ABSTRACT
Cartilage tissue engineering, with in vitro expansion of autologus chondrocytes, is a promising technique for tissue regeneration and is a new potential strategy to prevent and/or treat cartilage damage (e.g., osteoarthritis). The aim of this study was (i) to investigate and compare the effects of new biotechnological chondroitin (BC) and a commercial extractive chondroitin sulfate (CS) on human chondrocytes in vitro culture; (ii) to evaluate the anti‐inflammatory effects of the innovative BC compared to extractive CS. A chondrogenic cell population was isolated from human nasoseptal cartilage and in vitro cultures were studied through time‐lapse video microscopy (TLVM), immunohistochemical staining and cytometry. In order to investigate the effect of BC and CS on phenotype maintainance, chondrogenic gene expression of aggrecan (AGN), of the transcriptor factor SOX9, of the types I and II collagen (COL1A1 and COL1A2), were quantified through transcriptional and protein evaluation at increasing cultivation time and passages. In addition to resemble the osteoarthritis‐like in vitro model, chondrocytes were treated with IL‐1β and the anti‐inflammatory activity of BC and CS was assessed using cytokines quantification by multiplex array. BC significantly enhances cell proliferation also preserving chondrocyte phenotype increasing type II collagen expression up to 10 days of treatment and reduces inflammatory response in IL‐1β treated chondrocytes respect to CS treated cells. Our results, taken together, suggest that this new BC is of foremost importance in translational medicine because it can be applied in novel scaffolds and pharmaceutical preparations aiming at cartilage pathology treatments such as the osteoarthritis. J. Cell. Biochem. 117: 2158–2169, 2016. © 2016 The Authors. Journal of Cellular Biochemistry Published by Wiley Periodicals, Inc.
Keywords: BIOTECHNOLOGICAL CHONDROITIN, NASAL CHONDROCYTES, CHONDROITIN SULFATE, TIME‐LAPSE VIDEOMICROSCOPY, ECM BIOMARKERS, INFLAMMATION IN VITRO MODEL
Abbreviations
- AGN
aggrecan
- BC
biotechnological chondroitin
- COL1A1
type I collagen
- COL2A1
type II collagen
- CS
chondroitin sulfate
- DMEM
Dulbecco's Modified Eagle Medium
- ECM
extracellular matrix
- ERK1/2
extracellular‐signal‐regulated kinases 1/2
- FACS
Fluorescence‐activated cell sorting
- FBS
fetal bovine serum
- GAGs
glycosaminoglycans
- GAPDH
glyceraldehydes‐3‐phosphate dehydrogenase
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- P38MAPK
P38 mitogen‐activated protein kinases
- SOX‐9
Sry‐type HMG box
- TLVM
time‐lapse video microscopy
Cartilage is an avascular and aneural tissue, composed of chondrocytes surrounded by a viscous extracellular matrix (ECM). These are specialized cells responsible for production, maintaining, and remodeling of cartilage ECM [Chung and Burdick, 2008]. However, articular chondrocytes are rather difficult to culture, having a low proliferation capacity and losing their chondrogenic phenotype through dedifferentiation in monolayer cultures [Kuo and Lin, 2006]. In addition to articular cartilage, chondrocytes can be extracted from auricular cartilage [Malicev et al., 2009] and nasal septum, with some advantages as compared to articular chondrocytes, since they are easily harvested and grow for several days without significant reduction in cell viability [Chia et al., 2004]. The extracellular matrix is composed of fibrous proteins (collagen, elastin, fibronectin, and laminin) and glycosaminoglycans (GAGs), among which hyaluronan, chondroitin sulfate (CS), that are known to play a pivotal role in providing adequate mechanical properties [Plaas et al., 2001]. Chondroitin sulfate (CS) is a linear anionic polysaccharide composed of repeating disaccharide units of d‐glucuronic acid and N‐acetyl‐d‐galactosamine and present different sulfation patterns according to the extractive source. For commercial purposes, it is derived from bovine trachea, swine nasal septa, shark fins, fish cartilage, and by a semi‐synthetic approach [Schiraldi et al., 2010]. Unsulfated biotechnological chondroitin (BC) biopolymer has also been obtained in novel and patented biotechnological production processes, increasing application opportunities. Marketed as a food supplement and anti‐arthritic drug, recently, CS has received attention for novel/diverse medical device applications [Schiraldi et al., 2010]. It was demonstrated that CS reduced the nuclear translocation of NF‐kB in vitro [Du Souich et al., 2009; Calamia et al., 2010]. Recently, Calamia et al. [2012] have reported the effective use of CS coupled to glucosamine in the treatment of osteoarthritis (OA) for its anti‐inflammatory properties [Hochberg, 2012]. In osteoarthritis, CS may stimulate the synthesis of proteoglycans and decrease the catabolic activity of chondrocytes through inhibition of proteolytic enzymes and other factors that contribute to tissue degradation. The study of the effect of CS on anabolism and catabolism in cartilage tissue [Kwan et al., 2010] has suggested a positive role in balancing the two, thus delaying the pathology progression [Du Souich et al., 2009]. In addition, CS has been shown to have anti‐inflammatory effects on chondrocytes in vitro. Recently medical devices based on CS and hyaluronan have been used in intra‐articular viscosupplementation [Chen et al., 2011; Henrotin et al., 2012]. Being new biotechnological chondroitin un‐sulfated and thus similar to the hyaluronan, the aim of this study was to evaluate and compare the effects of BC respect to CS on human primary chondrocytes in vitro model in order to assess its biomedical potential for use in medical devices, scaffolds, or other active pharmaceutical principle.
MATERIALS AND METHODS
Biotechnological chondroitin (BC) (1% w/v) was specifically produced in our laboratory following the procedure described in Schiraldi et al. [2010]. In addition, specific purification steps, using activated carbon and ethanol precipitations, were used to reduce contamination such as o‐chain and endotoxins, host proteins, lipid A, 95 ± 5% pure chondroitin was obtained with endotoxin content lower than 0.05 EU/mg and used in the experiment in comparison to commercially available chondroitin sulfate (CS) (1% w/v) extracted from swine nose cartilage (Sigma–Aldrich, Milan, Italy). All solutions were obtained dissolving BC and CS (1% w/v) in the medium, pH = 6.5‐8.0 and osmolality = 300 mOsm. Solutions were tested for endotoxin content and microbiological counts after microfiltration (0.22 μm) in order to perform safe experiments (Pharma grade). Endotoxin concentration (EU/mL) was evaluated using the Limulus test and solutions were used only when titer was lower than 0.1 EU/mL. IL‐1β was obtained from Sigma–Aldrich.
ISOLATION AND EXPANSION OF HUMAN NASAL CHONDROCYTES
Nasoseptal cartilage was extracted from consenting human healthy volunteers, ranging from 20 to 50 years of age who were undergoing surgery procedures for functional respiratory limitation at Pellegrini Hospital. The study was approved by the Medical Ethics Committee of the Second University of Naples (AOU‐SUN registration No 0003711/2015). In particular, the patients enrolled in this study signed an informed consent form drafted following instructions from the SUN Internal Ethical Committee. Briefly, the cartilage was minced into small pieces and digested using 3 mg/mL collagenase Type I (Gibco, Invitrogen), 4 mg/mL dispase (Gibco, Invitrogen) in Phosphate‐Buffered Saline (PBS, PH 7.2, Lonza), and 0.2 mg/mL Gentamycin (Hospira) overnight at 37°C under a condition of gentle shaking. The released cells were separated by filtration through sterile filter (70 μm) and the cellular suspension was centrifuged (1500 rpm, 7 min). Cell pellet was re‐suspended in Dulbecco's Modified Eagle Medium (DMEM), supplemented with fetal bovine serum (FBS) (10% v/v), (Gibco, Invitrogen) penicillin‐streptomycin (1% v/v), Amphotericin B (1% v/v) (Lonza), then the cells were seeded in a T‐25 tissue culture flask and maintained at 37°C in a humidified atmosphere with 5% v/v CO2. The medium was changed every 3–5 days until cells reached ∼80% confluence. Afterward, cells were harvested with trypsin/EDTA 0.2 mg/mL and re‐seeded in appropriate tissue culture plates. Dissociation with trypsin followed by re‐seeding for cell expansion was named “passage.”
CELL PROLIFERATION ASSAY AND TIME‐LAPSE VIDEO‐MICROSCOPY
Chondrocytes at 2nd passage of culture, were grown to confluence for proliferation experiments; 1.0 × 104 cells/cm2 into 24‐well tissue plates were seeded. The culture medium was then removed and replaced by either fresh medium alone (control), or medium containing BC (1% w/v) or CS (1% w/v). To study cell growth and morphology changes in time, extracted chondrocytes were observed for 72 h, using a time‐lapse video microscopy apparatus. Briefly, as previously described in Schiraldi et al. [2012] [D'Agostino et al., 2015], the station was assembled with an inverted optical microscope with a CCD gray scale camera, a stage incubator (CO2, T and air control) and a thermostatic bath (Okolab). The CO2 microscope stage incubator maintained all the required environmental conditions for cell cultures (37°C and 5% CO2 in air, etc.), allowing prolonged observations of living cell behavior and events (e.g., proliferation, migration, elongation, cell morphology) to be performed. The instrument was connected to software OKO‐Vision 4.4.3 (composed of OKO‐Vision Time Lapse and OKO‐Vision Imaging). Time‐lapse video‐microscopy enabled both the contemporary incubation of more samples and also for a multiple‐field visualization within the same sample, thus ensuring the statistical significance of the experiments. Pictures were taken every 60 min throughout the entire interval of the experiment (72 h) and five representative fields of view for each well were selected and collected. All conditions were performed in triplicate to minimize variations. The recorded images were analyzed by counting using Image‐Pro® Plus 5.1 software, for cell image analysis (Media Cybernetics).
CELL SIZE ANALYSES
The morphological modifications (shape and dimensions) of primary chondrocytes were examined after 10 days of treatment with BC (1% w/v) and CS (1% w/v). Cell dimension analyses were performed measuring the major axis length during in vitro cell culture. In particular, in order to quantify cell elongation, 10× cell images were acquired from four non consecutive fields of view from each well. During experiments, at least two operators observed the images and pointed out six cells/field of view that were considered representative of the population, and the four that were highlighted by both observers were then subjected to measurements. Overall at least four cells per view‐field for each of three independent experiments were measured. The length of cellular major axis were measured using Image‐Pro® Plus 5.1 software (Media Cybernetics). Next, analyses from corresponding lengths of different samples were compared; data were reported as average cell length (μm) a three different passages (P2, P3, and P4).
FLUORESCENCE‐ACTIVATED CELL SORTING (FACS)
Chondrocytes, treated with BC (1% w/v), CS (1% w/v), and untreated (CTR) samples (at a density of 1 × 105 cells/sample) at the second passage of culture, were incubated with fluorescent‐labeled monoclonal antibody or respective isotype controls. The antibody used was anti‐collagen type II (COL2A1, AbCam). Secondary antibody was anti‐rabbit FITC (AbCam). For intracellular staining of COL2A1, cells were processed using the Fix & Perm Kit (Invitrogen) following the manufacturer's guidelines. The fluorescence associated to the cells was measured using FITC channel and calculated both as mean percentage of positivity for Type II collagen and as mean fluorescence intensity (MFI) for each sample. All data were acquired using FACS Aria III (BD) and analyzed using FCS version 3 software.
IN VITRO CARTILAGE INFLAMMATION MODEL
To establish the inflammation in vitro model, we used human chondrocytes primary cultures treated with IL‐1β (10 ng/mL) a key pro‐inflammatory cytokine involved in the osteoarthritis (OA) pathogenesis. A slight modification of the model described by Calamia et al. [2010] was applied. In particular, 2.5 × 104 cells/cm2 were seeded in 24 well tissue plates, when confluence of the cells was reached the medium was changed to FBS free medium containing biotechnological chondroitin (1% w/v) and/or chondroitin sulfate (1% w/v), nothing was added in the control wells. Two hours later, IL‐1β was added at 10 ng/mL to each well, except for the negative control wells (at least three per trial) and multiwells were incubated 24 h. In order to evaluate cell response in the diverse conditions of this in vitro inflammation assay, supernatants were collected for cytokines multiplex analyses. Beside oxidative stress of cells was also evaluated through gene expression quantification (RT‐PCR) of the enzyme superoxide dismutase 2 (SOD‐2) from cell extracts. The method is described in the successive paragraph.
QUANTITATIVE GENE EXPRESSION BY REAL‐TIME PCR
To evaluate changes in gene expression profile, total RNA from primary chondrocytes of serial passages (n = 4) was analyzed. For quantitative real time PCR, 2.5 × 104 cells/cm2, that correspond to 50,000 cells/well, were used. Primary chondrocytes treated for 4, 7, and 10 days with BC (1% w/v) and CS (1% w/v), were seeded in 24 well tissue plates, and total RNA was extracted using Trizol Reagent (Invitrogen), following manufacturer's instructions. Briefly, 200 μL of chloroform were added into the tube. The mixture was then shaken vigorously for 10 s followed by 10 min incubation. Then, it was centrifuged for 15 min at 4°C, 12.000g resulting in the formation of three distinct layers: RNA, protein, and DNA, respectively. The aqueous phase layer was carefully transferred into a new tube for each sample. Five hundred microliters of isopropanol were then mixed homogeneously into the tube to precipitate total RNA. Total RNA extracted from the samples were washed with 1.0 mL 75% ethanol and centrifuged at 12,000g for 5 min at 4°C. The supernatant was discharged and the RNA pellet was air‐dried for 25 min. The precipitated pellet was dissolved in 30μL nuclease free water (Sigma–Aldrich). The concentration of RNA extracted was determined spectrophotometrically using a Nanodrop Instrument (Celbio). One microgram of total RNA was reversely transcribed into cDNA with Reverse Transcription System Kit (Promega). Quantitative real‐time PCR was performed using IQ™ SYBR® Green Supermix (Bio‐Rad Laboratories) to evaluate cartilage‐specific markers of aggrecan core protein (AGN), type I (COL1A2) and type II collagen (COL2A1), SOX‐9 gene expression, and SOD‐2 for IL‐1β treatment. The primer sequences were designed by Beacon Designer™ software (Bio‐Rad Laboratories) and are shown in Table I. The expression of specific mRNA was normalized compared to the level of the housekeeping gene, human glyceraldehydes‐3‐phosphate dehydrogenase (GAPDH). All reactions were carried out in triplicate. The temperature of annealing and amplification protocol for each primer pair is reported in Table I. The efficiency (between 80% and 110%) and specificity of each primer set was verified with the use of a standard curve, Ct value versus serial dilution of total RNA and the fold‐change in gene expression was evaluated by using the comparative threshold method (ΔΔCt = difference of ΔCt between treated cells and non‐treated cells used as control) using Bio‐Rad iQ5 software (Bio‐Rad Laboratories).
Table I.
Primer Sequences Used in Real‐Time PCR for Quantitative Gene Expression Analysis
| Gene | Primer sequence | Temperature of annealing (°C) | Amplification protocol |
|---|---|---|---|
| GAPDH | Forward: 5′ TGCACCACCAACTGCTTAGC 3′ | 55 | 95°C 10 s, 55°C 30 s, 72°C 3 min, 40 cycles |
| Reverse: 5′ GGCATGGACTGTGGTCATGAG 3′ | |||
| Aggrecan (AGN) | Forward: 5′ TCGAGGACAGCGAGGCC 3′ | 56 | 95°C 10 s, 56°C 30 s, 72°C 3 min, 40 cycles |
| Reverse: 5′ TCGAGGGTGTAGCGTGTAGAG 3′ | |||
| Type I Collagen (COL1A1) | Forward: 5′ CAGCCGCTTCACCTACAGC 3′ | 56 | 95°C 10 s, 56°C 30 s, 72°C 3 min, 40 cycles |
| Reverse: 5′ TTTGTATTCAATCACTGTCTTGCC 3′ | |||
| Type II Collagen (COL2A1) | Forward: 5′ CAACACTGCCAACGTCCAGAT 3′ | 57 | 95°C 10 s, 57°C 30 s, 72°C 3 min, 40 cycles |
| Reverse: 5′ CTGCTTCGTCCAGATAGGCAA 3′ | |||
| SOX9 | Forward: 5′ AGACCTTTGGGATGCCTTAT 3′ | 55 | 95°C 10 s, 55°C 30 s, 72°C 3 min, 40 cycles |
| Reverse: 5′ TAGCCTCCCTCACTCCAAGA 3′ | |||
| SOD‐2 | Forward: 5′ CTGGACAAACCTCAGCCCTA 3′ | 55 | 95°C 10 s, 55°C 30 s, 72°C 3 min, 40 cycles |
| Reverse: 5′ TGATGGCTTCCAGCAACTC 3′ |
IMMUNOBLOTTING
Western blot analyses were performed following standard procedures. After 10 days of treatment with BC (1% w/v) and CS (1% w/v), chondrocytes were lysed in RIPA buffer (1×) (Cell Signaling Technology). Protein concentration was determined using the Bradford method [Bradford, 1976] and 60 µg intracellular proteins were loaded and resolved using 8% SDS–PAGE. The separated proteins were then transferred to nitrocellulose membrane (Amersham). The membrane was blocked in 5% milk, Tris‐buffered saline and 0.05% Tween‐20. Primary antibodies to detect type II collagen and type I collagen (Abcam) were used at 1:250 dilutions. Immunoreactive bands were detected by chemiluminescence using corresponding horseradish peroxidase‐conjugated secondary antibody (Santacruz Biotechnology; 1:5000 dilutions) and reacted with an ECL system (Chemicon‐Millipore). Protein levels were normalized with respect to the signal obtained with anti actin polyclonal antibody (Santacruz Biotechnology; 1:500 dilutions). The semi‐quantitative analysis of protein levels was carried out by the Gel Doc 2000 UV System and the Gel Doc EZ Imager, using quantity one software (Bio‐Rad Laboratories).
IMMUNOHISTOCHEMICAL ANALYSIS
Immunohistochemistry for type II collagen (Abcam) was performed on chondrocytes, treated with BC (1% w/v) and CS (1% w/v) and untreated, at 2nd passage of culture at 4, 7, and 10 days of culture, in 12 well plates and at 4th passage at 10 days of culture, fixed with 4% paraformaldehyde for 10 min at 4°C, rinsed in PBS, and permeabilizing with 0.1% Triton X‐100 (Sigma–Aldrich). For staining, the DAKO Cytomation En Vision + HRP kit AEC (DAKO) was used according to manufacturer's instructions. The type II collagen antibody (AbCam) was diluted 1:50 in PBS and incubated for 30′ at 4°C. The nuclei were stained with hematoxylin, and the cells were observed using an inverted light microscope (Nikon). For Type II collagen quantification, TIFF images were analyzed with ImageJ software.
IMMUNOFLUORESCENCE AND CONFOCAL MICROSCOPY
The nasal primary chondrocytes treated with BC (1% w/v) and CS (1% w/v) for 10 days and maintained for two and four passages in culture (p2 and p4), were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X‐100 in PBS. The cells were then incubated with anti‐type I collagen antibody (Abcam) overnight at 4°C followed by a corresponding secondary antibody for 2 h at room temperature. Nuclei were stained with 2′‐(4‐hydroxyphenyl)‐5‐(4‐methyl‐1‐piperazinyl)‐2,5′‐bi‐1H‐benzimidazole trihydrochloride hydrate, bisBenzimide (Hoechst, Sigma–Aldrich). Fluorescence images were captured using the confocal system (Zeiss).
BIO‐PLEX ASSAY
In order to evaluate inflammation in vitro chondrocytes were insulted by the addition of IL‐1β 50 ng/mL and after 24 h added with BC or CS at 1% w/v, except for the control wells. Cell supernatants were collected after 24h, centrifuged (3,000 rpm for 10 min at 4°C) and stored at −80°C until analyzed. Cytokines were evaluated using multiplex biometric ELISA‐based immunoassay, containing dyed microspheres conjugated with a monoclonal antibody specific for a target protein (Bio‐plex, Bio‐Rad Lab, Milan Italy). Cytokines production was quantified using 8‐plex immunoassay panel: IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, GM‐CSF, IFN‐γ, and TNF‐α. Each experiment was performed in triplicate and cytokines levels of all targets were determined using a Bio‐Plex array reader (Luminex, Austin, TX). The analytic concentrations were calculated using a standard curve according to the manufacturer's instructions.
STATISTICAL ANALYSIS
All experiments were performed in triplicate. Student's t‐test was used for statistical evaluation. The level of significance was set at P < 0.05.
RESULTS
HUMAN PRIMARY CHONDROCYTE CELL LINES
Primary chondrocyte cell lines were obtained from digested samples. In standard medium, without CS and BC addition, after 24 h of culture, cells adhered to the bottom of the flask and acquired polygonal morphology (data not shown). This feature was maintained up to the 4th passage. Then, cells changed their morphology, becoming fibroblast‐like. At 1st passage of culture, the cells were analyzed for type II collagen both at flow cytometry and immunohistochemistry. All samples tested were positive for type II collagen indicating chondrocytes lineage (data not shown—Supplementary Material Fig. S1).
CELL GROWTH AND CHONDROCYTE SIZE ANALYSES
To evaluate the effect of BC versus CS on chondrocytes proliferation, cell growth curves were performed (Fig. 1). CS promoted proliferation at early stages (up to 12–15 h), while BC exerted a more powerful stimulus after 24 h of incubation. In fact, at 24 h, CS treated wells increased cell number of 1.3‐fold while BC treated chondrocytes incresead twofold; proliferation rate was also compared and found to be statistically superior for BC treated samples (P < 0.05). On the contrary, at later stages of culture (from 24 to 96 h), BC treated chondrocytes nearly reached confluence respect to both untreated and CS treated cells covering only 52 ± 5% of each well area (Fig. 1A). In fact, the average doubling time was of approximately 68 ± 4 h for control 40 ± 3 h for BC treatment and 56 ± 3 h for CS treated ones (Fig. 1B). Cellular proliferation trend was confirmed by quantitative cell viability assay (data not shown) up to 7 days. MTT test results showed that BC slightly but significantly improved cell growth (110% vs. control), while CS was comparable to the control; however, it was noticed that both GAGs preserved the phenotype while untreated chondrocytes at 7 days already showed a morphological change (data not shown). These changes were also evaluated measuring the major axis of chondrocytes during culture at increasing time intervals. Image analyses showed that at increasing passages, (from 2 to 4), untreated and CS treated cells were elongated: a morphological sign of the differentiation toward fibroblast‐like phenotype. On the contrary, BC treated cells maintained a polygonal shape with a measure of major axis significantly lower than those of untreated and CS treated cells (4th passage of culture, 137.0 μm BC vs. 164.5 μm CS) (Fig. 2).
Figure 1.
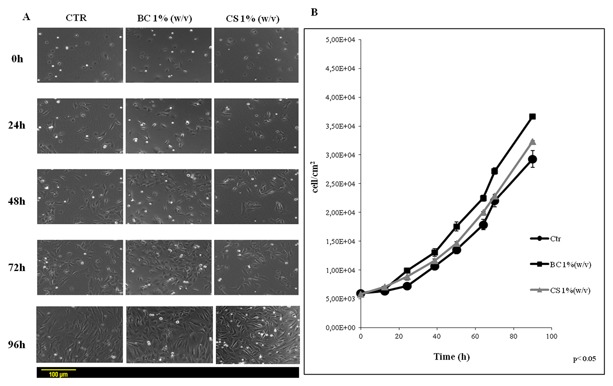
A: Representative micrographs pictures relative to cell growth in time lapse experiments at 0, 24, 48, and 72 of treatment with biotechnological chondroitin (BC 1% w/v) and chondroitin sulfate (CS 1% w/v). B: Proliferation curves for nasoseptal chondrocytes. The cells growth was obtained analyzing, by counting, three different wells for each treatment (Untreated‐cells = CTR), biotechnological chondroitin (BC 1% w/v) and chondroitin sulfate (CS 1% w/v) of the five different experiments. Data shown are means ± SD (P < 0.05).
Figure 2.
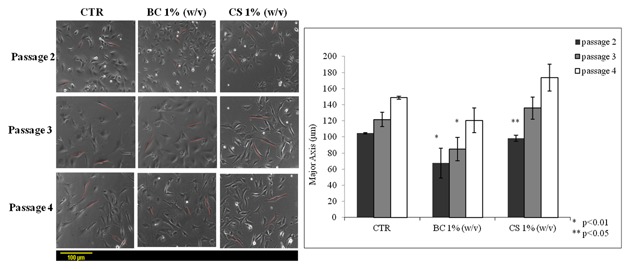
Comparative cell dimensions analysis relative to major axis between chondrocytes cultured a three different passages (p2, p3, p4) in presence of biotechnological chondroitin (BC 1% w/v) and chondroitin sulfate (CS 1% w/v) after 7 days of treatment. The data represent mean ± SD of three independent experiments. The groups (BC at passage 2 and 3 respect to control) are significantly different according to Student's t‐test (*P < 0.01) and (CS passage 2 respect to control) (**P < 0.05).
FACS ANALYSES
To assess the effect of BC versus CS on type II collagen expression, cytometric analyses at 2nd passage after 10 days of culture was performed. The results showed that the mean percentage of cells expressing type II collagen was 78.90 ± 1.1% for untreated primary chondrocytes, 93.1 ± 2.2% for BC treated primary chondrocytes and 80.07 ± 1.0% for CS treated primary chondrocytes indicating that BC induced an increase of type II collagen expression levels compared to CS as reported in Table II and Figure 3. In particular, the differences were statistically significant for BC treatment compared to untreated cells (P = 0.001).
Table II.
Type II Collagen Expression on Untreated BC and CS Human Primary Chondrocytes
| Type II collagen expression | |||
|---|---|---|---|
| Mean percentage (%) | MFI | P‐value | |
| Untreated chondrocytes | 78.90 ± 1.1 | 51,61 | |
| BC treated chondrocytes | 93.1 ± 2.2 | 69,7 | 0,001 |
| CS treated chondrocytes | 80.07 ± 1.0 | 21,84 | 0,05 |
P‐value is calculated respect to untreated chondrocytes.
Figure 3.
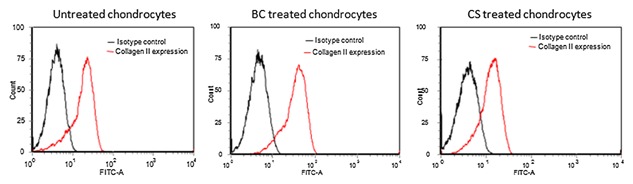
Flow cytometry. Type II collagen expression on untreated, BC‐treated and CS‐treated chondrocytes at 2° passage by flow cytometry. BC‐treated chondrocytes express type II collagen levels greater than both untreated and CS‐treated chondrocytes.
QUANTITATIVE RT‐PCR ANALYSIS
Aggrecan, types I and II collagen are main components of ECM of cartilages as well as Sox9 plays a key role in chondrogenesis. In order to evaluate the effect of BC and CS on these biomarkers, qRT‐PCR was performed. Molecular analyses showed that independently from the age of the culture, BC induced an increase of AGN mRNA levels that were higher than those of CS and untreated samples. In particular, at 2nd and 4th passages, AGN was highly expressed for BC and decreased in time. At 3rd passage, no difference in AGN levels for the diverse treatment was detectable at 4 and 7 days. On the contrary, at 10 days, their levels were strongly increased in BC treated chondrocytes (Fig. 4). COL2A1 expression levels were always higher than COL1A1, confirming chondrocyte phenotype. In particular, at the 2nd passage, at 4 days of culture, no significant differences in COL2A1 gene expression were observed between BC and CS. At 7 days, BC induced higher COL2A1 expression as well as at 10 days respect to the others. At 3rd passage, at all the time intervals observed BC promoted an increase of COL2A1 levels respect to CS. At 4th passage, similar expression trend for COL2A1 between BC and CS were detectable at 7 days, the 10 days results showed a higher expression for COL1A1 (always lower than the control), indicating a progressive phenotype modification occurring even in CS and BC treated samples (Fig. 4). Further analyses concerned Sox9, an important marker for activation of the COL2A1 gene during chondrogenesis in both human and mouse primary cells [Bell et al., 1997]. At 2nd passage, Sox9 gene expression was lower in BC than CS treated cells at 4 days. For 7 and 10 days, on the contrary, higher levels were observed in BC treated cells. At 3rd and passage, Sox9 levels were higher for treated samples respect to control only at 4 and 7 days of culture, respectively.
Figure 4.
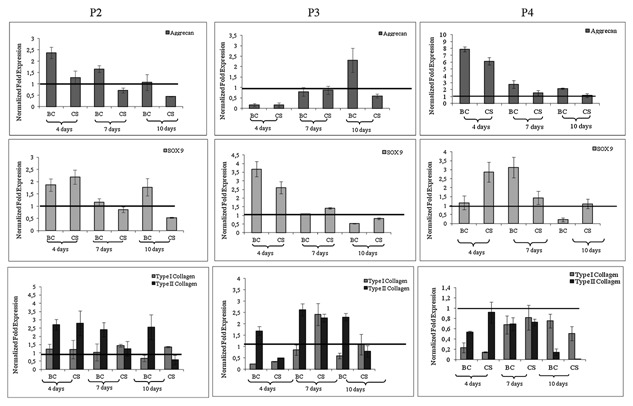
Quantitative RT‐PCR analysis of the expression levels respectively of Aggrecan (AGN), type I collagen (COL1A1), type II collagen (COL2A1), and SOX‐9 mRNA in the chondrocytes monolayer at passages 2 (p2), 3 (p3), and 4 (p4). The histogram reported the mean of six different experiments performed in triplicate. The groups (BC respect to IL‐1β and BC respect to CS) are significantly different according to Student's t‐test (# P < 0.01 and *P < 0.05).
In OA in vitro model, IL‐1β‐induced SOD‐2 gene expression and this was modulated by biotechnological chondroitin and chondroitin sulfate addition in the medium. In particular data showed an up‐regulation of SOD‐2 (data not shown—Supplementary Material Fig. S2) when the chondrocytes were treated with IL‐1β both glycosaminoglycans proved able to reduce SOD‐2 expression with a higher significance for BCs (P < 0.01 BC group respect to IL‐1β).
IMMUNOBLOTTING ANALYSIS
Western blot analysis revealed a slight increase in type II collagen protein level in BC (1% w/v) treated samples compared with control and CS 1% (w/v) at 4th passage after 10 days of treatment (densitometry evaluated a 1.2‐fold increase of BC respect to CS treated samples), but more interestingly it was found a remarkable lower collagen I level for BC treated cells (Fig. 5A and B). Western blot analyses are performed on chondrocytes treated with BC (1% w/v) and CS (1% w/v) at p2 and p4 of culture. Our results showed that at 2nd passage BC significantly increase COLII expression (10‐fold increase respect to CTR) and CS significantly increase COLI levels (eightfold increase respect to CTR). However, at 4th passage, only a slight increase in COLII protein level was found for BC treated samples compared with CS and control after 10 days of incubation (densitometry evaluated a 1.2‐fold increase of BC respect to CS treated samples). Interestingly, it was found a remarkable lower of COLI level for BC treated cells (Fig. 5A and B).
Figure 5.
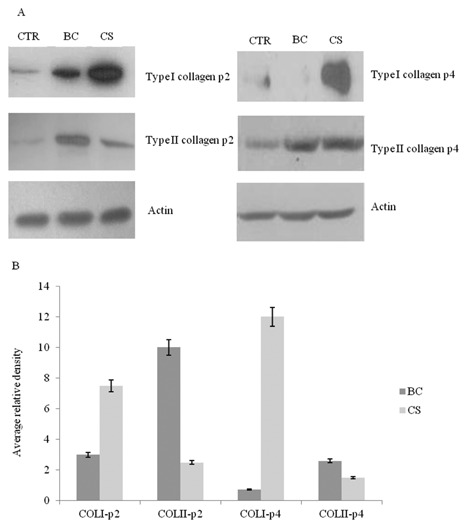
A: Representative western blot analysis of COL2A1, COL1A1 and actin housekeeping protein levels in chondrocytes (BC 1% w/v) and (CS 1% w/v) treated samples, after 10 days of treatment, compared to the control at passages 2 and 4. B: In order to measure protein expression levels (types I and II collagen), intensities of specific bands, corresponding to the proteins of interest are measured using commercially available software (Image J software). The histogram reported the mean of three different experiments performed in duplicate.
IMMUNOHISTOCHEMICAL ANALYSIS
Immunohistochemical experiments performed at 2° and 4° passage of culture at 4, 7, and 10 days showed that all cells, independently from time of culture, passage and treatment, expressed type II collagen. In particular, both at 2° and 4° passage of culture, BC treated chondrocytes showed a localization of type II collagen not only on surface of the cell membrane but also in the cytoplasm with formation of ECM increasing with culture time (Fig. 6 and data not shown—Supplementary Material Fig. S3). On the contrary, both untreated and CS treated chondrocytes showed a distribution of type II collagen especially on surface of cell membrane and not in cytoplasm without formation of ECM with time (Fig. 6A). Moreover, BC induced also an increase of type II Collagen expression compared to those of CS and untreated cells (Fig. 6B).
Figure 6.
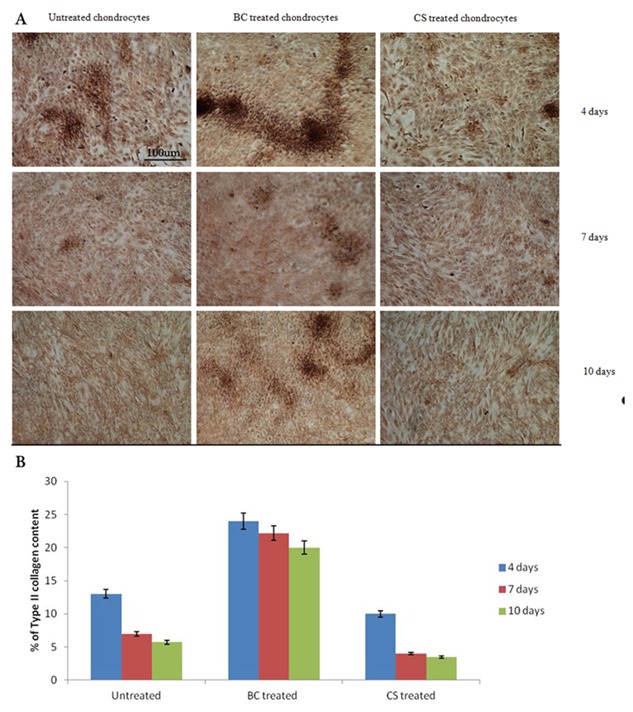
A: Immunohistochemical analysis, figure of Histology. Immunohistochemistry for type II collagen expression on untreated, BC‐treated and CS‐treated chondrocytes at 2° passage at 4, 7, and 10 days. BC treated chondrocytes express type II collagen levels greater than both untreated and CS treated chondrocytes with formation of ECM. Untreated and CS‐treated chondrocytes show a type II collagen distribution especially on surface of cell membrane and the shape of these cells results to be more fibroblast than polygonal. B: Quantification of type II collagen by Image J software, the graph showed the mean of two different experiments.
IL‐1β‐TREATED CHONDROCYTES: COMPARISON OF CYTOKINE PRODUCTION IN BC AND CS PRETREATED CELLS RESPECT TO CONTROL
In order to evaluate the effect of BC and CS on inflammation, bio‐plex assay (Fig. 7) was used to analyze cytokine levels (pg/mL) in IL‐1β‐treated chondrocytes with or without GAG addition. Among the 8 cytokines evaluated the ones that were not statistically different from the control are not shown. Our results proved that in the inflammatory in vitro model both BC and CS, reduced the effects of IL‐1β on primary chondrocytes. In particular, IL‐2 was significantly reduced (2.4‐ and 1.9‐fold) when the cells are treated with BC and CS, respectively; IL‐4 decreased 3.4‐fold for BC and 2.6‐fold for CS pre‐treatments, respectively; IFNγ (2.8‐ and 2.1‐fold) and TNF‐α (three‐ and twofold, respectively). All these cytokines were significantly (P < 0.01) reduced in presence of BC and CS respect to the negative control that resemble the OA pathology in vitro.
Figure 7.
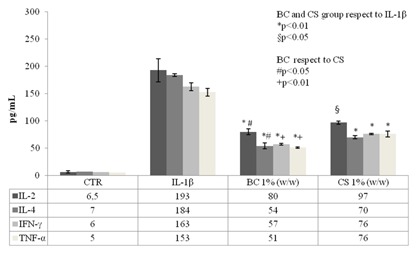
Cytokines production assay in chondrocytes cell supernatants. We reported the significant molecule levels in the control (untreated cells), in IL‐1β and in BC (1% w/w) and in CS (1% w/w) treatments. The graph showed the mean of two experiments performed in triplicate. The groups (BC and CS respect to IL‐1β) are significantly different (*P < 0.01 and § P < 0.05) and BC groups respect to CS in according to Student's t‐test (# P < 0.05 and + P < 0.01).
IMMUNOFLUORESCENCE ANALYSIS
To further determine the expression level of type I collagen and cytoplasmatic distribution, BC and CS treated chondrocytes, were observed at confocal microscopy for immunofluorescence analyses. Pictures showed that CS promoted major distribution of the protein into the cytoplasm of cells, respect to the control and BC treated cells (Fig. 8). The figure clearly provide evidence of an increase of expression of type I collagen in chondrocytes at the 4th passage respect to the 2nd, further supporting a different pattern of matrix formation and behavior in presence of CS and BC and at different passage of culture.
Figure 8.
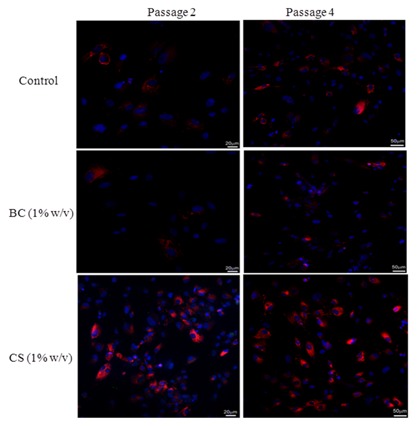
Confocal microscopy images. Type I collagen expression (red) in human primary nasal chondrocytes (untreated cells = control) treated with biotechnological chondroitin (BC 1% w/v) and chondroitin sulfate (CS 1% w/v). Cytoplasmatic distribution of type I collagen in chondrocytes at different passages of culture (p2–p4), after 10 days of treatment, was determined by immunofluorescnce as described above.
DISCUSSION
Cartilage injuries are highly prevalent in both young and elderly patient populations; hormonal, genetic, and inflammatory factors are known to increase the risk of osteoarthritis and influence the course of the disease. Cell‐based cartilage tissue engineering could provide permanent solutions in therapeutic applications to treat cartilage lesions and osteo‐chondral pathologies [Saadeh et al., 1999; Fuentes‐Boquete et al., 2008; Do Amaral et al., 2012]. Advances in alternative approaches, such as the optimization of chondrocyte isolation and characterization in vitro, represent the potential prospective to translate the in vitro models of cartilage regeneration into clinical practice [Shafiee et al., 2011; Oseni et al., 2013]. Recent evidence for osteoarthritis treatment supports the effectiveness of new medical devices combining chondroitin sulfate and hyaluronan. Therapy based on CS‐HA intra‐articular injections in both humans [Chen et al., 2011] and animals [Kwan et al., 2010] have changed the approach on the treatment of the disease, conferring a therapeutic outcome via anti‐inflammatory mediated mechanisms [Altman, 2010]. Recently in according to the study of Ferro et al. [2012], in the human bladder cancer, the use of CS in combination with traditional drugs could increase the anti‐tumor activity of these drugs in order to reduce their active concentrations and their toxic side effects.
In this study, we explored the efficacy of a new chondroitin obtained from biotechnological process (BC) compared to commercial chondroitin sulfate (CS) using in vitro expansion of human nasal cartilage derived cells [Hochberg et al., 2013]. This new available biotechnological chondroitin is produced by fermentation and is extensively purified in our laboratories [Schiraldi et al., 2010]. Although chondrocytes dedifferentiate during in vitro monolayer culture [Matmati et al., 2013], a biochemical relationship between the degree of chondrocytes dedifferentiation and their corresponding morphology, gene expression profile and proliferation rates is reported in this research. In fact, chondrocytes dedifferentiate gradually changing their structure from a spherical to a spindle‐shaped morphology and develop a fibroblast‐like aspect producing type I collagen and not type II collagen, normally found in articular cartilage [Munirah et al., 2010; Sheu et al., 2013]. This needs to be prevented for clinical applications, when chondrocytes, isolated from patients, need to be expanded for subsequent surgical replacement using biomimetic scaffolds, on engineered tissues [Shakibaei et al., 2006]. In fact, autologous chondrocyte transplantation (ACT) based on the association of biomaterials with autologous chondrocytes expanded in vitro is used to repair cartilage [Rutgers et al., 2013], but either the limited source of healthy primary cells and/or dedifferentiation of cells may lead to the failure of tissue repair [Kawasaki et al., 1999; Marlovits et al., 2004]. In this study, first of all, we characterized the cells derived from human nasal septum using type II collagen in order to select only chondrocytes [Benya et al., 1978]. Then, we analyzed the cell growth, dimensions, and specific biomarkers of chondrocyte phenotype when either BC or CS were used to supplement the culture medium. Overall, our study aimed at evaluating the potential effectiveness of the biotechnological chondroitin, respect to the well‐known CS to restore the chondrogenic phenotype considering the limited intrinsic healing property of the cartilage itself [Hicks et al., 2005; Lim et al., 2014; Demoor et al., 2014]. In proliferation studies, CS powers cell doubling compared to controls and even to BC, but this effect lasted only 15 h, whereas a prolonged effect was found for BC. The latter is un‐sulfated, similar to hyaluronan, this could allow different interaction with receptors also promoting diverse cell response. Another interesting result proved that CS and BC supplementation increased type II collagen expression in primary chondrocytes thus better preserving cell type, BC was effective up to P4 10 days of culture. In addition, BC supplement proved superior for the maintenance of chondrogenic phenotype at both morphological and biomarkers level. Molecular studies are complementary to cellular, histological/morphological ones. SOX factors bind to a COL2A1 enhancer segment regulating type II collagen expression during chondrogenesis and chondrocitic in vitro differentiation [Stokes et al., 2001; Kypriotou et al., 2003]. In this study, SOX‐9 transcription factor was activated and over‐expressed in BC and CS treated samples according to the results obtained for COL2A1. It should be underlined that the biomarker expression trends for the 4th passage at 10 days may suffer by the cell confluence that is reached at 5 ± 1 days for BC treated wells and only at 9 ± 1 for CS and control.
Well assessed in vitro model are also useful to predict to a certain extent the efficacy of novel molecules in vivo. Besides, the administration of glucosamine and chondroitin sulfate to patients affected by OA was shown to improve the production of synovial HA and then clarify the immediate clinical responses [David‐Raoudi et al., 2009; Campo et al., 2013].
Our results showed that both BC and CS decrease oxidative stress. In particular, BC significantly reduced SOD‐2 expression level (data not shown, Supplementary Material). In vitro studies described the anti‐inflammatory and the anti‐oxidant activity of glucosamine compared to chondroitin sulfate [Calamia et al., 2010]. Exploiting a similar cell based model here we compared BC and CS as anti‐inflammatory molecules. To induce inflammation, chondrocytes were treated with IL‐1β and when cells were in contact with BC and CS the inflammatory response was significantly reduced. In addition, our data showed a down regulation of IL‐2, IL‐4, IFNγ, and TNFα, in cell treated with BC, superior to the one found for CS.
In conclusion, our results suggest that BC (i) increase cell proliferation contemporarily preserving cell phenotype by exerting a beneficial effect on type II collagen expression up to 10 days of incubation; (ii) enhances their lifespan as demonstrated by the prevalence of chondrocyte phenotype after serial culture passages (p4); (iii) BC confirmed its biological activity respect to inflammation. Specifically, BC is better than CS in the reduction of cytokines levels in chondrocyte inflammation model in vitro. The outcomes of this research support the remarkable biotechnological chondroitin importance in translational medicine: its bioactivity can find numerous applications in medical‐devices and pharmaceutical preparations aiming at cartilage pathology treatments.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Material Fig. S1
Supplementary Material Fig. S2
Supplementary Material Fig. S3
ACKNOWLEDGMENTS
This research work was supported by the national grant PON01_1226 Nutrafast and the Regione Campania grant POR “BIP.” The authors are grateful to Dr. Alfano A., Della Corte K., and Diana P. for supplying pure biotechnological chondroitin. Dr. Urbanek K. for confocal microscopy images and Dr. Pirozzi A.V.A. for technical support in western blot analyses.
Conflicts of interest: The authors declare that they have no competing interests.
REFERENCES
- Altman RD. 2010. Pharmacological therapies for osteoarthritis of the hand: A review of the evidence. Drugs Aging 27(9):729–745. [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KKH, Wheatly SC, Ng LJ, Zhou S. 1997. SOX9 directly regulates the type‐II collagen gene. Nat Genet 16:174–178. [DOI] [PubMed] [Google Scholar]
- Benya PD, Padilla SR, Nimni ME. 1978. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 15:1313–1321. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 7(72):248–254. [DOI] [PubMed] [Google Scholar]
- Calamia V, Ruiz‐Romero C, Rocha B, Fernández‐Puente P, Mateos J. 2010. Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther 12(4):R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia V, Lourido L, Fernández‐Puente P, Mateos J, Rocha B. 2012. Secretome analysis of chondroitin sulfate‐treated chondrocytes reveals anti‐angiogenic, anti‐inflammatory and anti‐catabolic properties. Arthritis Res Ther 14(5):R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, D'Ascola A, Scuruchi M, Nastasi G, Micali A, Puzzolo D, Pisani A, Calatroni A, Campo S. 2013. The SOD mimic MnTM‐2‐PyP(5+) reduces hyaluronan degradation‐induced inflammation in mouse articular chondrocytes stimulated with Fe (II) plus ascorbate. Int J Biochem Cell Biol 45(8):1610–1619. [DOI] [PubMed] [Google Scholar]
- Chen L, Ling PX, Jin Y, Zhang TM. 2011. Hyaluronic acid in combination with chondroitin sulfate and hyaluronic acid improved the degeneration of synovium and cartilage equally in rabbits with osteoarthritis. Drug Discov Ther 5(4):190–194. [DOI] [PubMed] [Google Scholar]
- Chia SH, Schumacher BL, Klein TJ, Thonar EJ, Masuda K, Sah RL. 2004. Tissue‐engineered human nasal septal cartilage using the alginate‐recovered‐chondrocyte method. Laryngoscope 114(1):38–45. [DOI] [PubMed] [Google Scholar]
- Chung C, Burdick JA. 2008. Engineering cartilage tissue. Adv Drug Deliv Rev 60(2):243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino A, Stellavato A, Busico T, Papa A, Tirino V, Papaccio G, La Gatta A, De Rosa M, Schiraldi C. 2015. In vitro analysis of the effects on wound healing of high‐ and low‐molecular weight chains of hyaluronan and their hybrid H‐HA/L‐HA complexes. BMC Cell Biol 16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David‐Raoudi M, Deschrevel B, Leclercq S, Galéra P, Boumediene K, Pujol JP. 2009. Chondroitin sulfate increases hyaluronan production by human synoviocytes through differential regulation of hyaluronan synthases: Role of p38 and Akt. Arthritis Rheum 60(3):760–770. [DOI] [PubMed] [Google Scholar]
- Demoor M, Ollitrault D, Gomez‐Leduc T, Bouyoucef M, Hervieu M. 2014. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta 1840(8):2414–2440. [DOI] [PubMed] [Google Scholar]
- Do Amaral RJ, Pedrosa Cda S, Kochem MC, Silva KR, Aniceto M. 2012. Isolation of human nasoseptal chondrogenic cells: A promise for cartilage engineering. Stem Cell Res 8(2):292–299. [DOI] [PubMed] [Google Scholar]
- Du Souich P, García AG, Vergés J, Montell E. 2009. Immunomodulatory and anti‐inflammatory effects of chondroitin sulfate. J Cell Mol Med 13(8A):1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro M, Giuberti G, Zappavigna S, Perdonà S, Facchini G, Sperlongano P, Porto S, Di Lorenzo G, Buonerba C, Abbruzzese A, Altieri V, Caraglia M. 2012. Chondroitin sulphate enhances the antitumor activity of gemcitabine and mitomycin‐C in bladder cancer cells with different mechanisms. Oncol Rep 27(2):409–415. [DOI] [PubMed] [Google Scholar]
- Fuentes‐Boquete IM, Arufe Gonda MC, Díaz Prado SM, Hermida Gómez T, de Toro Santos FJ. 2008. Cell and tissue transplant strategies for joint lesions. Open Transplant J 2:21–28. [Google Scholar]
- Henrotin Y, Hauzeur JP, Bruel P, Appelboom T. 2012. Intra‐articular use of a medical device composed of hyaluronic acid and chondroitin sulfate (structovial CS): Effects on clinical, ultrasonographic and biological parameters. BMC Res Notes 5:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks DL, Sage AB, Schumacher BL, Sah RL, Watson D. 2005. Growth and phenotype of low‐density nasal septal chondrocyte monolayers. Otolaryngol Head Neck Surg 133(3):417–422. [DOI] [PubMed] [Google Scholar]
- Hochberg M, Chevalier X, Henrotin Y, Hunter DJ, Uebelhart D. 2013. Symptom and structure modification in osteoarthritis with pharmaceutical‐grade chondroitin sulfate: What's the evidence? Curr Med Res Opin 29(3):259–267. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. 2012. The rheumatologist's perspective. Osteoarthritis HSS J 8(1):35–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. 1999. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded incollagen gels. J Cell Physiol 179:142–148. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin CY. 2006. Effect of genipin‐crosslinked chitin‐chitosan scaffolds with hydroxyapatite modifications on the cultivation of bovine knee chondrocytes. Biotechnol Bioeng 95(1):132–144. [DOI] [PubMed] [Google Scholar]
- Kwan Tat S, Lajeunesse D, Pelletier JP, Martel‐Pelletier J. 2010. Targeting subchondral bone for treating osteoarthritis: What is the evidence? Best Pract Res Clin Rheumatol 24(1):51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypriotou M, Fossard‐Demoor M, Chadjichristos C, Ghayor C, de Crombrugghe B. 2003. SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol 22(2):119–129. [DOI] [PubMed] [Google Scholar]
- Lim EH, Sardinha JP, Myers S. 2014. Nanotechnology biomimetic cartilage regenerative scaffolds. Arch Plast Surg 41(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicev E, Kregar‐Velikonja N, Barlic A, Alibegović A, Drobnic M. 2009. Comparison of articular and auricular cartilage as a cell source for the autologous chondrocyte implantation. J Orthop Res 27(7):943–948. [DOI] [PubMed] [Google Scholar]
- Marlovits S, Hombauer M, Truppe M, Vècsei V, Schlegel W. 2004. Changes in the ratio of type‐I and type‐II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br 86(2):286–295. [DOI] [PubMed] [Google Scholar]
- Matmati M, Ng TF, Rosenzweig DH, Quinn TM. 2013. Protection of bovine chondrocyte phenotype by heat inactivation of allogeneic serum in monolayer expansion cultures. Ann Biomed Eng 41(5):894–903. [DOI] [PubMed] [Google Scholar]
- Munirah S, Samsudin OC, Aminuddin BS, Ruszymah BHI. 2010. Expansion of human articular chondrocytes and formation of tissue‐engineered cartilage: A step towards exploring a potential use of matrix‐induced cell therapy. Tissue Cell 42:282–229. [DOI] [PubMed] [Google Scholar]
- Oseni AO, Butler PE, Seifalian AM. 2013. Optimization of chondrocyte isolation and characterization for large‐scale cartilage tissue engineering. J Surg Res 1 181(1):41–48. [DOI] [PubMed] [Google Scholar]
- Plaas AH, West L, Midura RJ, Hascall VC. 2001. Disaccharide composition of hyaluronan and chondroitin/dermatan sulfate. Analysis with fluorophore‐assisted carbohydrate electrophoresis. Methods Mol Biol 171:117–128. [DOI] [PubMed] [Google Scholar]
- Rutgers M, Saris DB, Vonk LA, van Rijen MH, Akrum V. 2013. Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng A 19(1–2):59–65. [DOI] [PubMed] [Google Scholar]
- Saadeh PB, Brent B, Mehrara BJ, Steinbrech DS, Ting V. 1999. Human cartilage engineering: Chondrocyte extraction, proliferation, and characterization for construct development. Ann Plast Surg 42(5):509–513. [PubMed] [Google Scholar]
- Schiraldi C, Cimini D, De Rosa M. 2010. Production of chondroitin sulfate and chondroitin. Appl Microbiol Biotechnol 87(4):1209–1220. [DOI] [PubMed] [Google Scholar]
- Schiraldi C, Stellavato A, D'Agostino A, Tirino V, d'Aquino R. 2012. Fighting for territories: Time‐lapse analysis of dental pulp and dental follicle stem cells in co‐culture reveals specific migratory capabilities. Eur Cell Mater 23(24):426–440. [DOI] [PubMed] [Google Scholar]
- Shafiee A, Kabiri M, Ahmadbeigi N, Yazdani SO, Mojtahed M. 2011. Nasal septum‐derived multipotent progenitors: A potent source for stem cell‐based regenerative medicine. Stem Cells Dev 20(12):2077–2091. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Seifarth C, Thilo J, Rahmanzadeh M. 2006. Igf‐I extends the chondrogenic potential of human articular chondrocytes in vitro: Molecular association between Sox9 and Erk1/2§. Biochem Pharmacol 721:382–1395. [DOI] [PubMed] [Google Scholar]
- Sheu SY, Chen WS, Sun JS, Lin FH, Wu T. 2013. Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering. J Biomed Mater Res A 101(12):3457–3466. [DOI] [PubMed] [Google Scholar]
- Stokes DG, Liu G, Dharmavaram R, Hawkins D, Piera‐Velazquez S. 2001. Regulation of type‐II collagen gene expression during human chondrocyte de‐differentiation and recovery of chondrocyte‐specific phenotype in culture involves Sry‐type high‐mobility‐group box (SOX) transcription factors. Biochem J 360:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Material Fig. S1
Supplementary Material Fig. S2
Supplementary Material Fig. S3


