Figure 3.
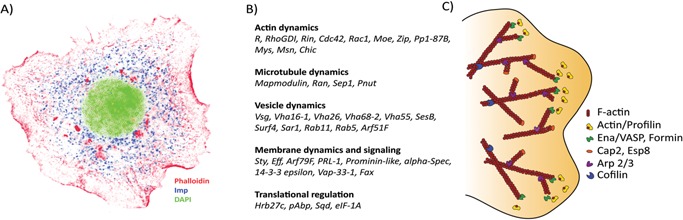
A: Drosophila Imp in cytoplasmic RNP granules. The picture shows a Drosophila S2 cell stained with DAPI (green), anti‐Imp antibody (blue) and phalloidin (red) (all in pseudo‐colors). Imp granules are about 100–200 nm large and located in the perinuclear region and close to the actin mesh. B: Transcripts associated with the Imp‐mediated RNA assemblage. Forty out of the 86 transcripts, identified by three different high‐throughput analyses, participate in growth cone dynamics 64. C: Key proteins involved in the formation and maintenance of F‐actin in lamellipodia. Several effector proteins are needed to nucleate, elongate, depolymerize, bundle and contract the actin filaments in order to reorganize the shape of the leading edge of a cell in response to external guidance cues. Profilin is responsible for the addition of actin monomers to the barbed end of growing filaments by interacting with the barbed‐end protector proteins, the formins and Ena/VASP 78. Formins and Ena/VASP also ensure continuous growth of the filament by inhibiting the binding of capping proteins, Cap2 and Esp8, otherwise capable of blocking the addition of actin monomers 79, 80. In lamellipodia, the mesh‐like form of F‐actin is accomplished by the dendritic nucleator proteins, the Arp2/3 complex, functioning as branch point holders, where they nucleate new filaments that branch off from pre‐existing ones 78. The recycling of actin subunits and actin remodelling is vital for movement of the cell. Cofilin in its unphosphorylated form binds to and twists the actin filament toward the pointed end, and the severing creates additional barbed ends, enhancing the turnover of actin subunits 81.
