Abstract
Objectives:
The purpose of this case-control study was to find a correlation between certain imaging findings in dental panoramic radiographs and the risk for developing a medication-related osteonecrosis of the jaw (MRONJ) in patients taking antiresorptive therapy (AT).
Methods:
Randomized and blinded dental panoramic radiographs of 60 patients undergoing antiresorptive drug treatment (36 patients with MRONJ, 24 patients without MRONJ) and of 60 patients without AT were analyzed by 3 experts for the following signs: sequestrum, osteosclerosis, difference in sclerosing of alveolar process and body of mandible, visible alveolar socket, enhancement and loss of lamina dura, enhancement of the oblique ridge, enhancement of the mandibular canal, proliferative periostitis and osteolytic processes at the cortex.
Results:
Signs were seen significantly more often in patients undergoing AT than in the control group (CG) (osteosclerosis p-value = 0.019, visible alveolar socket p-value = 0.001, enhancement of lamina dura p-value < 0.001, enhancement of the mandibular canal p-value = 0.025, proliferative periostitis p-value = 0.05 and osteolytic processes at the cortex p-value < 0.001). While there is no significant difference between the CG and the group of patients with AT without manifest MRONJ for any sign, the significance increases when taking the group of patients under AT with manifest MRONJ into consideration. In addition, if medication was administered for malignant reasons, the signs visible alveolar socket, enhancement of the lamina dura and the enhancement of the mandibular canal were seen significantly more often.
Conclusions:
The radiographic findings mentioned above are not indicators for the development of MRONJ, as they are seen only in patients with manifest osteonecrosis. However, these findings could be important to assess the dimension and potency of a MRONJ.
Keywords: panoramic radiography, MRONJ, radiographic findings, bisphosphonates, denosumab
Introduction
Bisphosphonates and denosumab (AMGEN, Thousand Oaks, CA) are acting as antiresorptive medications, which are used—owing to their capability to inactivate osteoclasts—in conditions with increased bone resorption such as osteoporosis, Paget's disease of bone and osseous metastases. Bisphosphonates are also indicated for the treatment of multiple myeloma.1,2 While bisphosphonates interfere within the mevalonate pathway inside the osteoclasts and lead to apoptosis of those cells, denosumab—a human monoclonal antibody—imitates the effect of osteoprotegerin within the receptor activator of NF-κB/RANK-ligand/osteoprotegerin pathway (RANK/RANKL/osteoprotegerin pathway), which inhibits the differentiation and function of osteoclasts.3,4 By reducing the bone resorption, both drugs cause a gain in bone mass. Unlike bisphosphonates, denosumab does not accumulate inside the bone.1
For the first time in 2003, Marx described that the side effect of bisphosphonates is the osteonecrosis of the jaw.5,6 Ever since, this clinical picture has been known as bisphosphonate-related osteonecrosis of the jaw (BRONJ).7,8 As it also appears in patients treated with denosumab—which got its approval for use in the European Union in 2010—the “American Association of Oral and Maxillofacial Surgeons” (AAOMS) recommend, in their 2014 updated position paper on BRONJs, the use of the term medication-related osteonecrosis of the jaw (MRONJ).1,4,8–10 MRONJ has been defined by the AAOMS as follows: “(1) current or previous treatment with antiresorptive […] agents; (2) exposed bone or bone that can be probed through an intraoral or extraoral fistula[e] in the maxillofacial region that has persisted for more than 8 weeks; and (3) no history of radiation therapy to the jaws or obvious metastatic disease to the jaws”.1
As the diagnosis of MRONJ is primarily made upon clinical signs, the early detection of the risk of developing an osteonecrosis in the maxillofacial area before showing exposed bones still seems to pose a challenge.1,11,12 One approach can be the detection of specific radiologic signs on dental panoramic radiographs, which has been recently described in the literature.11–20 The assumption that there are such signs to be found is based on the mode of action of the antiresorptive drugs, which lead to a gain of bone mass. Even though this takes place in all bones, osteonecroses are almost only seen in the jaws.21 There, the positive bone balance can result in trabecular bone alterations with a dense woven appearance (osteosclerosis), which may appear first at the alveolar process, a thickening of the lamina dura and/or the cortex in the area of the external oblique ridge. As a result of the more dense bone, the mandibular canal can appear enhanced with a stronger contrast towards the surrounding structures. A reduction in blood flow due to the gain in bone mass and the antiangiogenic effect of bisphosphonates can lead to sequestra, osteolytic processes and faulty bone healing (which could result in persisting alveolar sockets after extraction).11,13,14,17,20,22–24
The purpose of this study was to identify a correlation between those imaging findings in dental panoramic radiographs and the risk for developing a MRONJ in patients taking either bisphosphonates or denosumab. So far, there are only limited data available concerning this subject. Therefore, we designed this case-control study, in which 3 examiners were blinded towards 120 panoramic radiographs.
Methods and materials
Patients
Patient characteristics, dental history and data concerning antiresorptive therapy (AT) were obtained from the clinic internal electronic database of the Department of Oral- and Maxillo-facial Surgery of the University of Regensburg. We collected missing information from the referring dentists and doctors. The dental panoramic radiographs used in this study dated from the period December 2006 till April 2014. All data were used and analyzed anonymously according to the guidelines of the Ethics Committee of the University of Regensburg.
The most important criterion for inclusion into this study was the accessibility to a panoramic radiograph of very good quality. Patients for the review group (RG) needed to fulfil the following criteria: sufficient residual teeth and AT for at least 3 months. Within the RG, we differentiated between patients with manifest MRONJ (AT+MRONJ+) and those without manifest MRONJ (AT+MRONJ−). Dental panoramic radiographs for the control group (CG) needed to be from patients without antiresorptive treatment and sufficient residual teeth. Osteomyelitis, neoplastic lesions, osseous metastases or radiation therapy to the maxillofacial region, benign fibro-osseous lesions and a distinctive periodontitis were exclusion criteria for this study. The case number for each group was 60.
Methods
3 examiners, who were blinded, reviewed all 120 dental panoramic radiographs independently (maxillofacial surgeons from the University of Regensburg with 10–32 years' practical experience). Radiographic findings were documented, particularly the evidence of (1) sequestrum, (2) bone densification in the maxillofacial region (osteosclerosis), (3) difference in sclerosing of the alveolar process and body of the mandible, (4) visible alveolar socket, (5) enhancement and (6) loss of the lamina dura, (7) enhancement of the cortex of the external oblique ridge, (8) enhancement of the mandibular canal, (9) proliferative periostitis at the body of the mandible and (10) osteolytic processes at the cortex. Osteosclerosis—if present—was characterized for its graduation (light—strong) and localization (local—general). Figures 1–7 are shown as examples for the imaging findings mentioned above. The evaluation of the radiographs was performed following a certain scheme: all examiners analyzed the radiographs independently, while the results were documented in an Excel table. Only if all three examiners saw a sign in one radiograph, was it considered as “present”. Consequently, only if all three examiners corresponded that a sign was “not present”, was it considered as such. If one examiner differed from the others, the radiograph was excluded. For objectivity, the sign was statistically analyzed only when it was seen in >75% of all radiographs identically by all three examiners as “present” or “not present”. To determine whether any sign was able to act as an indicator for the development of MRONJ, we generated the p-value separately for the group of patients taking antiresorptive drugs without a manifest MRONJ (AT+MRONJ−) and the patients of the CG.
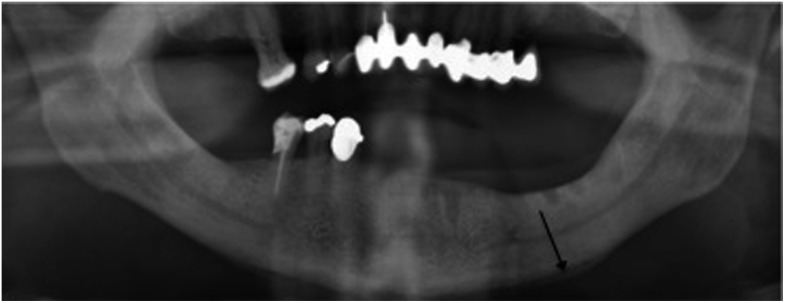
Figure 1.
Sequestrum (black arrow).
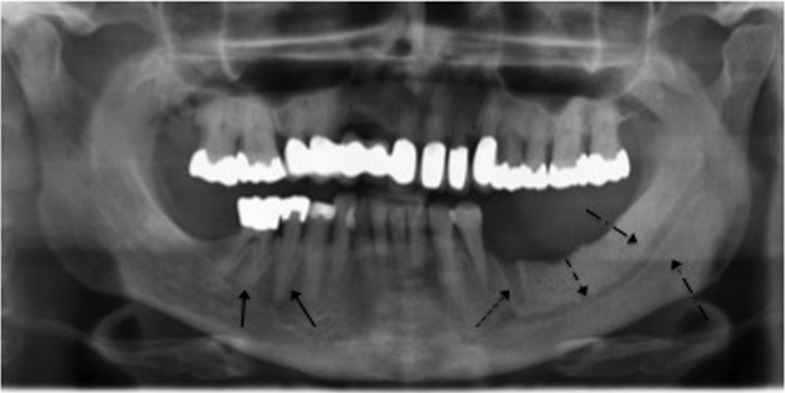
Figure 7.
Osteolytic processes at the cortex (black arrows).
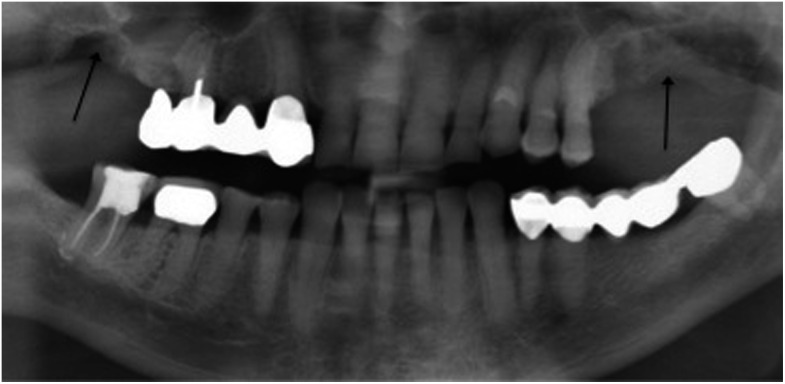
Figure 2.
Multiple imaging findings: osteosclerosis ( ), enhancement of the lamina
dura (
), enhancement of the lamina
dura ( ), visible
extraction socket (
), visible
extraction socket ( ) and enhancement of the mandibular canal (
) and enhancement of the mandibular canal ( ). The sclerosis is localized
on the left mandible and considered as strong sclerosis.
). The sclerosis is localized
on the left mandible and considered as strong sclerosis.
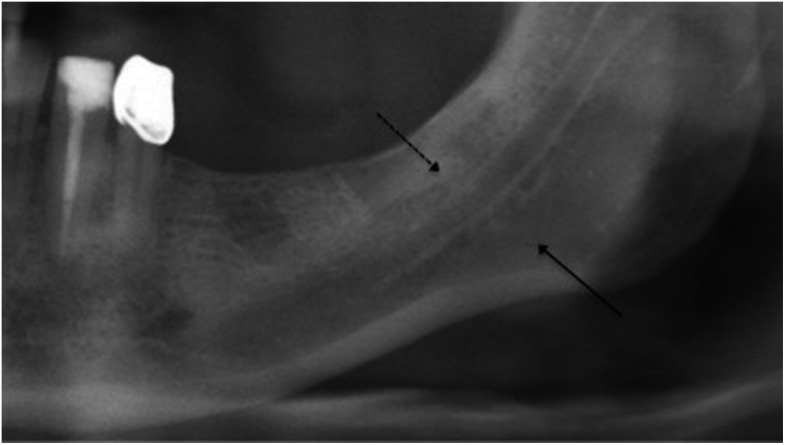
Figure 3.
Difference in sclerosis of the alveolar process and the body of the mandible (black arrows).
Figure 4.
The local sclerosing process at a former extraction site (black arrow).
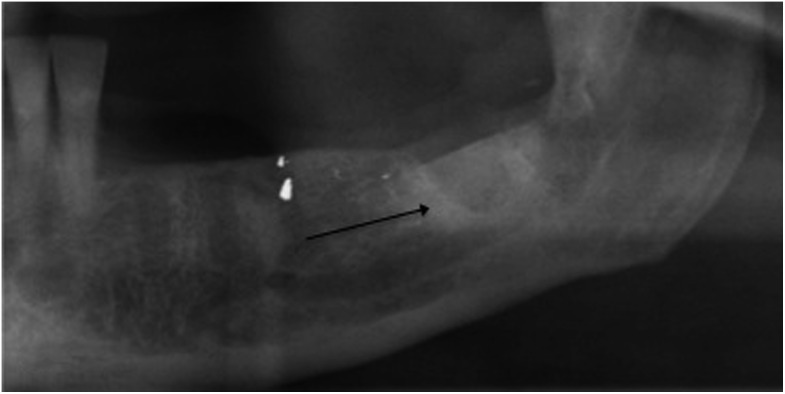
Figure 5.
Proliferative periostitis at the inferior border of the mandible (black arrow).
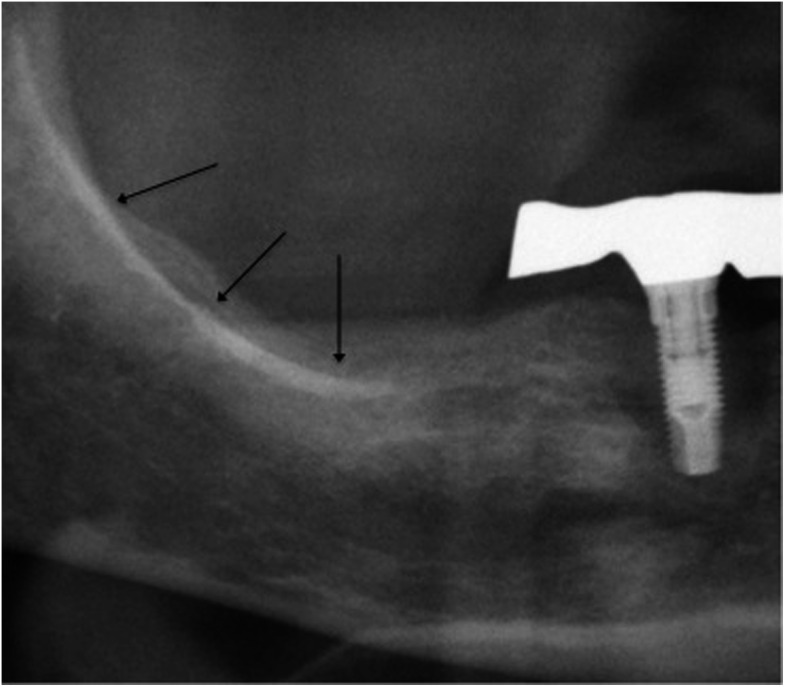
Figure 6.
Enhancement of the external oblique ridge (black arrows).
Statistics
Statistical analysis was performed using the statistical software SPSS® v. 22 for Microsoft (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) and Microsoft Excel® (Microsoft, Redmond, WA). Data were expressed as mean ± standard deviation Fisher's exact test and χ2 test were conducted for the comparison of measured data among different groups. A p-value ≤ 0.05 was considered as statistically significant. We also obtained the odds ratio, sensitivity and specificity. To determine a sufficient case number for this study, a statistician of the University of Regensburg performed sample size determination.
Results
Patient characteristics and data concerning the antiresorptive therapy
The mean age of the patients at the time the radiographs used were taken was 67.6 years in the RG and 63.7 years in the CG. In 40 cases within the RG, the patients were treated with antiresorptive drugs for malignant reasons; 18 patients were treated for benign reasons. In two cases, the AT-causing disease could not be identified. Six patients had multiple diseases that needed AT. Within the RG, 51 patients were treated with bisphosphonates (exact agents: for 24 patients, zoledronic acid; for 13 patients, alendronic acid; for 3 patients, risedronic acid; for 4 patients, pamidronic acid; for 2 patients, ibandronic acid; and for 5 patients, unknown; form of application: 20× oral, 31× i.v.), 2 patients received denosumab subcutaneously and in 7 cases, the medication was readjusted from nitrogen-containing bisphosphonate to denosumab. The mean duration of drug intake was 56.98 months (standard deviation = 51.09). Out of the 40 patients receiving AT for malignant reasons, 30 patients developed a manifest MRONJ, while 6 out of 18 patients receiving AT for benign reasons developed a manifest MRONJ (p-value = 0.004).
Imaging findings
The sign enhancement of the oblique ridge was the only one not assessed as identical by the reviewers concerning the 75% limit and was therefore excluded from statistical analysis.
Six out of nine signs were seen significantly more often in patients treated with antiresorptive drugs than in the CG (sclerosis, visible alveolar socket, an enhanced lamina dura, enhancement of the mandibular canal, proliferative periostitis at the body of the mandible and osteolytic processes at the cortex). In addition, the determination of the odds ratio showed an increased risk for developing any of the 9 signs under AT, except sclerosis (visible alveolar socket OR = 5; enhancement of the lamina dura OR = ∞; enhancement of the mandibular canal OR = 4.8; proliferative periostitis at the body of the mandible OR = ∞; and osteolytic processes at the cortex OR = 19.7). The rather rare frequency of all radiologic findings resulted in a low sensitivity with a high specificity for all signs as summarized—among the p-value and odds ratio—in Table 1. The evaluation of the p-value for the groups AT+MRONJ− and CG resulted in no significant difference for any sign (Table 1). Only the signs enhancement of the lamina dura (OR = ∞), osteolytic processes at the cortex (OR = 8.8) and visible alveolar socket (OR = 2.7) were detected more frequently within the AT+MRONJ− group.
Table 1.
Imaging findings. The p-value, odds ratio, specificity, sensitivity and the frequency for all signs are shown
| Imaging finding | p-value RGavs CG | p-value AT+MRONJ−vs CG | Odds ratio (95% CI) | pspecificity (%) | psensitivity (%) | Frequency RG (%) | Frequency CG (%) |
|---|---|---|---|---|---|---|---|
| Sequestrum | 0.09 | 1 | 3.2 (0.6–16.7) | 96 | 10 | 10 | 3.3 |
| Sclerosing | 0.019 | 1 | 2.76 (1.24–6.14) | 78 | 43 | 43.3 | 27.7 |
| Difference in sclerosing | 0.178 | 1 | 2.5 (0.8–7.6) | 92 | 18 | 18.3 | 8.3 |
| Alveolar socket | 0.001 | 0.173 | 5 (2.0–13.0) | 88 | 40 | 77.4 | 22.6 |
| Enhanced lamina dura | <0.001 | 0.074 | ∞ | 100 | 20 | 10 | 0 |
| Loss of lamina dura | 0.619 | 1 | 3.1 (0.3–30.7) | 98 | 5 | 5 | 1.7 |
| Enhanced mandibular canal | 0.025 | 0.614 | 4.8 (1.3–17.9) | 95 | 20 | 20 | 5 |
| Proliferative periostitis | 0.05 | b | ∞ | 100 | 8 | 25 | 1.7 |
| Osteolytic processes at cortex | <0.001 | 0.063 | 19.7 (2.5–154.5) | 98 | 25 | 4.2 | 0 |
AT, antiresorptive therapy; CG, control group; CI, confidence interval; MRONJ, medication-related osteonecrosis of the jaw; RG, review group.
RG includes the groups AT+MRONJ− and AT+MRONJ+.
Sign did not appear in the groups AT+MRONJ− and CG.
Alterations to the bony structure in the maxillofacial region are due to antiresorptive drugs, but appear particularly among patients with existing osteonecrosis. On closer examination of osteosclerosing processes, sclerosis did not increase significantly over the time period of antiresorptive drug intake. But, there was a significant increase over the time of visible sclerosing processes among the cohort of patients who had taken antiresorptive drugs for the therapy of malignant conditions (p-value = 0.008). No other sign showed a more frequent appearance over the duration of medication intake. The further analysis of the graduation and localization of osteosclerosis showed that osteosclerosis was not detected more frequently in more intense or larger occurences in the group of patients with AT and manifest MRONJ compared with the patients in the AT+MRONJ− group (data not shown).
The evaluation of imaging findings concerning the different subgroups of antiresorptive medications resulted in no significant difference among those groups, except for the enhancement of the mandibular canal (p-value = 0.047) (Table 2). None of the subgroups were associated with a more frequent occurrence of manifest MRONJ (Table 2). However, if medication was administered for malignant reasons, the signs visible alveolar socket, enhancement of the lamina dura and the enhancement of the mandibular canal were seen significantly more often (Table 2). Osteosclerosis was also detected more frequently in this group (OR = 2.87).
Table 2.
Imaging findings regarding different antiresorptive medications
| Imaging finding | AA |
ZA |
RA |
PA |
IA |
D |
p-valuea |
AT for benign
reasons |
AT for malignant
reasons |
Odds ratio (95%
CI) |
p-valueb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of imaging findings n (%) | Number of imaging findings n (%) | ||||||||||
| Sequestrum | 1 (7.6) | 3 (12.5) | 0 (0) | 0 (0) | 1 (50.0) | 0 (0) | 0.409 | 2 (11.1) | 4 (10.0) | 0.88 (0.14–5.35) | 1 |
| Sclerosing | 4 (30.7) | 13 (54.1) | 0 (0) | 1 (25.0) | 2 (100.0) | 4 (44.4) | 0.280 | 5 (27.7) | 21 (52.5) | 2.87 (0.86–9.57) | 0.090 |
| Difference in sclerosing | 3 (23.0) | 5 (20.8) | 0 (0) | 0 (0) | 0 (0) | 2 (22.2) | 0.878 | 3 (16.6) | 8 (20.0) | 1.25 (0.29–5.39) | 1 |
| Alveolar socket | 3 (23.0) | 14 (58.3) | 0 (0) | 0 (0) | 0 (0) | 5 (55.5) | 0.066 | 3 (16.6) | 21 (52.5) | 5.52 (1.38–22.1) | 0.020 |
| Enhanced lamina dura | 0 (0) | 7 (29.1) | 0 (0) | 2 (50.0) | 1 (50.0) | 2 (22.2) | 0.128 | 0 (0) | 12 (30.0) | ∞ | 0.011 |
| Loss of lamina dura | 0 (0) | 2 (8.3) | 0 (0) | 0 (0) | 0 (0) | 1 (11.1) | 0.847 | 0 (0) | 3 (7.5) | ∞ | 0.545 |
| Enhanced mandibular canal | 0 (0) | 8 (33.3) | 0 (0) | 0 (0) | 0 (0) | 4 (44.4) | 0.047 | 0 (0) | 12 (30.0) | ∞ | 0.011 |
| Proliferative periostitis | 0 (0) | 2 (8.3) | 0 (0) | 0 (0) | 1 (50.0) | 2 (22.2) | 0.169 | 1 (5.55) | 4 (10.0) | 1.88 (0.19–18.2) | 1 |
| Osteolytic processes at cortex | 4 (30.7) | 5 (20.8) | 0 (0) | 1 (25.0) | 1 (50.0) | 3 (33.3) | 0.866 | 5 (27.7) | 10 (25.0) | 0.86 (0.24–3.04) | 1 |
| AT+MRONJ+c | 4 (30.7) | 18 (75.0) | 1 (33.3) | 3 (75.0) | 2 (100.0) | 6 (66.6) | 0.137 | 6 (33.3) | 30 (75.0) | 6.00 (1.78–20.1) | 0.004 |
AA, alendronic acid; AT, antiresorptive therapy; CI, confidence interval; D, denosumab; IA, ibandronic acid; MRONJ, medication-related osteonecrosis of the jaw; PA, pamidronic acid; RA, risedronic acid; ZA, zoledronic acid.
p-value regarding different antiresorptive medications.
p-value regarding AT because of benign or malignant reasons.
Compared groups: AT+MRONJ+ vs AT+ MRONJ−.
Discussion
MRONJ—formerly known as BRONJ—is a severe side effect of medications, which manipulate the bone-remodelling process by inhibiting the function of osteoclasts such as bisphosphonates and the RANK ligand inhibitor denosumab, and the function of antiangiogenic medications, which inhibit angiogenesis.1 The manipulation of the osteoclast causes a gain in bone mass, which leads to a wide spectrum of radiographic features especially in the maxillofacial region of patients taking antiresorptive drugs. Those features have been described in the existing literature as focal or diffuse osteosclerosis, cortical disruptions, an increased lamina dura thickness and density, lack of bone fill in extraction sides (persisting alveolar socket), prominence of the inferior alveolar canal, periosteal bone formation, sequestration, widening of the periodontal ligament space and the loss of the trabecular structure.11–15,17–20,25–27
Hutchinson et al13 came to the conclusion that some of those radiologic signs can appear as early as in Stage 0 of the disease (as defined by the AAOMS) and are the same as seen in later stages.1 Hutchinson et al were not able to address causality, as the study was of only descriptive character and had no CG. In the cohort of patients who underwent AT within our study, some of the radiographic signs mentioned earlier appeared significantly more often (sclerosis, visible alveolar socket, enhanced lamina dura, enhancement of the mandibular canal, proliferative periostitis at the body of mandible and osteolytic processes at the cortex). All signs analyzed through this study showed an increased risk of development under bisphosphonate therapy and denosumab therapy. Nevertheless, patients under AT without MRONJ showed no significantly increased evidence of imaging findings compared with the control cohort. It may be possible that signs seen early in the disease are due to underlying dental conditions or anatomic variations.13–15,20,25 They are also seen in other conditions like osteoradionecrosis, osteomyelitis, Paget's disease of bone or cancer metastasis.15
Even though many studies have shown evidence of the existence of radiographic features among patients with MRONJ, the AAOMS still does not recommend using radiographic signs alone for case definition; nevertheless, they are aware of the risk of underestimating the true number of cases.1,11–15,17–20,25,26 As we have shown with this study, there are a higher number of imaging findings among patients with manifest MRONJ. Those findings may help estimate the real extent of the disease and may be a guide in finding the right therapy. Often, the area of the affected bone seen in radiographic imaging is larger than that when present in clinical examination.17,18 Patients in Stage 2 of the disease may as well already be a Stage 3 case. If surgical debridement is considered for these patients, it can result in a surgical resection.1
Which imaging technique that is the preferable one within the detection, staging and therapy decision has been part of the research about MRONJ. Some authors come to the conclusion that CT and CBCT are the methods of choice and superior to dental panoramic radiographs, as the panoramic image underestimates the extent of the lesion.11,18–20 An advantage of the computed techniques compared with panoramic radiograph is the standardized computed analysis and its third dimension. On the other hand, panoramic radiographs are readily accessible, have lower costs and less radiation exposure.17 As panoramic radiograph is the standard technique for the diagnosis of MRONJ at the Department of Oral and Maxillofacial Surgery of the University of Regensburg and readily available to mostly all dentists, we decided to evaluated the radiographic features within this dental image. Studies evaluating other imaging techniques such as CT, CBCT or MRI compared with the panoramic radiograph are often characterized by being of a descriptive character owing to a small cohort and a missing CG.11,18,19 Besides, the descriptions of imaging signs often lack details and are vaguely phrased. By performing a sample size determination, forming a CG and having three examiners who were blinded evaluate the panoramic radiographs independently, we tried to rule out most shortcomings of other studies.14 Nevertheless, the inconsistency concerning the type of medication is a limitation of this study. Different diseases and their respective stages need several therapeutic strategies, which contain a variety of antiresorptive medications of different potencies. Patients of the RG were treated with six different antiresorptive medications. This resulted in no significant occurrence of imaging findings (except enhancement of the mandibular canal). However, imaging findings can be seen more often among the cohort of patients with malignant diseases. This is most likely caused by more potent medications, which are administered in these cases. Likewise, it is obvious that this cohort of patients had a significantly higher risk of developing a MRONJ. In general, we have to act on the assumption that different types of medication cannot cause the same degree of osteosclerosis in the jaws. However, the incidence of MRONJ under denosumab is almost equal when compared with zoledronate therapy for malignant underlying diseases and the duration of the AT.28
Conclusion
The radiographs of patients undergoing AT with manifested osteonecrosis showed more frequently changes in the bone structure. The signs evaluated in this study can help to estimate the extent of the lesions and therefore should be included in the planning of therapy, especially if a surgical intervention is being considered. As the pathological imaging findings were not significantly increased but were partly more often seen among patients with AT without MRONJ, we concluded that the signs might not be able to act as indicators for this disease. Consequently, if pathological findings are present in a patient radiograph, the examiner should be aware of the possibility of having missed a hidden osteonecrosis. In addition, the radiologic findings should always be considered in conjunction with the clinical findings and symptoms of the patient.
Contributor Information
Christoph Klingelhöffer, Email: christoph.klingelhoeffer@ukr.de.
Manja Klingelhöffer, Email: m_klingelhoeffer@gmx.de.
Steffen Müller, Email: steffenmueller.mail@gmx.de.
Tobias Ettl, Email: tobias.ettl@ukr.de.
Ulrich Wahlmann, Email: ulrich.wahlmann@ukr.de.
References
- 1.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg 2014; 72: 1938–56. doi: http://dx.doi.org/10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 2.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008; 19: 733–59. doi: http://dx.doi.org/10.1007/s00198-007-0540-8 [DOI] [PubMed] [Google Scholar]
- 3.Pecherstorfer M. [Osteoporosis medication with a new effect mechanism.] J Mineral Metabolism Musculeskelal Disorders 2009; 16: 152–3. [Google Scholar]
- 4.Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: e437–42. doi: http://dx.doi.org/10.1016/j.oooo.2012.01.046 [DOI] [PubMed] [Google Scholar]
- 5.Marx R, Stern D. Oral an maxillofacial pathology: a rationale for diagnosis and treatment. Chicago: Quintessence; 2002. [Google Scholar]
- 6.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61: 1115–7. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62: 527–34. doi: http://dx.doi.org/10.1016/j.joms.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2007; 65: 369–76. doi: http://dx.doi.org/10.1016/j.joms.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Amgen.com. Prolia(R) (Denosumab) Granted Marketing Authorization in the European Union. Available from: http://investors.amgen.com/phoenix.zhtml?c=61656&p=irol-newsArticle&ID=1432232
- 10.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 2009; 67(Suppl. 5): 2–12. doi: http://dx.doi.org/10.1016/j.joms.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 11.Phal PM, Myall RW, Assael LA, Weissman JL. Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am J Neuroradiol 2007; 28: 1139–45. doi: http://dx.doi.org/10.3174/ajnr.A0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite AF, Ogata FDS, Melo NSD, Figueiredo PTDS. Imaging findings of bisphosphonate-related osteonecrosis of the jaws: a critical review of the quantitative studies. Int J Dentistry 2014; 2014: 11. doi: http://dx.doi.org/10.1155/2014/784348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson M, O'Ryan F, Chavez V, Lathon PV, Sanchez G, Hatcher DC, et al. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. J Oral Maxillofac Surg 2010; 68: 2232–40. doi: http://dx.doi.org/10.1016/j.joms.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 14.Rocha GCMA, Jaguar GC, Moreira CR, Neves EG, Fonseca FP, Pedreira EN. Radiographic evaluation of maxillofacial region in oncology patients treated with bisphosphonates. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114(Suppl. 5): S19–25. doi: http://dx.doi.org/10.1016/j.tripleo.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 15.Arce K, Assael LA, Weissman JL, Markiewicz MR. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J Oral Maxillofac Surg 2009; 67(Suppl. 5): 75–84. doi: http://dx.doi.org/10.1016/j.joms.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Stockmann P, Hinkmann FM, Lell MM, Fenner M, Vairaktaris E, Neukam FW, et al. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin Oral Investig 2010; 14: 311–7. doi: http://dx.doi.org/10.1007/s00784-009-0293-1 [DOI] [PubMed] [Google Scholar]
- 17.Treister N, Sheehy N, Bae EH, Friedland B, Lerman M, Woo S. Dental panoramic radiographic evaluation in bisphosphonate-associated osteonecrosis of the jaws. Oral Dis 2009; 15: 88–92. doi: http://dx.doi.org/10.1111/j.1601-0825.2008.01494.x [DOI] [PubMed] [Google Scholar]
- 18.Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, et al. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105: 358–64. doi: http://dx.doi.org/10.1016/j.tripleo.2007.08.040 [DOI] [PubMed] [Google Scholar]
- 19.Chiandussi S, Biasotto M, Dore F, Cavalli F, Cova MA, Di Lenarda R. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol 2006; 35: 236–43. doi: http://dx.doi.org/10.1259/dmfr/27458726 [DOI] [PubMed] [Google Scholar]
- 20.Bianchi SD, Scoletta M, Cassione FB, Migliaretti G, Mozzati M. Computerized tomographic findings in bisphosphonate-associated osteonecrosis of the jaw in patients with cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 249–58. [DOI] [PubMed] [Google Scholar]
- 21.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 2011; 26: 1871–82. doi: http://dx.doi.org/10.1002/jbmr.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood J, Bonjean K, Ruetz S, Bellahcène A, Devy L, Foidart JM, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 2002; 302: 1055–61. doi: http://dx.doi.org/10.1124/jpet.102.035295 [DOI] [PubMed] [Google Scholar]
- 23.Santini D, Vincenzi B, Hannon R, Brown J, Dicuonzo G, Angeletti S, et al. Changes in bone reorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncol Rep 2006; 15: 1351–7. [PubMed] [Google Scholar]
- 24.Misso G, Porru M, Stoppacciaro A, Castellano M, De Cicco F, Leonetti C, et al. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol Ther 2012; 13: 1491–500. doi: http://dx.doi.org/10.4161/cbt.22274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisdas S, Chambron Pinho N, Smolarz A, Sader R, Vogl TJ, Mack MG. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin Radiol 2008; 63: 71–7. doi: http://dx.doi.org/10.1016/j.crad.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 26.Wilde F, Heufelder M, Lorenz K, Liese S, Liese J, Helmrich J, et al. Prevalence of cone beam computed tomography imaging findings according to the clinical stage of bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 804–11. doi: http://dx.doi.org/10.1016/j.oooo.2012.08.458 [DOI] [PubMed] [Google Scholar]
- 27.Groetz KA, Al-Nawas B. Persisting alveolar sockets—a radiologic symptom of BP-ONJ? J Oral Maxillofac Surg 2006; 64: 1571–2. doi: http://dx.doi.org/10.1016/j.joms.2006.05.041 [DOI] [PubMed] [Google Scholar]
- 28.Qi WX, Tang LN, He AN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol 2014; 19: 403–10. doi: http://dx.doi.org/10.1007/s10147-013-0561-6 [DOI] [PubMed] [Google Scholar]