Abstract
Background
Chewing gum may stimulate gastrointestinal motility, with beneficial effects on postoperative ileus suggested in small studies. The primary aim of this trial was to determine whether chewing gum reduces length of hospital stay (LOS) after colorectal resection. Secondary aims included examining bowel habit symptoms, complications and healthcare costs.
Methods
This clinical trial allocated patients randomly to standard postoperative care with or without chewing gum (sugar‐free gum for at least 10 min, four times per day on days 1–5) in five UK hospitals. The primary outcome was LOS. Cox regression was used to calculate hazard ratios for LOS.
Results
Data from 402 of 412 patients, of whom 199 (49·5 per cent) were allocated to chewing gum, were available for analysis. Some 40 per cent of patients in both groups had laparoscopic surgery, and all study sites used enhanced recovery programmes. Median (i.q.r.) LOS was 7 (5–11) days in both groups (P = 0·962); the hazard ratio for use of gum was 0·94 (95 per cent c.i. 0·77 to 1·15; P = 0·557). Participants allocated to gum had worse quality of life, measured using the EuroQoL 5D‐3L, than controls at 6 and 12 weeks after operation (but not on day 4). They also had more complications graded III or above according to the Dindo–Demartines–Clavien classification (16 versus 6 in the group that received standard care) and deaths (11 versus 0), but none was classed as related to gum. No other differences were observed.
Conclusion
Chewing gum did not alter the return of bowel function or LOS after colorectal resection. Registration number: ISRCTN55784442 (http://www.controlled-trials.com).
Short abstract
No advantage observed
Introduction
Ileus is a clinical state characterized by nausea, vomiting, abdominal distension and an inability to pass stools/flatus. It can delay postoperative recovery, leading to an increase in length of hospital stay (LOS) and healthcare costs1, 2. The aetiology of ileus is multifactorial, but it may be reduced with minimally invasive surgery, avoidance of certain anaesthetic drugs and opiates, the use of regional anaesthetic blocks, early postoperative mobilization, and the use of prokinetic medication1, 2, 3, 4, 5. Early postoperative feeding improves clinical outcomes after surgery, but the risk of vomiting is increased6. Recommendations for early postoperative feeding are included in enhanced recovery programmes, which have been shown to reduce LOS by 2–3 days7, 8, 9.
Chewing gum is a type of sham feeding, and may reduce ileus without increasing the risk of vomiting. Potential mechanisms of action include the promotion of intestinal motility via cephalovagal stimulation and the secretion of various gastrointestinal hormones, saliva and pancreatic juice10. It has also been suggested that the hexitols in sugar‐free gum may play a role in resolving ileus through their osmotic effects11. Randomized clinical trials (RCTs), primarily in patients undergoing gastrointestinal surgery, but also in caesarean section or cystectomy, have investigated the effects of chewing gum on recovery. Meta‐analyses12, 13, 14, 15, 16, 17, 18 of these RCTs have generally shown that patients who chew gum after surgery have earlier return of intestinal function (earlier passage of flatus and stool) than those receiving standard postoperative management. In addition, some12, 15, 17, 18, although not all13, 14, 16, reported a shorter LOS with chewing gum. Sample sizes of RCTs in patients undergoing colorectal surgery have been relatively small, ranging from 19 to 16818. The aim of the present study was to look at the effects of chewing gum on postoperative recovery by conducting an adequately powered RCT of postoperative chewing gum versus standard care in patients after elective colorectal resection.
Methods
Participants were recruited from five hospitals in the UK (Bristol Royal Infirmary, Derriford Hospital Plymouth, Yeovil District Hospital, Torbay Hospital and Queen's Medical Centre Nottingham) between October 2010 and April 2013. Patients scheduled for an elective colorectal resection owing to colorectal neoplasia (invasive cancer or dysplasia), ulcerative colitis or diverticular disease were identified and asked to participate in the study. Potential participants were approached by clinical and research staff at their surgical outpatient appointment or at a preoperative assessment clinic. Patients were ineligible if they were aged less than 18 years, had Crohn's disease, were operated on as an emergency, were pregnant or lactating, were participating in another study that could undermine the scientific basis of the present trial, or were deemed unsuitable (for example, were incapable of providing adequate responses/information or consent to participate in the trial).
The trial was performed in accordance with the Declaration of Helsinki, and the ethical principles of the International Conference on Harmonization – Good Clinical Practice. All study procedures were approved by the National Research Ethics Service Committee South West (REC reference number 09/H0106/37), and all study participants provided written informed consent. The trial is registered in the ISRCTN registry (ISRCTN55784442).
Randomization
After written informed consent had been obtained, research staff used a computer‐based tool created within Microsoft® Access (Microsoft, Seattle, Washington, USA) to allocate participants randomly on a 1 : 1 basis to either standard care plus chewing gum or standard care alone. Encryption concealed allocation until the patient's details had been logged. Random block sizes of two, four or six were used, and randomization was stratified by hospital site and within sites by disease type (colorectal neoplasia, ulcerative colitis and diverticular disease).
Intervention
Participants allocated to chewing gum were asked to chew a stick of commercially available sugar‐free gum (Wrigley's 5; Wm Wrigley Jr, Plymouth, UK) for at least 10 min, four times a day for 5 consecutive days (or until discharge, if less than 5 days) from the first postoperative morning. The approximate cost per patient for a 5‐day course of gum was €1·61. Chewing gum was dispensed to participants by ward staff at each of the four drug rounds per day. Participants in the control group were asked not to chew gum during their hospital stay. No other alterations to perioperative care were made.
Acceptability and compliance
Acceptability of chewing gum was assessed via a brief interview with participants in the chewing gum arm after at least 1 complete day after operation. They were asked how they felt about chewing gum and whether they had any problems or difficulties chewing the gum. Compliance was assessed by asking participants to record when, and for how long, they chewed each piece of gum across all potential chewing occasions (taking into account whether discharge was before day 5). Participants in the standard care arm were asked at the time of discharge if they had chewed gum during their hospital stay, and if so when they had done so.
Blinding
The surgical nursing and medical team were blinded to treatment arm at the time of the operation. Members of the research team responsible for data collection were not blinded to treatment allocation as data on, for example, acceptability of gum were collected from study participants. As in other trials, given the nature of the intervention and the lack of a suitable placebo it was not possible to blind study participants to the treatment allocation. Due consideration was given to blinding the surgical team (those involved in determining suitability for discharge) to the treatment allocation after surgery, such as using empty boxes of ‘gum’ for control participants. However, all solutions were deemed impractical or impossible owing to the nature of the intervention and the need for labels within drug charts for dispensing gum. Nonetheless, by dispensing the chewing gum from the drugs trolley there were no open chewing gum boxes for the surgical team to see. Furthermore, patients and staff were instructed not to tell the surgical team which arm of the study they were in, and those in the chewing gum arm were asked to discard the wrapper straight away. The statistician was blinded to the treatment allocation during cleaning and recoding of the data, and the initial version of the main analyses (up to the point of submission of the final report to funders).
Data collection
Data were collected from several sources including interviews, questionnaires, and chewing gum log books completed by study participants. Baseline data were collected before surgery, and other data were collected from participants on days 1–5, and at 6 and 12 weeks after operation. In addition, study personnel extracted data from charts, medical notes and electronic records where available.
Outcome measures
Results for the primary outcome (LOS) and the following selected secondary outcomes (chosen based on other reported studies or outcomes that were deemed relevant) are reported here: passage of first bowel movement, passage of first flatus, and first day of auscultated bowel sounds; patient‐reported abdominal pain, nausea, vomiting, solid food consumption and tolerance, and quality of life; and clinical complications and death. LOS was calculated as the number of days from the date of operation to the date of discharge, transfer or death. Data used to generate LOS were double‐entered and any discrepancies rectified by checking the report form or with study sites to verify dates. Participant‐reported data for bowel movement, passage of flatus and vomiting were obtained from participant questionnaires on each of days 1–5 after operation. The first postoperative day on which a bowel movement or flatus was passed, and the presence or absence of vomiting on day 2 after surgery, were used in analyses. Medical records were used to determine the day (up to day 5) on which bowel sounds were first heard, and the first postoperative day on which they were heard was used in analyses.
The extent of abdominal pain and nausea was reported by study participants on each of days 1–5 using a visual analogue scale, where 0 per cent indicated no pain or no sickness at all, and 100 per cent a lot of pain or very sick. Data are presented for day 2 after operation. Dietary intake on each of days 1–5 was assessed by asking study participants to circle the amount (none; about a quarter; about a half; about three‐quarters; all) of each meal (breakfast, lunch and dinner) that they ate. The first postoperative day on which solid food was consumed or tolerated (defined as the consumption of at least half of 3 meals in a day without vomiting) was used in analyses.
Quality of life was assessed using the EuroQol (EQ) 5D‐3L questionnaire (EuroQol Group, Rotterdam, The Netherlands) at baseline, day 4, and 6 and 12 weeks after operation. The EQ‐5D‐3L™ summarizes health on a single index anchored at 1 (best health) and 0 (worst health)19.
Descriptive information reported on adverse event forms on days 1–5 after surgery was used to classify events according to the Dindo–Demartines–Clavien classification20. Adverse events were grouped into the following categories: ileus/nausea/vomiting (data on vomiting as a clinical complication within this category was taken from adverse event forms rather than using data from participant‐completed questionnaires), pneumonia, anastomotic leak, wound infection, other infection, bleeding, intra‐abdominal collection, bowel obstruction and death. The number of individuals per treatment group who experienced an adverse event of at least grade III was analysed. In addition, the number of individuals per treatment group who experienced each of the adverse events listed above, as well as the total number of individuals per treatment group who experienced an adverse event (irrespective of its nature), was analysed.
Power calculation and sample size
When the trial was designed, data from one study21 in a UK population were available for estimating power for the primary outcome (LOS), and additional data from a systematic review12 were available for selected secondary outcomes (time to first flatus and bowel movement). With a sample size of 400 (approximately 200 per treatment group), and using a two‐group Student's t test and 5 per cent two‐sided significance, the estimated power for detecting a mean difference of 1·5 days (s.d. 5 days) in LOS between treatment groups was 84 per cent. The estimated power for detecting a difference of 1 day between treatment groups for time to first flatus (s.d. 18·5 h) and time to first bowel movement (s.d. 34·4 h) was greater than 99 per cent.
Statistical analysis
Preoperative characteristics by treatment group were tabulated using mean(s.d.) for normally distributed data, median (i.q.r.) for non‐normally distributed data, and counts with percentages for categorical data. Data for LOS were skewed, with one participant having a hospital stay of 230 days. For LOS, Cox regression was used to test for equality of survival curves between treatment arms, and to calculate hazard ratios and 95 per cent confidence intervals (c.i.); Schoenfeld residuals were used to test the proportional hazards assumption. Discharges appeared as events, and deaths and transfers to other hospitals as censored observations. As a sensitivity analysis, deaths that occurred before discharge from hospital were considered to be a competing risk within a competing‐risks regression model.
Secondary outcomes were assessed using Student's t test for normally distributed continuous variables, Mann–Whitney U test for continuous variables with a non‐normal distribution and χ2 test for categorical variables. Data were analysed using Stata® release 13 (StataCorp, College Station, Texas, USA).
Economic evaluation
The mean healthcare cost and quality‐adjusted life‐years (QALYs) per patient in each arm of the study up to 12 weeks' follow‐up were estimated. Information on initial hospital stay and readmissions were extracted from the hospital records. Other service use (such as general practitioner visits and community care) was collected from participant‐completed questionnaires at 6 and 12 weeks. Unit costs were estimated from publicly available sources22, 23, 24 and the local hospital finance department. UK costs were converted to euros using 2014 exchange rates (£1 = €1·19). QALYs were estimated based on EQ‐5D‐3L™ responses at baseline, 4 days, 6 and 12 weeks. Cost‐effectiveness was summarized using the net monetary benefit statistic, assuming that the health service is able to pay £20 000 (approximately €23 700) per QALY gained25.
Results
A total of 412 patients were recruited between October 2010 and April 2013 (Fig. 1); follow‐up of the last participant was on 16 August 2013. Ten patients were withdrawn by investigators and not included in the analyses. Participant characteristics and baseline measures are shown in Table 1. Overall, there were slightly more men than women, most participants reported that they were Caucasian, and almost all had surgery for treatment of cancer. The trial arms were comparable across most demographic and clinical measures, although a slightly higher proportion of participants in the chewing gum arm had a stoma formed (36·9 versus 31·2 per cent).
Figure 1.
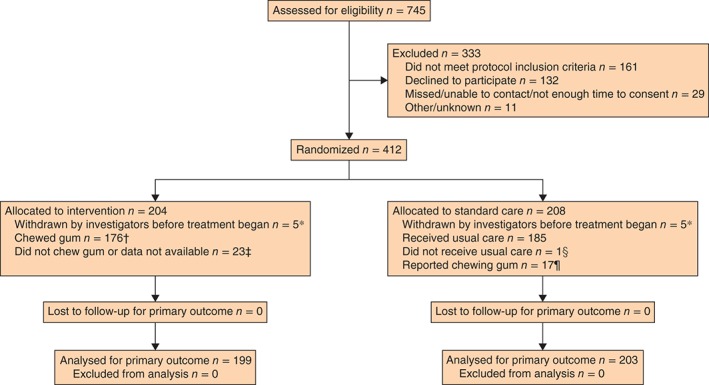
CONSORT diagram for the trial. *Did not undergo colorectal resection (7 patients), participating in another trial (1), had been operated on as an emergency (1), or deemed unsuitable based on intraoperative problems and subsequent intensive therapy unit admission (1). †On at least one occasion according to chewing gum log. ‡Chewing gum log showed that: gum had been received but there was no information on whether or not it had been chewed (4); gum had not been received (4, 1 of whom had not been given gum in error); no information on whether or not gum had been received or chewed (15). §Chewing gum provided in error. ¶Questionnaire on use of gum in standard care group indicated that gum had been chewed at least once during days 1–5 after surgery
Table 1.
Participant characteristics, baseline measures and operative data
| Overall proportion receiving gum* | Chewing gum (% of patients in group) | Standard care (% of patients in group) | |
|---|---|---|---|
| Age (years)† | 199 of 402 (49·5) | 65·5(14·1) | 66·9(11·6) |
| Sex | |||
| M | 111 of 230 (48·3) | 55·8 | 58·6 |
| F | 88 of 172 (51·2) | 44·2 | 41·4 |
| Ethnicity | |||
| Caucasian | 194 of 385 (50·4) | 98·5 | 96·5 |
| Black | 0 of 5 (0) | 0 | 2·5 |
| Other | 3 of 5 (60) | 1·5 | 1·0 |
| Level of education‡ | |||
| O‐level, GCSE, school certificate or less | 127 of 254 (50·0) | 67·6 | 67·6 |
| A‐level | 24 of 48 (50) | 12·8 | 12·8 |
| Degree | 37 of 74 (50) | 19·7 | 19·7 |
| Smoking status | |||
| Current smoker | 22 of 35 (63) | 11·2 | 6·6 |
| Former smoker | 100 of 210 (47·6) | 51·0 | 56·1 |
| Never smoked | 74 of 147 (50·3) | 37·8 | 37·2 |
| Body mass index (kg/m2)† | 199 of 400 (49·8) | 27·9(5·4) | 27·2(4·8) |
| EQ‐5D‐3 L™ quality‐of‐life score† | 194 of 389 (49·9) | 0·82(0·23) | 0·84(0·19) |
| ASA fitness grade | |||
| I | 28 of 49 (57) | 15·0 | 11·1 |
| II | 117 of 244 (48·0) | 62·6 | 66·8 |
| III | 42 of 84 (50) | 22·5 | 22·1 |
| Indication for surgery | |||
| Colorectal neoplasia | 184 of 372 (49·5) | 92·5 | 92·6 |
| Diverticular disease | 7 of 16 (44) | 3·5 | 4·4 |
| Ulcerative colitis | 8 of 14 (57) | 4·0 | 3·0 |
| Type of surgery | |||
| Open | 86 of 178 (48·3) | 43·4 | 45·5 |
| Laparoscopic | 78 of 159 (49·1) | 39·4 | 40·1 |
| Laparoscopically assisted | 22 of 43 (51) | 11·1 | 10·4 |
| Laparoscopic converted to open | 12 of 20 (60) | 6·1 | 4·0 |
| Stoma formed§ | |||
| Yes | 73 of 136 (53·7) | 36·9 | 31·2 |
| No | 125 of 264 (47·3) | 63·1 | 68·8 |
| Primary procedure | |||
| Total colectomy | 12 of 20 (60) | 6·1 | 3·9 |
| Right‐sided colectomy | 56 of 117 (47·9) | 28·4 | 30·1 |
| Left‐sided colectomy | 34 of 69 (49) | 17·3 | 17·2 |
| Rectal resection | 84 of 168 (50·0) | 42·6 | 41·4 |
| Other¶ | 11 of 26 (42) | 5·6 | 7·4 |
Values in parentheses are percentages; the overall number of patients was 402 (199 in chewing gum group and 203 in standard care group), but totals may not add up to this as there were some missing data.
Values are mean(s.d.).
O‐level, GCSE, school certificate: national school examinations at age 16 years; A‐level: national school examinations at age 18 years.
Data from day 1 after operation.
Includes partial resection and small bowel resection. EQ, EuroQol; ASA, American Society of Anesthesiologists.
Chewing gum
The majority of patients in the chewing gum arm (135; 77·1 per cent of those with available data) reported that they were happy to chew the gum and 122 (72·2 per cent of those with available data) said that they did not have any problems or difficulties. Data from chewing gum log books or acceptability of gum questionnaires showed that 176 participants chewed gum on at least one potential occasion, and 23 participants either did not chew gum or no data were available to ascertain whether they had or not (Fig. 1). For those with available data, gum was chewed on a median of 58 (i.q.r. 20–83) per cent of the occasions on which it had been received, for a mean(s.d.) of 12(7) min each time.
Seventeen participants (8·2 per cent) in the standard care arm reported that they had chewed gum during their hospital stay. Of these, one had been given trial gum in error, two said they chewed it on the first postoperative day and 13 reported that they chewed gum between days 2 and 5 after surgery. Data on when gum was chewed were not available for one individual.
Primary outcome
LOS ranged from 2 to 230 days. Median LOS in both treatment groups was 7 (i.q.r. 5–11) days (Table 2). The assumption of proportional hazards was met (χ2 = 0·03, P = 0·872). The estimated coefficient for discharge in the gum compared with standard care arm was −0·06 (95 per cent c.i. –0·26 to 0·14), and the associated hazard ratio was 0·94 (95 per cent c.i. 0·77 to 1·15; P = 0·557). Treating deaths as a competing risk, the coefficient for discharge in the gum compared with standard care arm was −0·15 (−0·33 to 0·03), and the associated sub‐hazard ratio was 0·86 (0·72 to 1·03; P = 0·102).
Table 2.
Comparison of primary outcome (length of stay) and selected secondary outcome measures between treatment groups
| Overall proportion receiving gum‡ | Chewing gum | Standard care | P # | |
|---|---|---|---|---|
| Length of postoperative hospital stay (days) | 199 of 402 (49·5) | 7 (5–11) | 7 (5–11) | 0·962 |
| Postoperative day bowel sounds first heard | 58 of 136 (42·6) | 2 (1–3) | 2 (1–3) | 0·619 |
| Postoperative day of first flatus | 130 of 268 (48·5) | 2 (2–3) | 2 (1–3) | 0·586 |
| Postoperative day of first bowel movement | 159 of 310 (51·3) | 2 (1–3) | 3 (1–4) | 0·153 |
| Postoperative day first ate solid food | 188 of 385 (48·8) | 1 (1–2) | 1 (1–2) | 0·920 |
| Postoperative day solid food tolerated§ | 131 of 260 (50·4) | 2 (1–3) | 2 (1–3) | 0·223 |
| Abdominal pain score (%)¶ | 154 of 324 (47·5) | 49 (22–69) | 43 (21–65) | 0·496 |
| Nausea score (%)¶ | 156 of 327 (47·7) | 9 (2–52) | 10 (2–51) | 0·662 |
| Vomiting on day 2 | 0·930** | |||
| Yes | 36 of 70 (51) | 18·3† | 16·8† | |
| No | 144 of 294 (49·0) | 73·1† | 74·3† | |
| Not known | 17 of 35 (49) | 8·6† | 8·9† | |
| EQ‐5D‐3 L™ quality‐of‐life score* | ||||
| Day 4 | 138 of 273 (50·5) | 0·44(0·35) | 0·50(0·34) | 0·116†† |
| 6 weeks | 163 of 328 (49·7) | 0·67(0·32) | 0·76(0·23) | 0·004†† |
| 12 weeks | 142 of 292 (48·6) | 0·74(0·31) | 0·80(0·21) | 0·036†† |
Values in parentheses are median (i.q.r.) unless indicated otherwise;
values are mean(s.d.);
values are percentage of patients in group.
Values in parentheses are percentages; the overall number of patients was 402 (199 in chewing gum group and 203 in standard care group), but totals may not add up to this as there were some missing data. For gum and standard care groups, bowel sounds, flatus and bowel movement events were reported as ‘not known’ for 92 and 80, 45 and 41, and one and two patients respectively, and data were missing on at least 1 relevant day for 49 and 45, 24 and 24, and 23 and 26 respectively; for bowel movement, event was reported as ‘no’ for 16 and 24 patients respectively.
Ate at least half of three meals in a day without vomiting.
Visual analogue scale score on day 2 after surgery (0 per cent, none at all; 100 per cent, a lot of pain or very sick).
Mann–Whitney U test, except
χ2 test and
t test.
Secondary outcomes
Bowel habit, bowel sounds and food consumption
There was a median of 2 days after operation until first occurrence of a bowel movement, flatus and bowel sounds among participants in both treatment groups (with the exception of day 3 for bowel movement in the standard care arm); there were no differences between groups (Table 2). Nor were there any differences between groups in median reported day of first consumption of solid food and toleration of solid food, which were days 1 and 2 respectively.
Abdominal pain, nausea, vomiting and quality of life
There were no differences between treatment groups in abdominal pain and nausea on day 2 after surgery: median scores were 49 and 9 per cent respectively for participants in the chewing gum group, and 43 and 10 per cent in the standard care group (Table 2). Data for all other days were similar (data not shown). Similar numbers of participants in both treatment groups reported having vomited on each of days 1–5 after operation; data for day 2 are shown in Table 2. There was no difference in quality of life between the treatment groups, assessed by means of the EQ‐5D‐3 L™, on day 4 after operation, but scores at 6 and 12 weeks were worse in the chewing gum group (Table 2).
Complications
During days 1–5 after operation, 22 individuals (16 gum, 6 standard care) had an adverse event of at least grade III (P = 0·027), but all were classed as not related, or unlikely to be related, to the intervention. Considering potentially relevant complications that had been grouped using data reported on adverse event forms, there were no differences between treatment groups in the number of documented episodes within each category (Table 3). Overall, 57 and 60 people in the gum and control groups respectively experienced at least one of the complications listed in Table 3 (P = 0·840). One complication in the chewing gum group was considered possibly related to the intervention (described as ‘postop ileus’ and included in the vomiting/nausea/ileus category), but all others were classed as not related, or unlikely to be related, to the intervention.
Table 3.
Number of documented episodes of selected complications between days 1 and 5 after surgery
| Total | Chewing gum | Standard care | P † | |
|---|---|---|---|---|
| Suspected or confirmed anastomotic leak | 13 | 6 | 7 | 0·815 |
| Bowel obstruction | 2 | 1 | 1 | – |
| Confirmed or suspected wound infection | 5 | 2 | 3 | – |
| Confirmed or suspected other infection | 20 | 9 | 11 | 0·691 |
| Vomiting*/nausea/ileus | 47 | 19 | 28 | 0·197 |
| Pneumonia | 17 | 8 | 9 | 0·847 |
| Bleeding | 16 | 8 | 8 | 0·958 |
| Intra‐abdominal collection | 1 | 1 | 0 | – |
| Other | 49 | 26 | 23 | 0·582 |
| Death | 3 | 3 | 0 | – |
Data on vomiting taken only from adverse event forms; no participant‐reported data used.
χ2 test; no formal statistical comparison was done when few participants experienced the complication.
Eleven patients died during the study, all in the chewing gum arm. Nine died before the 12‐week follow‐up (2 after discharge), and two died after the 12‐week follow‐up but before discharge from hospital. Detailed information on cause of death for ten of the 11 participants (medical records were not accessible for 1 individual) was reviewed by clinicians at each study site. Chewing gum was not considered related to the causes of death.
Economic evaluation
Mean hospital costs were slightly higher (by €596) for patients in the chewing gum group, but the difference was explained almost entirely by one patient who spent a long period in the intensive care unit (Table 4). Primary and community care use was also similar between the two arms of the trial. There was no difference in mean QALYs between the intervention and standard care arms (Table 4). Patients in the gum group had a lower net benefit (mean difference –€173, 95 per cent c.i. –€1103 to 757), indicating that gum chewing is unlikely to be cost‐effective.
Table 4.
Total and incremental costs and quality‐adjusted life‐years
| Overall proportion receiving gum* | Chewing gum† | Standard care† | Difference‡ | P | |
|---|---|---|---|---|---|
| Primary/community costs (€) | 151 of 326 (46·3) | 239(264) | 210(242) | 28 (−27, 83) | 0·316 |
| Hospital costs (€) | 199 of 402 (49·5) | 3773(8934) | 3177(5391) | 596 (−848, 2039) | 0·418 |
| Total health service costs (€) | 151 of 326 (46·3) | 3131(3117) | 2806(2598) | 325 (−298, 948) | 0·306 |
| QALYs | 90 of 193 (46·6) | 0·149(0·059) | 0·164(0·043) | −0·012§ (−0·026, 0·002) | 0·097 |
| Net monetary benefit (€) | 77 of 178 (43·3) | 874(2952) | 1048(3234) | −173 (−1103, 757) | 0·713 |
Values in parentheses are percentages; the overall number of patients was 402 (199 in chewing gum group and 203 in standard care group), but totals may not add up to this as there were some missing data.
Values are mean(s.d.);
values in parentheses are 95 per cent c.i.
Mean difference in quality‐adjusted life‐years (QALYs) after adjustment for baseline EuroQol 5D‐3 L™ score26.
Discussion
There were no differences in LOS or in most secondary outcomes between patients randomized to receive postoperative chewing gum and those allocated to standard care. There was a suggestion of more severe complications in the chewing gum group, but none was classed as related to chewing gum. There was no evidence to suggest substantial effects of chewing gum on overall costs. Chewing gum after colorectal surgery is unlikely to be cost‐effective.
Systematic reviews and meta analyses12, 15, 17, 18 of studies in specific surgery types (such as colorectal) or abdominal surgery in general have shown positive effects of chewing gum on outcomes such as return of bowel function and possibly also LOS, but issues such as heterogeneity and insufficient data on which to base conclusions have been highlighted15, 17, 18. The findings of the present trial are in agreement with a recent, relatively large trial (168 patients)27 of postoperative chewing gum in patients undergoing colorectal resection, which reported no effects of chewing gum on return of bowel function, LOS and other secondary outcomes, similar to those reported here. A potential reason for the lack of an effect of chewing gum in this trial is that all study sites routinely use enhanced recovery programmes that include early feeding. This may have negated any potential effects of chewing gum in the trial.
Few previous studies have assessed or reported on compliance with chewing gum regimens, which was very good in this trial. The gum was well tolerated by participants, with most reporting that they were happy to chew it. Although a large proportion of the participants did not achieve 100 per cent compliance, this was not surprising given that they had all undergone major colorectal surgery.
An unexpected finding in this trial was that all deaths during follow‐up were among participants in the chewing gum arm. However, a detailed investigation of the causes of death did not provide any information to suggest that the deaths were related to chewing gum. Furthermore, considering all potentially relevant complications, there were no differences between treatment groups, and only one (described as ‘postop ileus’) was considered possibly related to the intervention. Most other outcomes reported here were not substantially affected by treatment, or adversely affected by chewing gum. The results taken together suggest that the observed excess mortality in the intervention arm is a chance finding.
This study has some limitations. Given the nature of the intervention (chewing gum versus standard care) it was either not possible or very difficult to blind study participants or study personnel/ward staff. This limitation is common to most other similar studies17, 18. The lack of differences between treatment groups, especially in regard to outcomes unlikely to be influenced by knowledge of treatment allocation (such as anastomotic leak), combined with the fact that the findings of this trial are similar to those reported in other (albeit smaller) studies18, provides some reassurance that the findings may not have been subject to such bias. It is possible that knowledge of the treatment allocation may have altered participant responses, but the only difference between groups was a worse quality of life at 6 and 12 weeks in the chewing gum arm. A few individuals (less than 2·5 per cent) were withdrawn by investigators after randomization as they no longer met the protocol inclusion criteria, but the same number of patients was withdrawn from both treatment groups. The findings may not be generalizable to other populations, patients with other medical conditions and patients undergoing different procedures. The primary outcome was LOS, but a more appropriate primary outcome may have been a marker of postoperative ileus or clinical complications, as these would have been less influenced by local policies concerning discharge from hospital. Finally, relatively large amounts of data were missing for some of the secondary outcomes (such as bowel sounds), which may limit the interpretation of the findings.
A recent formal systematic review18 of the available evidence in this field included preliminary data from this trial. The overall findings of the review (which included all abdominal surgery) showed that chewing gum was associated with an earlier return of bowel function and a reduction in LOS of 0·7 (95 per cent c.i. –0·8 to −0·5) days, but there was little effect on complications18. This modest reduction in LOS was, however, based on poor‐quality evidence and the clinical importance of chewing gum may be diminished in the context of enhanced recovery programmes. Nonetheless, no serious adverse events were attributed to chewing gum. This suggests that chewing gum can be neither clearly recommended nor prohibited as a gastrointestinal stimulant.
Acknowledgements
The authors thank all of the study participants, without whom this study would not have been possible; the user representative whose contributions from the time of the grant application to the end of the trial were invaluable; the Research Nurses and associated study staff at the Bristol Royal Infirmary, Derriford Hospital Plymouth, Yeovil District Hospital, Torbay Hospital and Queen's Medical Centre Nottingham for data collection; and the Principal Investigators at all study sites for facilitating the recruitment of participants.
This is a summary of independent research funded by the Research for Patient Benefit Programme of the National Institute for Health Research (NIHR) (grant reference PB‐PG‐0807‐14113) and the NIHR Bristol Nutrition Biomedical Research Unit. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Disclosure: The authors declare no conflict of interest.
Preliminary findings presented to the 36th European Society for Clinical Nutrition and Metabolism Congress, Geneva, Switzerland, September 2014; published in abstract form as Clin Nutr 2014; 33(Suppl 1): S260
References
- 1. Bragg D, El‐Sharkawy AM, Psaltis E, Maxwell‐Armstrong CA, Lobo DN. Postoperative ileus: recent developments in pathophysiology and management. Clin Nutr 2015; 34: 367–376. [DOI] [PubMed] [Google Scholar]
- 2. Barletta JF, Senagore AJ. Reducing the burden of postoperative ileus: evaluating and implementing an evidence‐based strategy. World J Surg 2014; 38: 1966–1977. [DOI] [PubMed] [Google Scholar]
- 3. Story SK, Chamberlain RS. A comprehensive review of evidence‐based strategies to prevent and treat postoperative ileus. Dig Surg 2009; 26: 265–275. [DOI] [PubMed] [Google Scholar]
- 4. Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg 2003; 138: 206–214. [DOI] [PubMed] [Google Scholar]
- 5. Behm B, Stollman N. Postoperative ileus: etiologies and interventions. Clin Gastroenterol Hepatol 2003; 1: 71–80. [DOI] [PubMed] [Google Scholar]
- 6. Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta‐analysis. J Gastrointest Surg 2009; 13: 569–575. [DOI] [PubMed] [Google Scholar]
- 7. Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta‐analysis of randomized controlled trials. Clin Nutr 2010; 29: 434–440. [DOI] [PubMed] [Google Scholar]
- 8. Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta‐analysis of randomized controlled trials. Dis Colon Rectum 2013; 56: 667–678. [DOI] [PubMed] [Google Scholar]
- 9. Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011; (2)CD007635. [DOI] [PubMed] [Google Scholar]
- 10. Person B, Wexner SD. The management of postoperative ileus. Curr Probl Surg 2006; 43: 6–65. [DOI] [PubMed] [Google Scholar]
- 11. Tandeter H. Hypothesis: hexitols in chewing gum may play a role in reducing postoperative ileus. Med Hypotheses 2009; 72: 39–40. [DOI] [PubMed] [Google Scholar]
- 12. Chan MK, Law WL. Use of chewing gum in reducing postoperative ileus after elective colorectal resection: a systematic review. Dis Colon Rectum 2007; 50: 2149–2157. [DOI] [PubMed] [Google Scholar]
- 13. de Castro SM, van den Esschert JW, van Heek NT, Dalhuisen S, Koelemay MJ, Busch OR et al A systematic review of the efficacy of gum chewing for the amelioration of postoperative ileus. Dig Surg 2008; 25: 39–45. [DOI] [PubMed] [Google Scholar]
- 14. Purkayastha S, Tilney HS, Darzi AW, Tekkis PP. Meta‐analysis of randomized studies evaluating chewing gum to enhance postoperative recovery following colectomy. Arch Surg 2008; 143: 788–793. [DOI] [PubMed] [Google Scholar]
- 15. Noble EJ, Harris R, Hosie KB, Thomas S, Lewis SJ. Gum chewing reduces postoperative ileus? A systematic review and meta‐analysis. Int J Surg 2009; 7: 100–105. [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald JE, Ahmed I. Systematic review and meta‐analysis of chewing‐gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J Surg 2009; 33: 2557–2566. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Liu Y, Peng Q, Xie L, Wang J, Qin X. Chewing gum reduces postoperative ileus following abdominal surgery: a meta‐analysis of 17 randomized controlled trials. J Gastroenterol Hepatol 2013; 28: 1122–1132. [DOI] [PubMed] [Google Scholar]
- 18. Short V, Herbert G, Perry R, Atkinson C, Ness AR, Penfold C et al Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst Rev 2015; (2)CD006506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Reenen M, Oppe M. EQ‐5D‐3 L User Guide. Basic Information on How to Use the EQ‐5D‐3 L Instrument, Version 5.1. EuroQol Research Foundation: Rotterdam, 2015. [Google Scholar]
- 20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quah HM, Samad A, Neathey AJ, Hay DJ, Maw A. Does gum chewing reduce postoperative ileus following open colectomy for left‐sided colon and rectal cancer? A prospective randomized controlled trial. Colorectal Dis 2006; 8: 64–70. [DOI] [PubMed] [Google Scholar]
- 22. Personal Social Services Research Unit . Unit Costs of Health and Social Care 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014/ [accessed 5 March 2016]. [Google Scholar]
- 23. Turner J, O'Cathain A, Knowles E, Nicholl J, Tosh J, Sampson F et al Evaluation of NHS 111 Pilot Sites. Final Report. https://www.sheffield.ac.uk/polopoly_fs/1.227404!/file/NHS_111_final_report_August_2012.pdf [accessed 5 March 2016]. [Google Scholar]
- 24. Department of Health . National Schedule of Reference Costs for NHS Trusts and NHS Foundation Trusts 2013–2014. https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 [accessed 5 March 2016]. [Google Scholar]
- 25. National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 http://publications.nice.org.uk/pmg9 [accessed 5 March 2016]. [PubMed]
- 26. Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial‐based cost‐effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005; 14: 487–496. [DOI] [PubMed] [Google Scholar]
- 27. Lim P, Morris OJ, Nolan G, Moore S, Draganic B, Smith SR. Sham feeding with chewing gum after elective colorectal resectional surgery: a randomized clinical trial. Ann Surg 2013; 257: 1016–1024. [DOI] [PubMed] [Google Scholar]


