954 | SYNE2 | 0.639216 |
| METTL12 | 1.354466 | FAHD2B | 0.650478 |
| ELL3 | 1.333245 | MAFF | 0.654306 |
| HMGCS1 | 1.328731 | RND3 | 0.658228 |
| SNORD17 | 1.328284 | CADM4 | 0.660109 |
| IDI1 | 1.323846 | FAM131C | 0.660935 |
| GLYCTK | 1.32376 | JMJD7‐PLA2G4B | 0.66569 |
| TNFAIP8 | 1.315202 | JUB | 0.668008 |
| INSIG1 | 1.311661 | VLDLR | 0.668359 |
| LDHA | 1.306133 | PPP1R15A | 0.670045 |
| PTRH1 | 1.291859 | RHBDF1 | 0.671982 |
Table 8.
Genes selectively expressed in neuroendocrine marker‐positive (n = 11) or neuroendocrine marker‐negative (n = 4) (NE+ group/NE− group >3 or <1/3) lung cancer cell lines, showing positive or negative correlations with the expression of YAP1 (correlation coefficient [Correl] >0.3 or <−0.3)
| Upregulated genes | Downregulated genes | ||||
|---|---|---|---|---|---|
| Genes | NE+/NE− | Correl | Genes | NE+/NE− | Correl |
| RAB3A | 20.94204 | −0.60259 | YAP1 | 0.016573 | 1.000000 |
| SMAD9 | 5.662386 | −0.56706 | WTIP | 0.064979 | 0.870644 |
| HMGCS1 | 4.311002 | −0.34515 | MYL9 | 0.016699 | 0.816232 |
| TUBB3 | 3.536579 | −0.33223 | LRP1 | 0.297128 | 0.765227 |
| RHBDF1 | 0.133056 | 0.721056 | |||
| JUB | 0.062124 | 0.650110 | |||
| AMOTL2 | 0.051046 | 0.613924 | |||
| RND3 | 0.190660 | 0.434551 | |||
Genes shown in bold, NE+ group/NE− group >10 or <1/10, Correl >0.6 or <−0.6. Selected genes are among the top 20 upregulated or downregulated by shYAP1 in YAP1‐positive small‐cell lung cancer cell lines LCMA, SBC3, and SBC5.
Discussion
In the present study, we revealed that the loss of YAP1 correlated with the strong expression of neuroendocrine markers, and the knockdown of YAP1 induced the expression of the neuroendocrine marker RAB3A, suggesting the possible involvement of YAP1 in the regulation of neuroendocrine differentiation. We also showed that the loss of YAP1 is a promising predictor of chemotherapy responses in SCLC and LCNEC cases using a panel of high‐grade neuroendocrine tumor cell lines and sections.
Yes‐associated protein 1 functions as an oncogene; its overexpression overcomes cell contact inhibition, induces epithelial–mesenchymal transition, and promotes cancer cell proliferation and invasion.12, 13, 14 The strong expression of YAP1 has frequently been observed in various tumors, such as hepatocellular carcinoma, ovarian cancer, and NSCLC,12, 13, 14, 15, 16 and its overexpression in NSCLC has been correlated with a poor prognosis.14 However, in terms of SCLC, only a few studies have reported the tumor‐suppressive function of YAP1, inducing apoptosis in combination with p73.17, 18 Wu et al.17 reported that some single nucleotide polymorphisms within the promoter region of YAP1 were associated with the downregulation of the gene and survival of SCLC patients. Nishikawa et al.18 found that the downregulation of YAP1 by ASCL1 through the activation of mir375 inhibited apoptosis. However, no studies have focused on the role of YAP1 in neuroendocrine differentiation until now. Our analysis of 15 high‐grade neuroendocrine tumor cell lines and 41 NSCLC cell lines showed the loss of YAP1 in all cell lines strongly expressing neuroendocrine markers. Our immunohistochemical analysis of 71 high‐grade neuroendocrine tumors also revealed an inverse correlation between YAP1 and neuroendocrine markers, and also that the number of YAP1‐positive and neuroendocrine marker‐positive cases is more limited than negative cases. We also showed that the knockdown of YAP1 induced the neuroendocrine marker, the RAB3A gene. RAB3A is a synaptic vesicle‐specific protein, specifically expressed in normal neuroendocrine cells and malignant neuroendocrine tumors. These results suggest that YAP1 is involved in the repression of neuroendocrine differentiation. In this study, we did not stain carcinoid tumors for YAP1, but could confirm that carcinoid tumors were completely negative for YAP1 in the human protein atlas database (http://www.proteinatlas.org/), which suggested that loss of YAP1 was not specific to high‐grade neuroendocrine tumors, but common in neuroendocrine tumors.
Unlike the cell lines, there were 5 YAP1‐positive and ASCL1‐positive cases among 71 cases, which suggested that loss of YAP1 would occur after ASCL1 expression. It would be consistent with the report that ASCL1 induced suppression of YAP1 through mir375 in SCLC.17 However, 16 YAP1‐negative and ASCL1‐negative cases were also found among 71 cases, which suggested that, although some high‐grade neuroendocrine tumors would lose ASCL1 expressions in the progression, the expressions of YAP1 would not be recovered. ASCL1‐induced suppression through miRNA could not efficiently explain the complete loss of YAP1 in high‐grade neuroendocrine tumors. We were suspicious of the involvement of DNA methylation, but DNA methylation inhibitor, 5‐aza‐2‐deoxycytidine treatment did not upregulate YAP1 gene expression in YAP1‐negative SCLC cell lines (data not shown). We also could find no specific genetic abnormalities in the YAP1 gene in YAP1‐negative SCLC cell lines by mRNA‐Seq. We need to study further to find the mechanism that causes the loss of YAP1.
Our survival analysis of 71 high‐grade neuroendocrine tumors revealed that YAP1 is a useful marker for stratifying high‐grade neuroendocrine tumors into chemosensitive and chemoresistant groups. Our survival curves in Figure 5(a) also showed that, among YAP1‐positive cases, the adjuvant chemotherapy group had a slightly worse prognosis than the non‐adjuvant chemotherapy group, which suggested that adverse drug reactions may exceed the beneficial effects of platinum‐based chemotherapy in YAP1‐positive high‐grade neuroendocrine tumors. Recently, YAP1 has been attracting attention as a key molecule to determine the resistance of various tumors to platinum, including NSCLC, oral cancer, cervical cancer, thyroid cancer, and ovarian cancer.27, 28, 29, 30 Cheng et al.26 showed that the downregulation of YAP1 by verteporfin (a YAP1 inhibitor) sensitized cells to DNA‐damaging agents.
In the present study, the knockdown of YAP1 by shYAP1 did not induce sensitivity to cisplatin, and also did not induce neuroendocrine markers other than the RAB3A gene in YAP1‐positive SCLC cell lines. These results suggest that the knockdown of YAP1 is necessary, but not sufficient for inducing neuroendocrine differentiation. WWTR1 (TAZ), YAP1 homologue, was also lost in neuroendocrine marker‐positive cell lines. Interestingly, the expression levels of STMN2 (SCG10), one of the regulators of neuroendocrine secretion,31 increased more than 3‐fold only in SBC5, the YAP1‐positive and WWTR1‐negative SCLC cell line, by shYAP1 treatment, and high‐level expressions of STMN2 were characteristically shown in neuroendocrine marker‐positive SCLC cell lines (data not shown). These results suggested that suppression of both of YAP1 and WWTR1 might be important for inducing neuroendocrine differentiation. We did not focus on TEAD2 in this report, but the cell lines with high level expressions of neuroendocrine markers characteristically showed complete loss of TEAD2 gene expression. TEAD2 is transcription factor correlated with neuronal development.32 We need to elucidate each role of YAP–TEAD1‐4 or WWTR1–TEAD1‐4 complex‐mediated transcription, to reveal the meaning of loss of WWTR1 and TEAD2 in SCLC; this will be the focus of our future study.
Recently, YAP1 has been reported to inhibit the squamous differentiation of LKB1‐deficient lung adenocarcinomas.33 YAP1 must be a key regulator of differentiation, and searching for genetic changes in addition to the loss of YAP1 that induce neuroendocrine differentiation will help to elucidate its mechanism of action.
In summary, the loss of YAP1 may define a unique subset of high‐grade neuroendocrine tumors. These tumors strongly express neuroendocrine markers and show chemosensitivity.
Disclosure Statement
Matsubara Daisuke supported by the Smoking Research Foundation.
Abbreviations
- AMOTL2
angiomotin‐like 2
- ASCL1
Achaete‐scute homolog 1
- CAV
cyclophosphamide + doxorubicin + vincristine
- CBDCA
carboplatin
- CDDP
cisplatin
- CPT11
irinotecan
- GEM
gemcitabine
- LATS1/2
large tumor suppressor 1/2
- LCNEC
large‐cell neuroendocrine carcinoma
- mir
miRNA
- mRNA‐Seq
mRNA sequencing
- NCAM
neural cell adhesion molecule
- NSCLC
non‐small‐cell lung cancer
- SCLC
small‐cell lung cancer
- TEAD2
TEA domain transcription factor 2
- TMA
tissue microarray
- VNR
vinorelbine
- VP16
etoposide
- WWTR1
WW domain containing transcription regulator 1
- YAP1
yes‐associated protein 1
Supporting information
Appendix S1. Materials and methods.
Fig. S1. Electron microscopy of xenograft tumors of VMRC‐LCD lung cancer cell line.
Fig. S2. Patient survival of YAP1‐negative high‐grade neuroendocrine tumor cases, according to neuroendocrine expression levels.
Fig. S3. Hierarchical cluster analysis of 15 high‐grade neuroendocrine lung tumor cell lines using genes upregulated or downregulated by shYAP1.
Table S1. Gene expression data of Hippo pathway molecules, neuroendocrine markers, and the myc family in 15 high‐grade neuroendocrine tumor cell lines.
Table S2. Gene expression data of Hippo pathway molecules, neuroendocrine markers, and the myc family in 41 non‐small‐cell lung cancer cell lines.
Table S3. Gene expression analysis data of SBC3‐, SBC5‐, and LCMA‐shControl and shYAP1 cell lines. The genes with signals greater than or equal to 1 are listed.
Table S4. Expression data of genes listed in Table 7 in 15 high‐grade neuroendocrine tumor cell lines.
Acknowledgments
This study was supported in part by the Japan Society for the Promotion of Science (KAKENHI grant nos. 16K08672, 90198424, 25460432, and 30182108), Grants for Research on Human Genome Tailor‐made from the Ministry of Health, Labor, and Welfare of Japan, the Smoking Research Foundation, and the Foundation for the Development of the Community.
Cancer Sci 107 (2016) 1527–1538
Funding Information
Japan Society for the Promotion of Science (KAKENHI grant nos. 16K08672, 90198424, 25460432, and 30182108); Ministry of Health, Labor, and Welfare of Japan; Smoking Research Foundation; Foundation for the Development of the Community.
References
- 1. Travis WD, Brambilla E, Burke AP et al WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th edn Lyon, France: IARC, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Sundstrøm S, Bremnes RM, Kaasa S et al Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small‐cell lung cancer: results from a randomized phase III trial with 5 years' follow‐up. J Clin Oncol 2002; 20: 4665–72. [DOI] [PubMed] [Google Scholar]
- 3. Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol 2010; 21 (Suppl 7): vii65–71. [DOI] [PubMed] [Google Scholar]
- 4. Igawa S, Watanabe R, Ito I et al Comparison of chemotherapy for unresectable pulmonary high‐grade non‐small cell neuroendocrine carcinoma and small‐cell lung cancer. Lung Cancer 2010; 68: 438–45. [DOI] [PubMed] [Google Scholar]
- 5. Iyoda A, Hiroshima K, Moriya Y et al Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006; 82: 1802–7. [DOI] [PubMed] [Google Scholar]
- 6. Yamazaki S, Sekine I, Matsuno Y et al Clinical responses of large cell neuroendocrine carcinoma of the lung to cisplatin‐based chemotherapy. Lung Cancer 2005; 49: 217–23. [DOI] [PubMed] [Google Scholar]
- 7. Rossi G, Cavazza A, Marchioni A et al Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRα, PDGFRβ, and met in large‐cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005; 23: 8774–85. [DOI] [PubMed] [Google Scholar]
- 8. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–57. [DOI] [PubMed] [Google Scholar]
- 9. Zhao B, Wei X, Li W et al Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao B, Lei QY, Guan KL. The Hippo‐YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol 2008; 20: 638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self‐renewal. Nat Cell Biol 2011; 13: 877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zender L, Spector MS, Xue W et al Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 2006; 125: 1253–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Overholtzer M, Zhang J, Smolen GA et al Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA 2006; 103: 12405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Dong Q, Zhang Q et al Overexpression of yes‐associated protein contributes to progression and poor prognosis of non‐small‐cell lung cancer. Cancer Sci 2010; 101: 1279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu MZ, Yao TJ, Lee NP et al Yes‐associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009; 115: 4576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall CA, Wang R, Miao J et al Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010; 70: 8517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu C, Xu B, Yuan P et al Genome‐wide interrogation identified YAP1 variants associated with survival of small‐cell lung cancer patients. Cancer Res 2010; 70: 9721–9. [DOI] [PubMed] [Google Scholar]
- 18. Nishikawa E, Osada H, Okazaki Y et al miR‐375 is activated by ASH1 and inhibits YAP1 in a lineage dependent manner in lung cancer. Cancer Res 2011; 71: 6165–73. [DOI] [PubMed] [Google Scholar]
- 19. Tatematsu T, Sasaki H, Shimizu S et al Investigation of neurotrophic tyrosine kinase receptor 1 fusions and neurotrophic tyrosine kinase receptor family expression in non‐small‐cell lung cancer and sensitivity to AZD7451 in vitro. Mol Clin Oncol 2014; 2: 725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee M, Draoui M, Zia F et al Epidermal growth factor receptor monoclonal antibodies inhibit the growth of lung cancer cell lines. J Natl Cancer Inst Monogr 1992; 13: 117–23. [PubMed] [Google Scholar]
- 21. Matsubara D, Ishikawa S, Sachiko O et al Co‐activation of epidermal growth factor receptor and c‐MET defines a distinct subset of lung adenocarcinomas. Am J Pathol 2010; 177: 2191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsubara D, Ishikawa S, Oguni S et al Molecular predictors of sensitivity to the MET inhibitor PHA665752 in lung carcinoma cells. J Thorac Oncol 2010; 5: 1317–24. [DOI] [PubMed] [Google Scholar]
- 23. Matsubara D, Kishaba Y, Ishikawa S et al Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci 2013; 104: 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito T, Williams‐Nate Y, Iwai M et al Transcriptional regulation of the CADM1 gene by retinoic acid during the neural differentiation of murine embryonal carcinoma P19 cells. Genes Cells 2011; 16: 791–802. [DOI] [PubMed] [Google Scholar]
- 25. Hamanaka W, Motoi N, Ishikawa S et al A subset of small cell lung cancer with low neuroendocrine expression and good prognosis: a comparison study of surgical and inoperable cases with biopsy. Hum Pathol 2014; 45: 1045–56. [DOI] [PubMed] [Google Scholar]
- 26. Culine S, Rousseau‐Merck MF, Honoré N et al Specific expression of the ras‐related rab3A gene in human normal and malignant neuroendocrine cells. Cancer 1992; 70: 2552–6. [DOI] [PubMed] [Google Scholar]
- 27. Cheng H, Zhang Z, Rodriguez‐Barrueco R et al Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget 2015; 22: 28977–88. doi: 10.18632/oncotarget.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshikawa K, Noguchi K, Nakano Y et al The Hippo pathway transcriptional co‐activator, YAP, confers resistance to cisplatin in human oral squamous cell carcinoma. Int J Oncol 2015; 46: 2364–70. [DOI] [PubMed] [Google Scholar]
- 29. Lorenzetto E, Brenca M, Boeri M et al YAP1 acts as oncogenic target of 11q22 amplification in multiple cancer subtypes. Oncotarget 2014; 5: 2608–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xia Y, Chang T, Wang Y et al YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One 2014; 9: e91770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahapatra NR, Taupenot L, Courel M et al The trans‐Golgi proteins SCLIP and SCG10 interact with chromogranin A to regulate neuroendocrine secretion. Biochemistry 2008; 47: 7167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaneko KJ, Kohn MJ, Liu C, DePamphilis ML. Transcription factor TEAD2 is involved in neural tube closure. Genesis 2007; 45: 577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Y, Zhang W, Han X et al YAP inhibits squamous transdifferentiation of Lkb1‐deficient lung adenocarcinoma through ZEB2‐dependent DNp63 repression. Nat Commun 2014; 5: 4629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Materials and methods.
Fig. S1. Electron microscopy of xenograft tumors of VMRC‐LCD lung cancer cell line.
Fig. S2. Patient survival of YAP1‐negative high‐grade neuroendocrine tumor cases, according to neuroendocrine expression levels.
Fig. S3. Hierarchical cluster analysis of 15 high‐grade neuroendocrine lung tumor cell lines using genes upregulated or downregulated by shYAP1.
Table S1. Gene expression data of Hippo pathway molecules, neuroendocrine markers, and the myc family in 15 high‐grade neuroendocrine tumor cell lines.
Table S2. Gene expression data of Hippo pathway molecules, neuroendocrine markers, and the myc family in 41 non‐small‐cell lung cancer cell lines.
Table S3. Gene expression analysis data of SBC3‐, SBC5‐, and LCMA‐shControl and shYAP1 cell lines. The genes with signals greater than or equal to 1 are listed.
Table S4. Expression data of genes listed in Table 7 in 15 high‐grade neuroendocrine tumor cell lines.


