Abstract
Objectives
Neoadjuvant chemotherapy (NAC) before radical cystectomy is the standard of care for muscle-invasive bladder cancer (MIBC). Many patients are referred to an academic medical center (AMC) for cystectomy but receive NAC in the community setting. This study examines if administration of NAC in the community is associated with differences in type of NAC received, pathologic response rate (pT0), and time to cystectomy as compared to NAC administered at an AMC.
Methods
We performed a retrospective study of patients with MIBC (cT2a-T4-Nx-M0) referred to a single AMC between 1/2012 and 1/2014 who received NAC. We analyzed chemotherapy received, time to cystectomy, pT0, and survival in patients who received NAC in our AMC compared to those treated in the community.
Results
In all, 47 patients were analyzed. A similar total dose of cisplatin (median: 280 mg/m2 for both groups, P =0.82) and pT0 rate (25% vs. 29%, P =0.72) were seen in patients treated in our AMC and the community. However, administration of NAC in the community was associated with a prolonged time to cystectomy compared with that in our AMC (median number of days 162 vs. 128, P < 0.01). This remained significant after adjusting for stage, comorbidity status, and distance to the AMC (P =0.02). Disease-free survival and overall survival did not differ.
Conclusion
Patients with MIBC treated with NAC in the community as compared to an AMC received similar chemotherapy and achieved comparable pT0 rates, indicating effective implementation of NAC in the community. However, NAC in the community was associated with longer time to cystectomy, suggesting a delay in the transition of care between settings.
Keywords: Cystectomy, Neoadjuvant therapy, Urinary bladder neoplasms, Time factors, Quality indicators
1. Introduction
Bladder cancer is the 6th most common cancer in the United States, with an estimated 74,690 new cases and 15,580 deaths for 2014 [1]. Although most cases are noninvasive at initial diagnosis, nearly 25% of these cases progress to muscle-invasive bladder cancer (MIBC) and 20% to 30% of patients have MIBC at the time of diagnosis [2].
Treatment of MIBC requires coordinated multidisciplinary care that often stretches across practice settings. Neoadjuvant chemotherapy (NAC) before radical cystectomy represents a standard of care for MIBC based on randomized clinical trials and a meta-analysis [3–5]. The gradual but steady increase in implementation of NAC over the last 15 years has made transitions in care more common and important [6]. Many patients are referred to an academic medical center (AMC) upon diagnosis of MIBC for consideration of cystectomy because many community urologists do not routinely perform cystectomies, and centers with high surgical volume have improved perioperative mortality and long-term mortality rates [7–9]. Many of these patients return to community oncologists for NAC before cystectomy, with others receiving NAC at the AMC in which they undergo surgery.
Treatment of patients with MIBC at different centers raises a potential issue of treatment coordination and delays [10,11]. A treatment delay of more than 3 months from initial diagnosis to cystectomy has been associated with decreased overall survival (OS) in several studies of patients who did not receive NAC [12,13]. There is also an association of a treatment delay with worse pathologic stage at the time of cystectomy [12–19]. An association of delay with adverse outcomes in patients receiving NAC, however, is less well studied.
It is not known if patients who are referred to the community setting for NAC have a delay in cystectomy compared with patients who remain at the referral AMC for NAC. It is also not known if delay to cystectomy is associated with a decrease in survival or pathologic response rate (pT0) in patients who receive NAC in these different settings. The goals of this retrospective study are to examine time to cystectomy, chemotherapy received, pT0, and survival in patients who receive NAC before cystectomy in the community setting compared to an AMC.
2. Materials and methods
2.1. Study population
We performed a retrospective chart review at a single academic institution using the University of North Carolina Genitourinary OncoLogy Database (UNC GOLD) [20], a clinical database that captures all patients seen in the UNC multidisciplinary genitourinary oncology clinic. This study was approved by the Institutional Review Board at UNC.
A query of UNC GOLD for all MIBC (T2-T4-Nx-M0) cases between January 1, 2012 and January 2, 2014 revealed 94 patients recommended to undergo cystectomy at their initial visit after diagnosis. A medical record chart review of these patients revealed that 47 of 94 patients (50%) received NAC—these patients formed our study cohort. Of those who did not receive NAC, the most common reasons included renal dysfunction (26%), patient preference (21%), and hearing loss (14%).
2.2. Study variables
Data collected included patient demographics, stage, comorbidity score, site of NAC delivery (i.e., AMC or community), chemotherapeutic regimen, time to cystectomy, pathologic response rate, disease-free survival (DFS), and OS. Time to cystectomy was calculated from the day of initial consultation with medical oncology at our institution to the day of cystectomy. Stage was determined by American Joint Committee on Cancer TNM stage recorded before NAC (clinical stage) and after cystectomy (pathologic stage). Comorbidity score was calculated using age-adjusted Charlson Comorbidity index score [21]. Distance to the AMC was calculated from the site of each patient’s residence to our institution. The date of cessation of NAC was defined as the last date the patient received any chemotherapy. The NAC regimen for each patient was recommended by the medical oncologist at our AMC for all patients. Pathologic response rate was defined as pT0 (pT0-N0-M0) at the time of cystectomy. Rate of <pT2 (pT0-T1-N0-M0) at the time of cystectomy was also examined. Patients with available data were evaluated for bladder cancer progression/recurrence and survival.
The primary exposure variable was whether NAC was given at AMC or a community oncology practice. The primary outcome was time to cystectomy from date of initial visit at AMC. Secondary outcomes were pathologic response rate and total dose of cisplatin received with NAC, DFS, and OS.
2.3. Statistical analysis
Descriptive statistics were obtained for continuous and categorical variables. Means and standard deviation were used for continuous variables and frequencies for categorical variables. Fisher’s exact tests were used to compare categorical variables and Wilcoxon rank sums were used for continuous variables. Multivariable linear regression was used for the model with outcome of time to cystectomy in days. Cox proportional hazards analysis was used for DFS and OS calculations. All statistical analysis was conducted using SAS software (SAS Institute Inc., Cary, NC) and a P < 0.05 was considered statistically significant.
3. Results
In all, 47 patients received NAC. Of these, 31 patients (66%) received NAC in the community and 16 (34%) received NAC at our AMC. Of the patients treated at our AMC, 3 received NAC as part of a clinical trial. Demographic variables are listed in Table 1 and were similar between those who received NAC at our AMC and those treated in the community. Patients treated with NAC in the community lived at a greater distance from our AMC than those treated at our institution (130 vs. 58 miles, P =0.0003).
Table 1.
Patient characteristics of patients who received NAC in our AMC or in the community
| Demographic | Overall population (n = 47) | NAC in community (n = 31) | NAC in AMC (n = 16) | P value |
|---|---|---|---|---|
| Age, median (range) | 61 (31–86) | 64 (31–86) | 58 (36–74) | 0.18 |
| Male gender | 34 (72.3%) | 22 (71.0%) | 12 (75.0%) | 1.0 |
| Race | ||||
| White | 42 (89.4%) | 29 (93.6%) | 13 (81.3%) | 0.17 |
| Black | 4 (8.5%) | 1 (3.2%) | 3 (18.8%) | |
| Smoking status | ||||
| Current | 13 (27.7%) | 10 (32.3%) | 3 (18.8%) | 0.46 |
| Former | 28 (59.6%) | 18 (58.1%) | 10 (62.5%) | |
| Never | 6 (12.8%) | 3 (9.7%) | 3 (18.8%) | |
| BMI, kg/m2, median (range) | 30 (15–43) | 30 (19–43) | 29 (15–38) | 0.40 |
| Marital status | ||||
| Married | 30 (69.8%) | 20 (71.4%) | 10 (66.7%) | 0.74 |
| Not married | 13 (30.2%) | 8 (28.6%) | 5 (33.3%) | |
| No insurance | 3 (6.4%) | 2 (6.5%) | 1 (6.3%) | 1.0 |
| Age-adjusted CCI score, median (range) | 5 (2–13) | 5 (2–13) | 3.5 (2–8) | 0.17 |
| Distance to AMC, miles (range) | 83 (1–277) | 130 (36–277) | 58 (1–126) | 0.0003 |
| Stage at diagnosis | ||||
| 2 | 36 (76.6%) | 24 (77.4%) | 12 (75%) | 0.75 |
| 3 | 4 (8.5%) | 2 (6.5%) | 2 (12.5%) | |
| 4 | 7 (14.9%) | 5 (16.1%) | 2 (12.5%) | |
| Preoperative creatinine, mg/dl median (range) | 0.97 (0.58–1.55) | 0.99 (0.59–1.55) | 0.96 (0.58–1.41) | 0.45 |
| Histology | ||||
| Urothelial | 45 (95.7%) | 29 (93.5%) | 16 (100%) | 0.54 |
| Other | 2 (4.3%) | 2 (6.5%) | 0 (0%) | |
All but 1 patient received cisplatin-based combination chemotherapy and the regimen of gemcitabine and cisplatin (GC) was the most commonly administered (85% of patients received GC with most of them using every 3 wk dosing intervals). The median total dose of cisplatin received during NAC was 280 mg/m2 in both groups with no difference in dose between those who were treated at our AMC and those treated in the community (P =0.83). The median number of cycles was 4 in both groups with no statistical difference between groups (P =0.09). The single patient who received carboplatin instead of cisplatin was treated in the community. The median number of days between starting and ending NAC was not different between the 2 groups (P =0.33).
Of the original 47 patients, 38 underwent cystectomy at our AMC and had pathology available for review. One patient underwent cystectomy at an outside institution and pathology results were not available. Because of progression of disease during NAC (4 patients), patient preference (3 patients), and death unrelated to NAC (1 patient), 8 patients did not undergo cystectomy. Of the patients who received NAC in the community, 81% eventually underwent cystectomy compared with 88% of patients at our AMC.
The median number of days between the initial visit with medical oncology at our AMC and cystectomy was significantly longer in the patients who were referred to the community setting for NAC than those who received NAC at our AMC (162 vs. 128 d, P =0.006). The median number of days between diagnostic TURBT and cystectomy was also significantly longer if NAC was given in the community setting (189 vs. 151 d, P =0.015). After controlling for stage at diagnosis, comorbidity score, and distance to the AMC, those who received NAC at our AMC had their cystectomy an average of 43 days earlier (P =0.015). Increasing stage and comorbidity score were associated with longer time to cystectomy on univariable analysis, although this association was not statistically significant in the multivariable model (P =0.14 and P =0.26, respectively). Distance to the AMC was not associated with time to cystectomy (P =0.63). In all, 6 patients had a delay of longer than 6 months between initial visit and cystectomy. Of these, 5 had NAC administered in the community. Delays were due to scheduling issues (4 patients) and complications (1 myocardial infarction requiring CABG, and 1 cerebral vascular event). The numbers of days of delay in each step of the treatment process are reported in Table 2. The time from initial visit with medical oncology to starting NAC was significantly longer in the group treated in the community (P =0.002) but the delay in other steps of the process did not independently reach significance.
Table 2.
Median number of days of delay in each step of process of receiving NAC in our AMC and in community setting
| NAC in AMC | NAC in community | P value | |
|---|---|---|---|
| Initial visit to starting NAC | 11 | 21 | 0.002 |
| Starting to ending NAC | 64 | 68 | 0.33 |
| Ending NAC to urology visit | 24 | 30 | 0.48 |
| Urology visit to cystectomy | 32 | 37.5 | 0.18 |
| Initial visit to cystectomy | 128 | 162 | 0.006* |
P =0.015 after adjusting for stage, comorbidity status, and distance to AMC.
Of the patients who received NAC at our AMC, 50% had <pT2 and 29% had pT0 at the time of cystectomy. Of the patients who received NAC in the community, 50% had <pT2 and 25% had pT0 at time of cystectomy (Table 3). There was no difference in pathologic response rate between the groups (P =1.0 and P =0.81 for <pT2 and pT0, respectively).
Table 3.
Pathologic response rate at cystectomy and median dose of cisplatin received in those who received NAC in AMC vs. that in the community
| NAC in AMC (n = 14) | NAC in community (n =24) | P value | |
|---|---|---|---|
| pT0 at time of cystectomy | 4 (28.6%) | 6 (25.0%) | 0.81 |
| <pT2 at time of cystectomy | 7 (50.0%) | 12 (50.0%) | 1.0 |
| Median total dose of cisplatin received, mg/m2 | 280 | 280 | 0.82 |
Of those patients who received NAC at our AMC and in the community, 4 (25%) and 3 (10%) are known to have passed away at the last follow-up, respectively. Median OS was not reached in either group and the difference in OS was not statistically significant (P =0.20) (Fig. 1). DFS was also not different between the groups (P =0.50). Survival analyses included the patients who did not undergo cystectomy in addition to those who did. Median follow-up was 9.7 months in the group that received NAC at our institution and 6.7 months in the group that received NAC in the community.
Fig. 1.
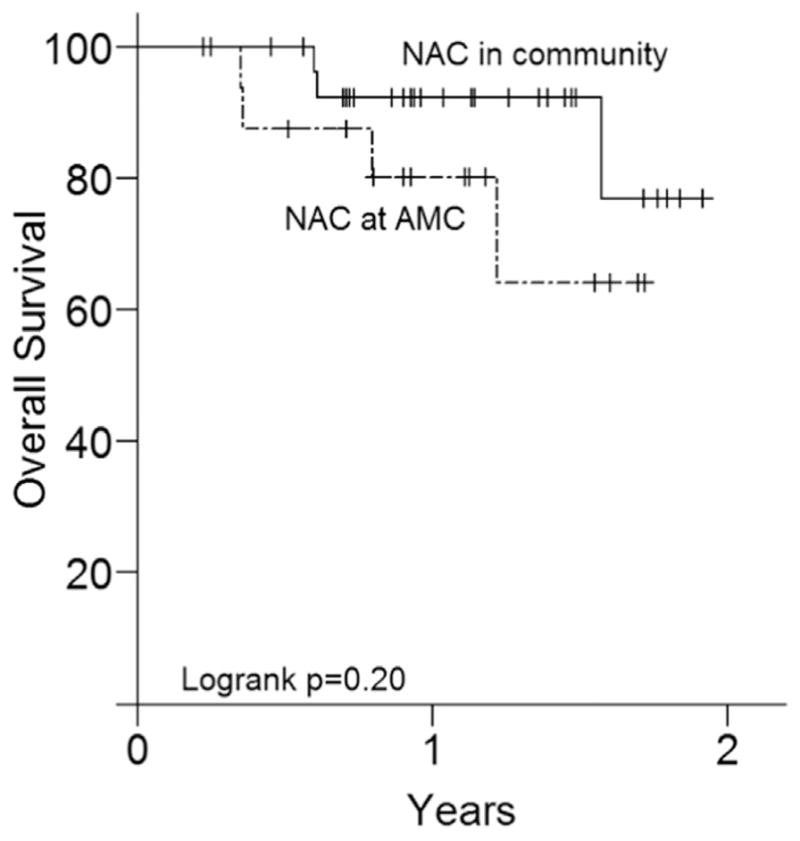
Overall survival in patients who received NAC in AMC and in the community.
4. Discussion
The current study demonstrated that time to cystectomy is delayed in patients with MIBC who were referred to the community setting to receive NAC compared with those who received it at an AMC. However, patients in the community and AMC received similar chemotherapy regimens and dosing, and there was no difference in pathologic response rate (including pT0), DFS, or OS.
When each step in the process of NAC administration was examined, the only significant time difference between those who received NAC at our institution and in the community was the delay between initial visit with medical oncology at our AMC and date of starting chemotherapy. This suggests that the delay to cystectomy is related to poor transitions of care between the AMC and community setting, as opposed to differences in the administration of chemotherapy or complications during treatment. This is supported by our findings that the total dose of cisplatin, number of cycles, and chemotherapy regimens administered were not different between the 2 groups, suggesting that the implementation and administration of NAC in the community setting was effective and similar to that in the academic setting. Lee et al. [13] have also suggested that delay to cystectomy is commonly due to avoidable issues such as scheduling and not due to patient-derived factors. It should be noted that the duration of chemotherapy (median: 64–68 d) would be the expected value for patients receiving 4 cycles of GC NAC because the date of cessation of NAC was defined as that of the last dose of chemotherapy administered. There was a delay of more than 30 days in both groups between the urology visit after cessation of NAC and cystectomy, suggesting that delays in scheduling of cystectomy may contribute to the time to cystectomy in all patients. Although the patients who received NAC in the community lived a greater distance from our AMC, the difference in time to cystectomy between the 2 groups remained significant even after adjusting for distance, further suggesting that the transition of care between healthcare settings was driving the delay.
Our study is in agreement with a recent analysis by Tomaszewski et al. [10] using the National Cancer DataBase showing that patients with MIBC who changed hospitals between diagnosis and definitive therapy (which included NAC or radical cystectomy) were more likely to experience a treatment delay of 3 months or longer. In that study, care transitions were associated with black race, male gender, Medicare or no insurance status, and treatment at an AMC. Our study did not find that such certain subgroups were more likely to have NAC at an outside facility, although it was not powered for such conclusions. Our study adds the knowledge that although a change in institution during treatment is associated with a delay, the actual chemotherapy regimens and dosages are consistent across settings.
Alva et al. [22] conducted a single-institution retrospective review and found that administration of NAC at an outside facility did not delay time between NAC completion and cystectomy. However, they did note that scheduling issues occurred disproportionately in the group of patients who received NAC outside their institution, suggesting the need for improved communication in care transitions. Our study suggested that a large portion of the delay experienced by patients treated at an outside facility may occur before initiating NAC, which was not examined in the Alva study.
The clinical relevance of a 43-day delay in cystectomy in patients who received NAC at an outside facility is not clear. The median number of days from initial visit to cystectomy at our AMC was 128, which is in keeping with the observed time from randomization to cystectomy in trials of NAC [3]. We found that pathologic response rate, DFS, and OS were not different depending on the site of NAC administration, suggesting that the observed delay did not translate to observable clinical significance. Our sample size may not be large enough to see an effect of delay to cystectomy on pathologic response rate and survival although Alva et al. [22] similarly did not see an association between time to cystectomy after NAC termination and survival. However, there are several studies demonstrating an association between inferior OS and delay to cystectomy of more than 12 weeks and OS appears to start decreasing at approximately 40 days after diagnosis in patients who do not receive NAC [12,14,15,23]. The acceptable time of delay to cystectomy remains unclear in patients undergoing NAC.
A limitation of this retrospective chart review is that we cannot be certain that patients treated with NAC at our AMC are the same as those treated at a community site, introducing the potential for unmeasured confounders. For example, although we had insurance information, we were unable to fully account for socioeconomic status in our analysis. In a study of breast cancer, several socioeconomic factors such as lack of insurance and low education were associated with increased time to surgery after diagnosis and increased time to adjuvant chemotherapy after surgery [24]. Another major limitation in our study is the limited follow-up data due to the fact that many patients received postcystectomy care at an outside facility. Our survival analysis is therefore limited.
Despite these limitations, we believe our institution is representative of a typical AMC. Our pathologic response rates are in keeping with observed rates in clinical trials of NAC (pT0 was 32.5% in a large randomized trial, 28.5% in a meta-analysis of 13 trials, and 26.3% in our study) [3]. In addition, 50% of patients with MIBC in our study received NAC, which is well above the national average [6].
5. Conclusion
Overall, the current study demonstrates a delay in time to cystectomy when NAC is administered in the community as compared with an AMC without a clear association with adverse outcomes. Transitions of care appear to be responsible for this delay. Future efforts should be directed toward ensuring fluid and efficient care transitions for patients with MIBC. The study also demonstrates that appropriate administration of NAC occurs in the community setting. Subsequent research should focus on the association between delay in cystectomy and survival outcomes in patients receiving NAC.
Acknowledgments
M.I.M. has received research funding from Pfizer (United States), BIND Therapeutics, Dendreon, Exelixis, Johnson and Johnson (United States), Astellas Pharma US, Mirati Therapeutics, Merck (United States), and Cerulean Pharma, Inc. M.E.N. was supported in part by the American Cancer Society, United States (Grant no. MRSG-13-154-01-CPPB) and the Urology Care Foundation/Astellas. A.B.S. was supported in part by the National Center for Research Resources, United States, the National Center for Advancing Translational Sciences, United States, National Institutes of Health, United States, through Grant no. KL2TR000084, and PCORI, United States. Y.E.W. has received research funding from Johnson and Johnson (United States) and Astellas Pharma US.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–8. [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4.International Collaboration of Trialists. Neoadjuvant cisplatin methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial, International collaboration of trialists. Lancet. 1999;354:533–40. [PubMed] [Google Scholar]
- 5.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neo-adjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 6.Zaid HB, Patel SG, Stimson CJ, Resnick MJ, Cookson MS, Barocas DA, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology. 2014;83:75–80. doi: 10.1016/j.urology.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 8.Konety BR, Dhawan V, Allareddy V, Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. 2005;173:1695–700. doi: 10.1097/01.ju.0000154638.61621.03. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer. 2013;119:3546–54. doi: 10.1002/cncr.28235. [DOI] [PubMed] [Google Scholar]
- 10.Tomaszewski JJ, Handorf E, Corcoran AT, Wong YN, Mehrazin R, Bekelman JE, et al. Care transitions between hospitals are associated with treatment delay for patients with muscle invasive bladder cancer. J Urol. 2014;192:1349–54. doi: 10.1016/j.juro.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liedberg F, Anderson H, Mansson W. Treatment delay and prognosis in invasive bladder cancer. J Urol. 2005;174:1777–81. doi: 10.1097/01.ju.0000177521.72678.61. discussion 81. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. 2009;182:1318–24. doi: 10.1016/j.juro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. 2006;175:1262–7. doi: 10.1016/S0022-5347(05)00644-0. discussion 7. [DOI] [PubMed] [Google Scholar]
- 14.Fahmy N, Kassouf W, Jeyaganth S, Amin M, Mahmud S, Steinberg J, et al. An analysis of preoperative delays prior to radical cystectomy for bladder cancer in Quebec. Can Urol Assoc J. 2008;2:102–8. doi: 10.5489/cuaj.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a surveillance, epidemiology, and end results-medicare analysis. Cancer. 2009;115:988–96. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May M, Nitzke T, Helke C, Vogler H, Hoschke B. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. 2004;38:231–5. doi: 10.1080/00365590410029141. [DOI] [PubMed] [Google Scholar]
- 17.Chang SS, Hassan JM, Cookson MS, Wells N, Smith JA., Jr Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol. 2003;170:1085–7. doi: 10.1097/01.ju.0000086828.26001.ca. [DOI] [PubMed] [Google Scholar]
- 18.Hara I, Miyake H, Hara S, Gotoh A, Okada H, Arakawa S, et al. Optimal timing of radical cystectomy for patients with invasive transitional cell carcinoma of the bladder. Jpn J Clin Oncol. 2002;32:14–8. doi: 10.1093/jjco/hyf002. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110–5. doi: 10.1016/S0022-5347(05)64047-5. discussion 5. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher SA, Smith AB, Matthews JE, Potter CW, Woods ME, Raynor M, et al. Roadmap for the development of the University of North Carolina at Chapel Hill Genitourinary OncoLogy Database—UNC GOLD. Urol Oncol. 2014;32(32):e1–9. doi: 10.1016/j.urolonc.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–92. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 22.Alva AS, Tallman CT, He C, Hussain MH, Hafez K, Montie JE, et al. Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer: a multidisciplinary approach. Cancer. 2012;118:44–53. doi: 10.1002/cncr.26240. [DOI] [PubMed] [Google Scholar]
- 23.Mahmud SM, Fong B, Fahmy N, Tanguay S, Aprikian AG. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol. 2006;175:78–83. doi: 10.1016/S0022-5347(05)00070-4. discussion. [DOI] [PubMed] [Google Scholar]
- 24.Liederbach E, Sisco M, Wang C, Pesce C, Sharpe S, Winchester DJ, et al. Wait times for breast surgical operations, 2003–2011: a report from the National Cancer Data Base. Ann Surg Oncol. 2015;22(3):899–907. doi: 10.1245/s10434-014-4086-7. [DOI] [PubMed] [Google Scholar]


