Abstract
The prognosis of pancreatic cancer is extremely poor compared to other cancers. One of the reasons for this is the difficulty of early diagnosis. Surveillance using cancer biomarkers and image diagnosis can enable early detection and has improved the prognosis of hepatocellular carcinoma in Japan. However, it is very difficult to detect pancreatic cancer at an early stage using cancer biomarkers and image diagnosis alone. Fucosylation is one of the most important types of glycosylation involved in cancer and inflammation. We have developed a novel glycocancer biomarker, fucosylated haptoglobin (Fuc‐Hpt), and have investigated its usefulness for the diagnosis of pancreatic cancer over approximately 10 years. Recently, we also found that most pancreatic tissues surrounding pancreatic cancer exhibit chronic pancreatitis with fibrosis and/or fatty degeneration. Certain forms of chronic pancreatitis might indicate high risk for the development of pancreatic cancer. In this review, we provide a historical summary of our research on Fuc‐Hpt as a cancer biomarker, and discuss a potential early detection system for pancreatic cancer.
Keywords: Cancer biomarker, fucosylation, glycosylation, haptoglobin, pancreatic cancer
Recent advances in medicine have improved the survival rates of patients with various types of cancers. However, the prognosis of pancreatic cancer is dismal and the 5‐year survival rate remains at approximately 5%.1 This poor prognosis is due to the difficulties encountered in the early diagnosis of this cancer type. In most cases, patients with pancreatic cancer come to a hospital at an advanced stage and the tumors are inoperable. In pancreatic cancer patients, even when small tumors are detected using various types of image diagnosis, a high rate of tumor recurrence is often observed following surgical operation. This is likely because small metastatic lesions, which are not detected with image diagnosis prior to surgical operation, already exist in distant organs such as the liver and lung at the time of surgery. Moreover, pancreatic cancer is very resistant to chemotherapy and radiotherapy.1 Therefore, more than 80% of patients suffer relapse after resection. Although recent approaches, such as polychemotherapy or strategies leading to the improved effect of gemcitabine, can substantially improve the prognosis, novel approaches and/or concepts are required to completely overcome the challenges in diagnosing and treating pancreatic cancer. There are no enclosure methods to identify high‐risk groups for pancreatic cancer, as in the case of the enclosure of chronic viral hepatitis patients for hepatocellular carcinoma. The genetic background of pancreatic cancer is complicated,2, 3, 4 and the interaction between cancer cells and immune cells in their microenvironment plays a pivotal role in pancreatic carcinogenesis.5 In this review, we introduce a possible disease associated with pancreatic cancer, a potential strategy for the early diagnosis of pancreatic cancer, and a non‐invasive but highly effective method for therapy, all from the viewpoint of glycobiology. Much of the material in this review is speculation based on available evidence, with the aim of spurring the research community to discuss possibilities for the early detection and pre‐emptive management of pancreatic cancer.
Pathogenesis of Pancreatic Cancer
Although many researchers have investigated the pathogenesis of PDAC, thus far, the crucial evidence pointing to causative inflammation and/or infection for PDAC has not yet been uncovered as it has in the case of hepatocellular carcinoma, gastric cancer, and cervical cancer. It is thought that the accumulation of multiple gene mutations induces PDAC. The pathogenesis of PDAC might be similar to that of colorectal cancer in terms of the multistep process of carcinogenesis proposed by Vogelstein et al.6 The PanIN theory is a classical concept describing the pathogenesis of PDAC. According to this theory,7, 8 invasive PDAC develops step by step, with the involvement of PanIN I, II, and III. This phenomenon is observed in the mouse model of pancreatic cancer. However, pathological analysis of pancreatic tissue from patients who did not suffer from pancreatic diseases revealed many PanIN lesions, especially in older patients. Some pathologists suggest that PanIN is just a hyperplasia of the pancreas, and not a premalignant lesion of PDAC. This concept is similar to that of colon polyps, which represent merely benign disease, with only a few cases of colon polyps developing into cancer through a multistep process of oncogene mutation.
Most cases of PDAC exhibit oncogene mutations in K‐ras and inactivation of the CDKN2 gene, which encodes the cell cycle regulator protein P16.9 However, K‐ras mutation alone is insufficient to cause the development of PDAC, as evidenced by the fact that mice transgenic for K‐ras in the pancreas do not develop spontaneous PDAC.10 However, additional mutations of tumor suppressor genes, such as p16 or p53, can induce PDAC in K‐ras‐mutated transgenic mice. Cerulein‐induced chronic inflammation,11, 12 alcohol administration,13 and a high‐fat diet14 also caused PDAC in K‐ras‐mutated transgenic mice, suggesting that the accumulation of gene mutations resulting from environmental changes is required for the development of PDAC. Thus, a gene manipulation mouse model of PDAC provides a variety of information on PDAC pathogenesis. How these basic pieces of evidence apply to human pancreatic cancer is a very important question. However, pancreatic biopsy for premalignant diseases such as chronic pancreatitis is clinically difficult because of the high risk it poses to patients. This is a key point to be considered in PDAC research.
Identification of Fuc‐Hpt as a novel type of cancer biomarker for PDAC
Oligosaccharide attachment is one of the most important post‐translational modifications of proteins, and there are two main types of protein glycosylation, N‐linked and O‐linked glycosylation.15 Among the various types of glycosylation, fucosylation on both N‐glycans and O‐glycans is closely related to cancer and inflammation.16 It is known that serum levels of fucosylated proteins are higher in cancer patients. To detect fucosylated glycoproteins, AAL is commonly used in glycoproteomic analysis. Aleuria aurantia lectin recognizes many types of linkages such as α1‐2, α1‐3/1‐4, and α1‐6 fucose. To identify serum fucosylated proteins in patients with PDAC, we used AAL blot analysis.17 As shown in Figure 1(a), an approximately 40‐kDa protein was strongly fucosylated in pancreatic cancer patients. There was no change in the protein levels of 40‐kDa proteins in Coomassie Brilliant Blue staining. Based on results from mass spectrometry and N‐terminal sequence analyses, this 40‐kDa protein was identified as the haptoglobin β‐chain. The haptoglobin β‐chain has four potential N‐glycosylation sites. As shown in Figure 1(b), site‐directed oligosaccharide analysis with mass spectrometry showed that the glycan on site 3 had a characteristic structure including fucosylation, compared to glycans on other sites.18 A glycan structure, 4F‐2 was observed only at site 3 of haptoglobin glycans of PDAC patients. 4F‐2 indicates a tetra‐antennary structure of N‐glycans with two fucoses. Based on tandem mass spectrometry analysis, this glycan structure is suggested to be the Lewis Y‐type (Fucα1‐2Galβ1‐4GlcNAcα1‐3Fuc). For the quantitative determination of serum Fuc‐Hpt, we established a lectin–antibody ELISA method, as shown in Figure 2. The bottom of a 96‐well ELISA plate was coated with the Fab fragment of anti‐human haptoglobin IgG, because the Fc portion of IgG has the fucosylated oligosaccharide. The coated plate had previously been blocked with PBS containing 3% BSA for 1 h, followed by washing with PBS containing 0.1% Tween 20. Sera diluted to 1/125–1/625 were used in this lectin–antibody ELISA. The conditioned medium of a pancreatic cancer cell line that was transfected with the human haptoglobin expression vector was used as the standard for lectin–antibody ELISA.19 Our previous study investigated the effect of interfering substrates such as formagine, bilirubin F/C, and hemoglobin in the lectin–antibody ELISA and found that the addition of hemoglobin reduced Fuc‐Hpt levels at half‐time.20
Figure 1.
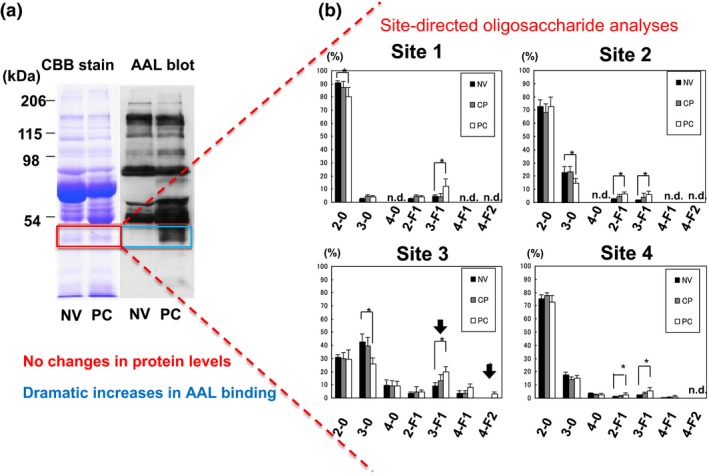
Identification of fucosylated haptoglobin and site‐directed oligosaccharide analyses of haptoglobin. (a) An approximately 40‐kDa protein was specifically fucosylated in the sera of pancreatic cancer (PC) patients, whereas its protein level was almost the same. This figure is adapted from Okuyama et al.17 with slight modification, with permission from Wiley. (b) Site‐specific oligosaccharide analyses with mass spectrometry on purified oligosaccharides from sera of normal volunteers (NV), patients with chronic pancreatitis (CP), and those with PC. Site 3 is susceptible to fucosylation, and the glycan structure 4F‐2 was specifically detected at this site. These data adapted from Nakano et al.18 with slight modification, with permission from Wiley. Numbers on the x‐axis in each figure part indicate branching on N‐glycans; F indicates the number of fucose molecules attached on each N‐glycan. n.d., not detected.
Figure 2.
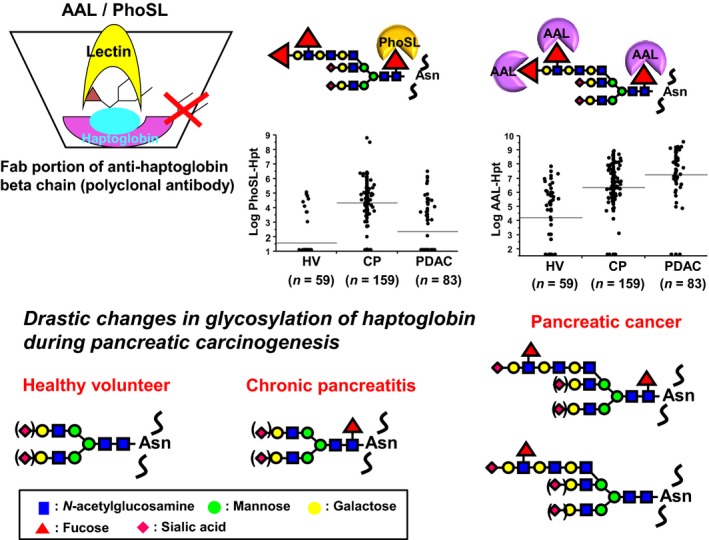
Lectin–antibody ELISA for fucosylated haptoglobin and changes in the fucosylated haptoglobin glycosylation pattern in pancreatic carcinogenesis. A polyclonal antibody for the haptoglobin β‐chain was coated onto the ELISA plate after deleting the Fc portion of the antibody. Biotinylated Aleuria aurantia lectin (AAL)/Pholiota squarrosa lectin (PhoSL) was used as the second antibody to detect fucosylation. PhoSL recognizes core fucose, whereas AAL recognizes all types of fucosylation. In normal controls, serum haptoglobin is scarcely fucosylated and core fucosylation levels of haptoglobin are increased at the stage of chronic pancreatitis. In contrast, an increase in the Lewis type of fucosylation was observed on haptoglobin, which might be a sign of distant metastasis to the liver. Asn, Asparagine; CP, chronic pancreatitis; HV, healthy volunteers; PDAC, pancreatic ductal adenocarcinoma.
Clinical Application of Lectin–Antibody ELISA of Fuc‐Hpt as a Cancer Biomarker
When we measured serum Fuc‐Hpt in patients with pancreatic cancer on a larger scale, the ROC curve showed that the diagnostic value of serum Fuc‐Hpt from pancreatic cancer patients from normal volunteers was almost the same as that of CA19‐9.20 The area under the curve of the ROC analysis for the diagnosis of pancreatic cancer was improved, compared to the first model of the lectin–antibody ELISA kit. This is because the numbers of patients at advanced stages of pancreatic cancer were increased in this multi‐institutional joint research. Levels of Fuc‐Hpt increased with progression through the clinical stages of pancreatic cancer.21 More interestingly, a combination of carcinoembryonic antigen and Fuc‐Hpt is a marker for poor prognosis following surgery in patients with colorectal cancer.17 It is speculated that metastatic cancer in the liver produces Fuc‐Hpt. Therefore, it is thought that Fuc‐Hpt is not a cancer biomarker for early diagnosis of pancreatic cancer, but a biomarker for early detection of liver metastasis of pancreatic/colon cancer that cannot be detected in the image diagnosis. Most pancreatic cancer cells did not express haptoglobin mRNA, as evidenced by RT‐PCR analysis.17 We recently found that the secretion of hepatic glycoproteins is regulated by fucosylation in the normal liver.22 That is to say, fucosylation is a possible signal for the polarized secretion of fucosylated glycoproteins into bile ducts in the liver.23 When inflammation‐induced levels of fucosylated proteins in hepatocytes or transformation of hepatocytes induce a loss of cell polarity, fucosylated proteins are secreted into blood vessels. According to these theories, Fuc‐Hpt could be produced in metastatic lesions of colon or pancreatic cancer in the liver. This hypothesis should be investigated by immunohistochemical study, using an antibody specific for Fuc‐Hpt if possible. Ballooning hepatocytes are known as a typical pathological characteristic of NASH.24, 25 In ballooning hepatocytes, the microtubule cytoskeleton is destroyed,26 and polarized secretion of fucosylated glycoprotein is destroyed. We found that Fuc‐Hpt can be used in the detection of ballooning hepatocytes in the livers of patients with NASH, for example, by liquid biopsy.27 Thus, AAL‐reactive Fuc‐Hpt is an index for hepatocyte deformity with local inflammation in the liver. However, because AAL recognizes both α1‐3/1‐4 fucosylation and α1‐6 fucosylation, the significance of each of the types of linkage in fucosylation should be clarified. To this end, a Fuc‐Hpt antibody for each linkage type should be developed. A summary of our clinical study of Fuc‐Hpt as a cancer biomarker is shown in Table 1. Although the value of the area under the ROC curve of Fuc‐Hpt is relatively high in the comparative analysis between PDAC patients with healthy volunteers, serum Fuc‐Hpt level is increased in several kinds of cancer patients, especially at the advanced stage.21 This is a limitation of Fuc‐Hpt as a cancer biomarker for pancreatic cancer.
Table 1.
Summary of the clinical study of fucosylated haptoglobin as a cancer biomarker
| Methods | Patient number | AUC | Sensitivity, % | Specificity, % | Reference |
|---|---|---|---|---|---|
| AAL lectin blot | PC 49, HV 30 | − | 57 | 97 | 17 |
| AAL lectin–antibody ELISA | PC 63, HV 22 | 0.63 | 50 | 91 | 21 |
| AAL lectin–antibody ELISA | PC 63, CP 72 | 0.63 | 50 | 79 | 21 |
| Improved AAL lectin–antibody ELISA | PC 300, HV 315 | 0.91 | 85 | 82 | 20 |
| PhoSL lectin–antibody ELISA | PC 55, HV 60 | 0.68 | 60 | 83 | 36 |
| Improved AAL lectin–antibody ELISA | PC 83, HV 59 | 0.84 | 76 | 80 | 37 |
| Improved AAL lectin–antibody ELISA | PC 83, CP 159 | 0.65 | 52 | 78 | 37 |
| Improved PhoSL lectin–antibody ELISA | PC 83, HV 59 | 0.63 | 41 | 85 | 37 |
| Improved PhoSL lectin–antibody ELISA | PC 83, CP 159 | 0.81 | 69 | 84 | 37 |
AAL, Aleuria aurantia lectin; CP chronic pancreatitis; HV, healthy volunteers; PC, pancreatic cancer; PhoSL, Pholiota squarrosa lectin.
Chronic Pancreatitis is a Next‐Generation Target for High‐Risk Groups of Pancreatic Cancer
Chronic pancreatitis has been identified as a strong risk factor for PDAC occurrence.28 Chronic pancreatitis is morphologically defined as progressive pancreatic fibrosis and inflammation that is accompanied by the atrophy of pancreatic exocrine cells.29, 30 However, pancreatic biopsy is limited due to its invasiveness. A diagnosis of chronic pancreatitis is clinically made based on characteristic symptoms of patients and several kinds of imaging examinations. However, many patients with gastroenterological and hepatic diseases have minimal symptoms in the early or mild conditions. Endoscopy and biopsy as well as blood testing can diagnose inflammation in these organs. Recently, we found that most pancreatic tissues surrounding pancreatic cancer had chronic pancreatitis with fibrosis and/or fatty degeneration.31 However, these patients had no clinical history of chronic pancreatitis, suggesting that the incidence of subclinical pancreatitis in pancreatic cancer patients might be fairly common. More interestingly, the pathological changes of subclinical pancreatitis were observed in cases of mortality due to diseases other than pancreas disease. Although the main cause of chronic pancreatitis is alcohol consumption, it is thought that other causes such as viral/bacterial infection as well as fatty regeneration might be involved. Non‐alcoholic fatty pancreas disease was recently recognized as a new clinical entity among obesity‐related disease.32 In patients with NAFPD, the level of pancreatic fatty degeneration increases with the degree of obesity, and obesity is known to increase the PDAC mortality rate.33 Non‐alcoholic fatty liver disease can progress to liver cirrhosis and hepatocellular carcinoma.34 We hypothesize that NAFPD could likewise progress to chronic pancreatitis and PDAC; however, how this progression may occur remains unknown. To solve this question, the cause of non‐alcoholic fatty liver disease/NAFPD should be clarified.
Development of a Novel Biomarker for Chronic Pancreatitis
As shown above, we found that Fuc‐Hpt is a novel type of cancer biomarker for pancreatic cancer. To detect fucosylation, we used AAL, which recognizes all type of fucosylation. In general, α1‐3/α1‐4 fucose is attached on branched N‐glycans/O‐glycans. Alpha1‐6 fucose (core fucose) is attached to both bi‐antennary and tri/tetra‐antennary glycan structures. Levels of branched glycans are increased in malignant transformation, and total fucose levels are increased. Previously we found that PhoSL more specifically recognizes core fucose.35 When PhoSL was used in lectin–antibody ELISA for Fuc‐Hpt instead of AAL, detected Fuc‐Hpt levels were 10‐fold lower, and the results of investigating Fuc‐Hpt as a cancer biomarker were therefore different.36 Because PhoSL‐reactive Fuc‐Hpt was not associated with the clinical stage of cancer, cells that produce this type of Fuc‐Hpt would not be cancer cells.
Next, we measured serum Fuc‐Hpt levels in patients with chronic pancreatitis and PDAC, using both AAL–antibody and PhoSL–antibody ELISA.37 Very interestingly, AAL‐reactive Fuc‐Hpt levels were significantly increased in patients with chronic pancreatitis, compared to healthy volunteers, and were further increased in PDAC patients. In contrast, PhoSL‐reactive Fuc‐Hpt levels were significantly higher in patients with chronic pancreatitis compared to healthy volunteers and PDAC patients (Fig. 2). Multivariate analyses showed that PhoSL‐reactive Fuc‐Hpt was an independent determinant for the diagnosis of chronic pancreatitis. According to glycan changes on haptoglobin in pancreatic diseases, we speculated that AAL‐reactive Fuc‐Hpt is produced from liver metastasis of pancreatic cancer, and PhoSL‐reactive Fuc‐Hpt is produced from infiltrated lymphocytes in chronic pancreatitis tissue. While AAL‐reactive Fuc‐Hpt is slightly increased following surgery for pancreatic cancer, PhoSL‐reactive Fuc‐Hpt is slightly decreased following surgery (EM, YK, HE, NH, KK, ST, RF, TM, manuscript in preparation). Recently, we reported that serum Mac‐2 binding protein is increased in patients with chronic pancreatitis, similar to PhoSL‐reactive Fuc‐Hpt.38 In this case, we hypothesize that the liver produces Mac‐2‐binding protein in patients with chronic pancreatitis, because these patients probably have steatohepatitis. Serum Mac‐2‐binding protein level is dramatically increased in NASH patients.39, 40 When we study the pathophysiology of pancreatic diseases, the correlation between liver and pancreas should be considered.
Perspectives from the Study of PDAC in Terms of Early Detection and Preemptive Medicine
Recently, the concept of “early chronic pancreatitis” has been established by the Japanese Pancreas Society.41 It remains unknown whether early chronic pancreatitis or subclinical pancreatitis develops into symptomatic pancreatitis. Even though subclinical pancreatitis is diagnosed using biomarkers and/or ultrasonography, it remains unknown whether the incidence of PDAC in patients with subclinical pancreatitis patients is high, as with the relationship between viral hepatitis and hepatocellular carcinoma. According to a variety of gene‐manipulating PDAC mouse models, at least one or more factors are required for progression to PDAC from chronic pancreatitis. It is reported that familial history of PDAC is involved in the incidence of PDAC. If a patient receives a diagnosis of chronic pancreatitis and has a high risk of PDAC based on genetic background, he/she should have the whole genome sequenced to determine whether there is an abnormality in a tumor suppressor gene. Such patients are good candidates for pre‐emptive therapy. On such pre‐emptive approach is cancer vaccination. Once pancreatic cancer cells develop into a solid tumor, it is difficult to treat with immunotherapy, because immune cells cannot attack cancer cells in the case of pancreatic cancer due to their microenvironment/stromal cells including fibrosis.42 We have developed a unique cancer vaccination therapy using porcine oligosaccharide types.43, 44 A possible strategy for early detection and preemptive medicine for PDAC is shown in Figure 3. A novel treatment for subclinical pancreatitis is also a candidate for pre‐emptive medicine. In most cases, once PDAC is detected using image diagnosis such as computed tomography, MRI, and PET, micrometastasis is observed in different organs. Limited PDAC, which might be genetically mild, could be completely cured by surgical resection.
Figure 3.
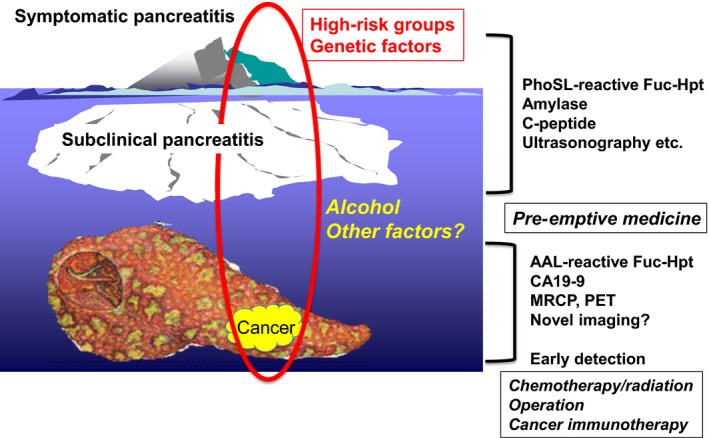
Strategy of early detection and pre‐emptive therapy for pancreatic cancer. To improve the current poor prognosis of pancreatic cancer, high‐risk groups of pancreatic cancer should be defined using a combination of several kinds of biomarkers and ultrasonography. Pre‐emptive medicine is an ideal tool to prevent pancreatic cancer. Although distant metastasis cannot be identified using image diagnosis such as computed tomography, MRI, or PET, dramatic increases in Aleuria aurantia lectin (AAL)‐reactive fucosylated haptoglobin (Fuc‐Hpt) might be an indication that therapies other than surgical operation are needed. MRCP, magnetic resonance cholangiopancreatography; PhoSL, Pholiota squarrosa lectin.
Sialyl Lewis A antigen is well known as CA19‐9, and is a representative cancer biomarker for PDAC, although there are several problems in its specificity for PDAC diagnosis.45 We have found a novel type of CA19‐9 carrier molecule: microlipids containing CA19‐9.46 Levels of this lipid type of CA19‐9 are specifically increased in the sera of patients with PDAC. Certain kinds of novel proteins are involved in supplying membranes with CA19‐9. Inhibition of this membrane supply system could be a novel strategy for PDAC treatment. Recently, we found that fucosylation is a common type of glycosylation seen in PDAC cancer stem cells, which were isolated by three different methods for establishing cancer stem cells.47 Considering these findings together, fucosylation could be a key type of glycosylation in PDAC.
Closing
A search in PubMed reveals over 10 000 reviews on PDAC, including many excellent reviews from a genetic perspective. Although the current review includes many hypotheses, glycobiology and glycotechnology (glycoscience) would be a promising tool for the management of PDAC. As a result of this application of glycoscience, a relationship between NAFPD and chronic pancreatitis might open a window for PDAC research similar to that of NASH‐derived hepatocellular carcinoma. Recent advances in cancer medicine have overcome 50% of cancer types, and this percentage will increase further in the next 10–20 years. Pancreatic ductal adenocarcinoma is probably the final target in which medical researchers still need to clarify a precise etiology, find a method for early detection, and provide more effective therapy. We believe that the early detection of PDAC using glycobiomarkers to identify high‐risk groups for PDAC could provide a solution and future directions for PDAC research.
Disclosure Statement
The authors have no conflicts of interest.
Abbreviations
- AAL
Aleuria aurantia lectin
- Fuc‐Hpt
fucosylated haptoglobin
- NAFPD
non‐alcoholic fatty pancreas disease
- NASH
non‐alcoholic steatohepatitis
- PanIN
pancreatic intraneoplasia
- PDAC
pancreatic ductal adenocarcinoma
- PhoSL
Pholiota squarrosa lectin
- ROC
receiver–operating characteristic
Acknowledgments
We would like to thank Naoyuki Taniguchi (Rikken), Naofumi Uozumi, Hitoshi Matsumoto, Shinichiro Shinzaki, Shinji Takamatsu, Kenta Moriwaki, Masahiro Tanemura, Hiroaki Nagano, Hidetoshi Eguchi, Noriko Okuyama, Yuri Takeda, Kanako Azuma, Makiko Ueda, Tomohiro Maekawa (Osaka University), Miyako Nakano (Hiroshima University), Yasuhiko Tomita (Osaka Medical Center for Cancer and Cardiovascular Diseases), and many members in our laboratory for performing and helping with the PDAC and glycobiology investigations (titles omitted). This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grant No. JP16H05226) and the Princess Takamatsu Cancer Research Fund.
Cancer Sci 107 (2016) 1357–1362
Funding Information
Japan Society for the Promotion of Science; Princess Takamatsu Cancer Research Fund.
References
- 1. Garrido‐Laguna I, Hidalgo M. Pancreatic cancer: from state‐of‐the‐art treatments to promising novel therapies. Nat Rev Clin Oncol 2015; 12: 319–34. [DOI] [PubMed] [Google Scholar]
- 2. Jones S, Zhang X, Parsons DW et al Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321: 1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexandrov LB, Nik‐Zainal S, Wedge DC et al Signatures of mutational processes in human cancer. Nature 2013; 500: 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waddell N, Pajic M, Patch AM et al Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 2012; 18: 4266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vogelstein B, Fearon ER, Hamilton SR et al Genetic alterations during colorectal‐tumor development. N Engl J Med 1988; 319: 525–32. [DOI] [PubMed] [Google Scholar]
- 7. Hruban RH, Adsay NV, Albores‐Saavedra J et al Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001; 25: 579–86. [DOI] [PubMed] [Google Scholar]
- 8. Basturk O, Hong SM, Wood LD et al A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 2015; 39: 1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falasca M, Kim M, Casari I. Pancreatic cancer: current research and future directions. Biochim Biophys Acta 2016; 1865: 123–32. [DOI] [PubMed] [Google Scholar]
- 10. di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology 2013; 144: 1220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guerra C, Schuhmacher AJ, Canamero M et al Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K‐Ras oncogenes in adult mice. Cancer Cell 2007; 11: 291–302. [DOI] [PubMed] [Google Scholar]
- 12. Daniluk J, Liu Y, Deng D et al An NF‐kappaB pathway‐mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Investig 2012; 122: 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu S, Chheda C, Ouhaddi Y et al Characterization of mouse models of early pancreatic lesions induced by alcohol and chronic pancreatitis. Pancreas 2015; 44: 882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khasawneh J, Schulz MD, Walch A et al Inflammation and mitochondrial fatty acid beta‐oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A. 2009; 106: 3354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem 1988; 57: 785–838. [DOI] [PubMed] [Google Scholar]
- 16. Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem 2008; 143: 725–9. [DOI] [PubMed] [Google Scholar]
- 17. Okuyama N, Ide Y, Nakano M et al Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int J Cancer 2006; 118: 2803–8. [DOI] [PubMed] [Google Scholar]
- 18. Nakano M, Nakagawa T, Ito T et al Site‐specific analysis of N‐glycans on haptoglobin in sera of patients with pancreatic cancer: a novel approach for the development of tumor markers. Int J Cancer 2008; 122: 2301–9. [DOI] [PubMed] [Google Scholar]
- 19. Miyoshi E, Shinzaki S, Moriwaki K, Matsumoto H. Identification of fucosylated haptoglobin as a novel tumor marker for pancreatic cancer and its possible application for a clinical diagnostic test. Methods Enzymol 2010; 478: 153–64. [DOI] [PubMed] [Google Scholar]
- 20. Kamada Y, Kinoshita N, Tsuchiya Y et al Reevaluation of a lectin antibody ELISA kit for measuring fucosylated haptoglobin in various conditions. Clin Chim Acta 2013; 417: 48–53. [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto H, Shinzaki S, Narisada M et al Clinical application of a lectin‐antibody ELISA to measure fucosylated haptoglobin in sera of patients with pancreatic cancer. Clin Chem Lab Med 2010; 48: 505–12. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa T, Uozumi N, Nakano M et al Fucosylation of N‐glycans regulates the secretion of hepatic glycoproteins into bile ducts. J Biol Chem 2006; 281: 29797–806. [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa T, Moriwaki K, Terao N et al Analysis of polarized secretion of fucosylated alpha‐fetoprotein in HepG2 cells. J Proteome Res 2012; 11: 2798–806. [DOI] [PubMed] [Google Scholar]
- 24. Kleiner DE, Brunt EM, Van Natta M et al Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–21. [DOI] [PubMed] [Google Scholar]
- 25. Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol 2010; 16: 5286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon Y, Torok N, Krueger E, Oswald B, McNiven MA. Ethanol‐induced alterations of the microtubule cytoskeleton in hepatocytes. Am J Physiol 1998; 274: G757–66. [DOI] [PubMed] [Google Scholar]
- 27. Kamada Y, Akita M, Takeda Y et al Serum fucosylated haptoglobin as a novel diagnostic biomarker for predicting hepatocyte ballooning and nonalcoholic steatohepatitis. PLoS One 2013; 8: e66328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowenfels AB, Maisonneuve P, Cavallini G et al Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993; 328: 1433–7. [DOI] [PubMed] [Google Scholar]
- 29. Ammann RW. A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas 1997; 14: 215–21. [DOI] [PubMed] [Google Scholar]
- 30. Sarles H. Definitions and classifications of pancreatitis. Pancreas 1991; 6: 470–4. [DOI] [PubMed] [Google Scholar]
- 31. Tomita Y, Azuma K, Nonaka Y et al Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas 2014; 43: 1032–41. [DOI] [PubMed] [Google Scholar]
- 32. van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas 2010; 39: 1185–90. [DOI] [PubMed] [Google Scholar]
- 33. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 34. Bugianesi E, Leone N, Vanni E et al Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002; 123: 134–40. [DOI] [PubMed] [Google Scholar]
- 35. Kobayashi Y, Tateno H, Dohra H et al A novel core fucose‐specific lectin from the mushroom Pholiota squarrosa . J Biol Chem 2012; 287: 33973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimomura M, Nakayama K, Azuma K et al Establishment of a novel lectin‐antibody ELISA system to determine core‐fucosylated haptoglobin. Clin Chim Acta 2015; 446: 30–6. [DOI] [PubMed] [Google Scholar]
- 37. Ueda M, Kamada Y, Takamatsu S et al Specific increase in serum core‐fucosylated haptoglobin in patients with chronic pancreatitis. Pancreatology 2016; 16: 238–43. [DOI] [PubMed] [Google Scholar]
- 38. Maekawa T, Kamada Y, Ebisutani Y et al Serum Mac‐2 binding protein is a novel biomarker for chronic pancreatitis. World J Gastroenterol 2016; 22: 4403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamada Y, Fujii H, Fujii H et al Serum Mac‐2 binding protein levels as a novel diagnostic biomarker for prediction of disease severity and nonalcoholic steatohepatitis. Proteomics Clin Appl 2013; 7: 648–56. [DOI] [PubMed] [Google Scholar]
- 40. Kamada Y, Ono M, Hyogo H et al A novel noninvasive diagnostic method for nonalcoholic steatohepatitis using two glycobiomarkers. Hepatology 2015; 62: 1433–43. [DOI] [PubMed] [Google Scholar]
- 41. Shimosegawa T. To establish the “early chronic pancreatitis”. Nihon Shokakibyo Gakkai zasshi = Jpn J Gastroenterol 2016; 113: 1–10. [DOI] [PubMed] [Google Scholar]
- 42. DuFort CC, DelGiorno KE, Hingorani SR. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology 2016; 150: 1545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanemura M, Miyoshi E, Nagano H et al Role of alpha‐gal epitope/anti‐Gal antibody reaction in immunotherapy and its clinical application in pancreatic cancer. Cancer Sci 2013; 104: 282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanemura M, Miyoshi E, Nagano H et al Cancer immunotherapy for pancreatic cancer utilizing alpha‐gal epitope/natural anti‐Gal antibody reaction. World J Gastroenterol 2015; 21: 11396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herreros‐Villanueva M, Gironella M, Castells A, Bujanda L. Molecular markers in pancreatic cancer diagnosis. Clin Chim Acta 2013; 418: 22–9. [DOI] [PubMed] [Google Scholar]
- 46. Uozumi N, Gao C, Yoshioka T et al Identification of a novel type of CA19‐9 carrier in human bile and sera of cancer patients: an implication of the involvement in nonsecretory exocytosis. J Proteome Res 2010; 9: 6345–53. [DOI] [PubMed] [Google Scholar]
- 47. Terao N, Takamatsu S, Minehira T et al Fucosylation is a common glycosylation type in pancreatic cancer stem cell‐like phenotypes. World J Gastroenterol 2015; 21: 3876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


