Abstract
Objective
To examine the effect of maternal exposure to asthma medications on the risk of congenital anomalies.
Design
Meta‐analysis of aggregated data from three cohort studies.
Setting
Linkage between healthcare databases and EUROCAT congenital anomaly registries.
Population
519 242 pregnancies in Norway (2004–2010), Wales (2000–2010) and Funen, Denmark (2000–2010).
Methods
Exposure defined as having at least one prescription for asthma medications issued (Wales) or dispensed (Norway, Denmark) from 91 days before to 91 days after the pregnancy start date. Odds ratios (ORs) were estimated separately for each register and combined in meta‐analyses.
Main outcome measures
ORs for all congenital anomalies and specific congenital anomalies.
Results
Overall exposure prevalence was 3.76%. For exposure to asthma medication in general, the adjusted OR (adjOR) for a major congenital anomaly was 1.21 (99% CI 1.09–1.34) after adjustment for maternal age and socioeconomic position. The OR of anal atresia was significantly increased in pregnancies exposed to inhaled corticosteroids (3.40; 99% CI 1.15–10.04). For severe congenital heart defects, an increased OR (1.97; 1.12–3.49) was associated with exposure to combination treatment with inhaled corticosteroids and long‐acting beta‐2‐agonists. Associations with renal dysplasia were driven by exposure to short‐acting beta‐2‐agonists (2.37; 1.20–4.67).
Conclusion
The increased risk of congenital anomalies for women taking asthma medication is small with little confounding by maternal age or socioeconomic status. The study confirmed the association of inhaled corticosteroids with anal atresia found in earlier research and found potential new associations with combination treatment. The potential new associations should be interpreted with caution due to the large number of comparisons undertaken.
Tweetable abstract
This cohort study found a small increased risk of congenital anomalies for women taking asthma medication.
Keywords: Asthma medication, congenital anomalies, first trimester exposure, inhaled beta‐2 agonists, inhaled corticosteroids
Tweetable abstract
This cohort study found a small increased risk of congenital anomalies for women taking asthma medication.
Introduction
Use of medication in pregnancy may also expose the fetus to the medication. Exposure during the first trimester of pregnancy has the potential to increase the risk of congenital anomalies. Pregnant women with asthma often have to continue their medication during pregnancy.1 Asthma treatment today is mainly administered by inhalation therapy, which reduces the systemic effects of the medications. The treatment is based on a stepwise approach. Asthma is managed with inhaled short‐acting beta‐2‐agonists (SABA) for symptom relief, with the addition of inhaled corticosteroids and other medicines, preferably as inhalation medicine, when control becomes reduced.2 Previous studies have shown a slightly increased risk of congenital anomalies in infants born to women with asthma 3, 4 but it is still unclear whether the aetiology is the asthma disease (inflammation, hypoxia), the medications used for treatment or a combination.
A literature review found nine significant associations for first trimester exposure to beta‐2‐agonists with the following specific congenital anomalies: spina bifida, cleft palate, cleft lip with or without cleft palate, severe congenital heart disease (CHD), tetralogy of Fallot, oesophageal atresia, anal atresia, omphalocele and gastroschisis.5 For exposure to inhaled corticosteroids, five associations have been published: cleft palate, cleft lip with and without cleft palate, anal atresia, omphalocele and hypospadias. The EUROmediCAT case‐malformed control study including 13 European congenital anomaly registries with 1301 malformed registrations exposed to asthma medication, was able to confirm two of these previous findings: cleft palate and gastroschisis, both after exposure to inhaled beta‐2‐agonists.5 That study could not assess total congenital anomaly risk, as it did not include non‐malformed controls.
Asthma has been reported with increasing prevalence over the last decades,6 including an Increase in the prevalence of asthma in pregnancy.7 Therefore, more fetuses are now exposed to asthma medications during pregnancy.
The aim of this EUROmediCAT study is to evaluate the risk of all and specific congenital anomalies in women reported as exposed to asthma medications in the beginning of pregnancy using data from prescription databases linked to birth registries and EUROCAT congenital anomaly registries in three European regions and countries where such linkage was possible.
Material and methods
Study design
Odds ratios (ORs) for a major congenital anomaly and a number of specific congenital anomalies with exposure to asthma medications in the first trimester of pregnancy were estimated in each of three population‐based cohorts and the estimates were combined in meta‐analyses.
Setting
Three congenital anomaly registers in Norway, Wales and the Funen region of Denmark, all contributors to the European Network EUROCAT (European Surveillance of Congenital Anomalies), were linked to their source populations and healthcare databases. All three congenital anomaly registers are population‐based and collect data on all major congenital anomaly cases for all pregnancy outcomes [live births, fetal deaths from 20 weeks of gestation and terminations of pregnancy at any gestational age (GA) for fetal anomalies] in their respective populations.8 The EUROCAT registries are based on multiple data sources and detailed description of the registries and the common methodology has been published previously.9 Prescription data, also population‐based, were collected prospectively in all healthcare databases. The Danish and Norwegian prescription registries capture information on pharmacy redemption of prescriptions, and capture all dispensed prescriptions from all pharmacies. Only medicines given to inpatients during a hospital stay are missed. The Wales database captures prescriptions issued in primary care.10
For Norway, the Medical Birth Registry of Norway,11 containing all EUROCAT congenital anomaly cases, was linked to the Norwegian Prescription Database, which included prescriptions redeemed at a pharmacy since 2004,12 and to the National Education Database, which provides information on maternal educational level at the time of linkage. In Wales, existing routinely collected data from the all‐Wales health and social care linked electronic databank [the Secure Anonymised Information Linkage (SAIL)] were interrogated.13, 14 Data from the Office of National Statistics birth and deaths registry, the National Community Child Health Database (NCCHD), the Patient Episode Database for Wales, and primary care records were linked to the Congenital Anomaly Register and Information Service for Wales (CARIS). Primary care prescribing records were available from 91 days before pregnancy start date to delivery for 117 717/357 989 (33%) live‐ and stillbirths and for terminations of pregnancy for fetal anomalies. Socioeconomic position was captured as Townsend scores, ranks and quintiles.15
For Denmark, live‐ and stillbirths in the county of Funen recorded in the Danish Medical Birth Registry16 and TOPFA recorded in the National Patient Register were linked to the National Prescription Registry17 and to the Danish EUROCAT registry, covering congenital anomaly cases in Funen. Maternal education at time of pregnancy end was used as a measure of socioeconomic status.
Databases were linked using unique personal identifiers and anonymised. Ethical and data access approvals were obtained for each database from the relevant governance infrastructures.
Study population
The study population consisted of all liveborn infants, stillbirths/late fetal deaths after 20 completed weeks of gestation in the three populations and terminations of pregnancies at any GA due to fetal anomaly (TOPFA) recorded in the EUROCAT registry, with delivery date or date of termination between 1 January 2000 and 31 December 2010. As the Norwegian Prescription Database was established in 1 January 2004, Norway was only able to contribute data for pregnancies where the date of last menstrual period was 1 April 2004 or later. For Wales, fetal deaths were only included from 24 completed weeks of pregnancy.
Pregnancy start date was computed primarily based on ultrasound estimation of GA using information on date of last menstrual period where this was missing. If both were missing (0.34% in Denmark, 2.9% in Wales), Denmark and Wales used median GA for their registries for live births, fetal deaths and terminations, respectively, and Norway discarded the observation (0.56%).
Exposure
Exposure was defined as the mother having redeemed (Denmark and Norway) or been issued (Wales) at least one prescription for an asthma medication (ATC code R03) in the time period from 91 days before to 91 days after pregnancy start date. Exposure was grouped based on 5‐digit ATC codes into six asthma medication classes: all asthma medications (ATC‐code R03), inhaled beta‐2‐agonists (R03AC), beta‐2‐agonists for systemic use (R03CC), inhaled glucocorticoids (R03BA), combined long‐acting beta‐2‐agonists and inhaled steroids [R03AK or a prescription of both inhaled steroids and a long acting beta‐2‐agonists (R03AC12‐R03AC13)] and leukotriene receptor antagonists (R03DC). Inhaled beta‐2‐agonists were further divided into two groups: short‐acting beta‐2‐agonists (SABA) and long‐acting beta‐2‐agonists (LABA) (ATC codes R03AC02‐R03AC04 and R03AC12‐R03AC13, respectively). Exposure to systemic glucocorticoids in combination with an asthma medication was analysed as a separate outcome, presumed to be an indicator of high asthma severity.
Infants were excluded if information on maternal prescriptions was missing or incomplete, or if the mother had a prescription for an antiepileptic or antidiabetic medications between 91 days before and 91 days after pregnancy start date. These exclusions were applied to both groups (exposed and non‐exposed to asthma medications).
After review of the results, a sensitivity analysis was performed with a more strict definition of exposure. Here exposure was defined as a prescription for an asthma medication (ATC code R03) in the time period from 91 days before to 91 days after pregnancy start date and at least two prescriptions for the same medication at two different time periods from 1 year before pregnancy to the end of pregnancy.
Outcomes
The outcome was specific major congenital anomalies recorded in the EUROCAT registry for each region, classified according to EUROCAT standard subgroups.9 Infants and fetuses with diagnosed chromosomal, genetic or teratogenic anomalies or with skeletal dysplasias were excluded (known aetiology).
Statistical analyses
As none of the registries was able to send data on individuals out of the country, analyses were done on aggregated data. Odds ratios for each registry were computed and combined using Mantel–Haenszel methods with variable continuity corrections for empty cells proportional to size of case group relative to control group and summing to 1.18 Confidence intervals for combined estimates were calculated using Cornfield approximation. The I 2‐statistic, describing the percentage of variation explained by heterogeneity, was computed for the meta‐analyses.
For outcomes and exposures with at least 15 exposed cases, logistic regression was used to adjust for maternal age and socioeconomic position, again running analyses in each registry separately and combining estimates in a meta‐analysis using a random effects model.
Odds ratio (OR) of exposure to asthma medications in general, or to each of seven asthma medication classes, was calculated for each of 62 EUROCAT congenital anomaly subgroups. Only associations with at least three exposed cases are reported. Due to the large number of tests, 99% confidence intervals are shown. As the outcomes are rare, ORs are close approximations to relative risks.
The software used for combining estimates was STATA13. Plots were created in R and modified in Inkscape.
Results
Of the 519 242 infants and fetuses included in the study, 19 513 mothers had a prescription for asthma medication within the defined exposure period from 91 days before to 91 days after the pregnancy start date, giving an overall exposure prevalence at 3.76%. Exposure prevalence by region was 3.66% in Denmark, 2.85% in Norway and 6.51% in Wales (Table 1). Inhaled beta‐2‐agonists were the most frequently prescribed asthma medications (2.93% exposed), 1.16% were exposed to inhaled steroids and 1.19% to combination treatments (inhaled steroids and LABA). Socio‐demographic data by region are presented in Table S1.
Table 1.
Use of asthma medications in three populations of pregnant women (Norway 2004–2010, Wales and Funen, Denmark 2000–2010)
| Norway | Wales | Funen, Denmark | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of infants and fetuses | 346 808 | 115 988 | 56 446 | ||||||||||
| ATC codes | Exposed | Exposed to only this asthma medication | Exposed | Exposed to only this asthma medication | Exposed | Exposed to only this asthma medication | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Exposed to any asthma medications | R03 | 9898 | 2.85 | 7544 | 6.51 | 2068 | 3.66 | ||||||
| Inhaled beta‐2‐agonists | R03AC | 6939 | 2.00 | 3865 | 1.11 | 6862 | 5.92 | 3073 | 2.65 | 1423 | 2.52 | 750 | 1.33 |
| Short‐acting beta‐2‐agonists (SABA) | R03AC02‐R03AC07 | 6488 | 1.87 | 3600 | 1.04 | 6789 | 5.86 | 3037 | 2.62 | 1294 | 2.29 | 694 | 1.23 |
| Long‐acting beta‐2‐agonists (LABA) | R03AC12‐R03AC13 | 655 | 0.19 | 265 | 0.08 | 431 | 0.37 | 36 | 0.03 | 200 | 0.35 | 57 | 0.10 |
| Inhaled corticosteroids | R03BA | 1908 | 0.55 | 552 | 0.16 | 3265 | 2.82 | 396 | 0.34 | 851 | 1.51 | 279 | 0.49 |
| Systemic beta‐2‐agonists | R03CC | 119 | 0.03 | 104 | 0.03 | 31 | 0.03 | 25 | 0.02 | 140 | 0.25 | 6 | 0.01 |
| Combinations LABA and inhaled corticosteroids | R03AK or both LABA and R03BA | 4178 | 1.20 | 2197 | 0.63 | 1558 | 1.34 | 255 | 0.22 | 480 | 0.85 | 280 | 0.50 |
| Leukotriene‐receptor antagonists | R03DC | 581 | 0.17 | 112 | 0.03 | 137 | 0.12 | 15 | 0.01 | 43 | 0.08 | 6 | 0.01 |
| Systemic corticosteroidsa | H02ABa | 539 | 0.16 | 504 | 0.43 | 78 | 0.14 | ||||||
Only in combination with other asthma drugs.
Among the exposed pregnancies, 650 fetuses and infants were registered with a major congenital anomaly. Exposure to any asthma medication (ATC code R03) compared with no exposure was associated with an increased risk of any congenital anomaly: adjusted OR (adjOR) = 1.21 (99% CI 1.09–1.34). Table 2 presents ORs and adjORs for a major congenital anomaly as one group after exposure to different types of asthma medications.
Table 2.
Association of major congenital anomaly with prescription of different types of asthma medications (OR and adjusted OR), Norway, Wales and Funen, Denmark, and combined
| Medication | Exposed cases | Meta‐analysis OR (99% CI) | Norway OR (99% CI) | Wales OR (99% CI) | Denmark OR (99% CI) | I2 | Meta‐analysis Adj OR (99% CI)a | I 2 | |
|---|---|---|---|---|---|---|---|---|---|
| All anomalies | Asthma medications | 650 | 1.22 (1.10–1.35) | 1.23 (1.06–1.44) | 1.22 (1.04–1.43) | 1.13 (0.79–1.63) | 0 | 1.21 (1.09–1.34) | 0 |
| All anomalies | Inhaled beta‐2‐agonists | 512 | 1.21 (1.08–1.36) | 1.22 (1.02–1.46) | 1.21 (1.02–1.43) | 1.18 (0.77–1.81) | 0 | 1.19 (1.06–1.34) | 0 |
| All anomalies | Short‐acting beta‐2‐agonists | 492 | 1.21 (1.07–1.37) | 1.21 (1.01–1.46) | 1.22 (1.03–1.45) | 1.12 (0.71–1.77) | 0 | 1.19 (1.06–1.35) | 0 |
| All anomalies | Long‐acting beta‐2‐agonists | 42 | 1.20 (0.80–1.80) | 1.24 (0.71–2.19) | 1.03 (0.52–2.05) | 1.56 (0.6–4.05) | 0 | 1.22 (0.82–1.82) | 0 |
| All anomalies | Inhaled corticosteroids | 202 | 1.19 (0.98–1.43) | 1.12 (0.78–1.59) | 1.26 (1.00–1.6) | 0.98 (0.54–1.77) | 0 | 1.20 (1.00–1.44) | 0 |
| All anomalies | Combination treatments | 202 | 1.21 (1.00–1.46) | 1.16 (0.92–1.47) | 1.19 (0.84–1.68) | 1.78 (0.98–3.23) | 30 | 1.21 (1.00–1.45) | 37 |
| All anomalies | Systemic corticosteroids | 47 | 1.51 (1.03–2.22) | 1.60 (0.92–2.79) | 1.40 (0.8–2.45) | 1.71 (0.00–6.96) | 0 | 1.48 (1.01–2.17) | 0 |
Adjusted for period, maternal age, socio‐economic status.
Figure 1 shows meta‐analysis ORs and 99% confidence intervals for each of the medication groups and each of the anomaly subgroups. Table 3 shows combined OR estimates and the I 2‐statistic (the percentage of the variation explained by heterogeneity) for all statistically significant associations. For the associations with high heterogeneity, the explanation is partly sparse data and partly actual heterogeneity; all associations with I 2 > 50 had very high OR in Denmark based on very few cases. Among these, the significant associations with limb reduction were not present in Norway (apart from for exposure to systemic steroids). There were no associations with syndactyly in the Norwegian data. and no significant association in the Welsh data. There were no associations with club foot and aortic valve atresia/stenosis in the Welsh data (data not shown). For severe CHD, common AV canal and aortic valve atresia/stenosis (both included in severe CHD subgroup), the increased odds seems to be associated with exposure to the combination of inhaled steroids and long‐acting beta‐2‐agonists. For limb reduction defects the odds are significantly increased after exposure to beta‐2‐agonists, but with no increased odds for inhaled steroids. The associations with renal dysplasia seem driven by exposure to short‐acting beta‐2‐agonists (2.37; 99% CI 1.20–4.67). There were no significant associations after exposure to long‐acting beta‐2‐agonists, but the total number of exposed fetuses was small.
Figure 1.
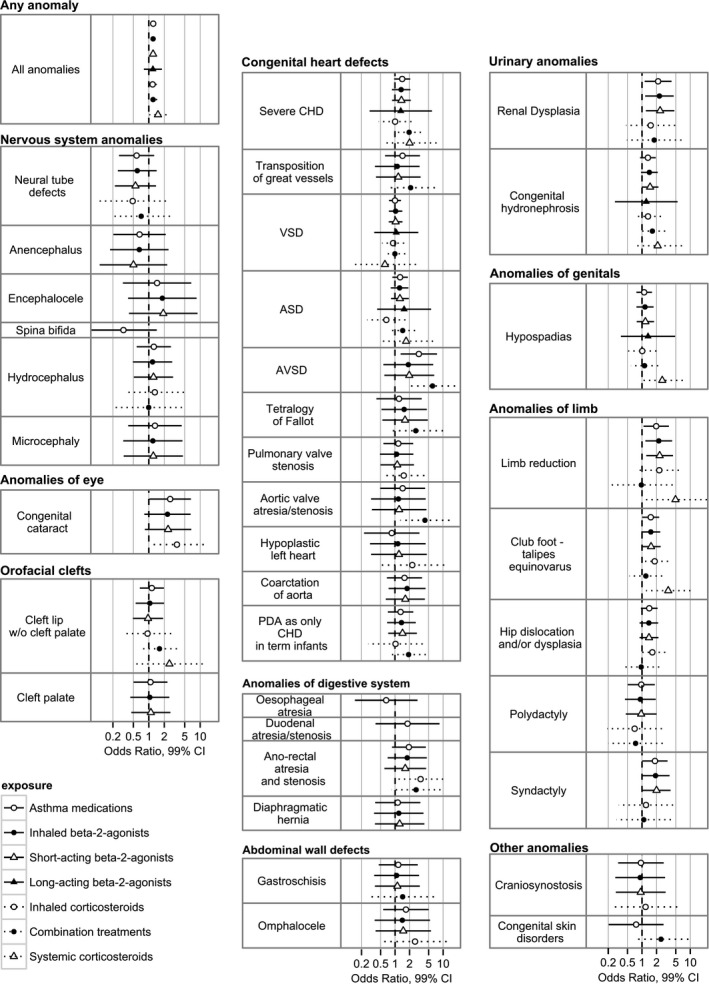
Associations between use of the seven asthma medication groups and each anomaly subgroup with at least three exposed cases: Results from Norway, Wales and Funen, Denmark combined.
Table 3.
Meta‐analysis odds ratios for associations between congenital anomaly subgroups and prescription of asthma medications, showing associations significant at the 1% level. Adjusted odds ratios given for anomalies with at least 15 exposed cases
| Anomaly | Medication | Exposed cases | Meta‐analysis ORa (99% CI) | I 2 |
|---|---|---|---|---|
| Congenital cataract | Any asthma medications | 9 | 2.61 (1.03–6.61) | NA |
| Inhaled corticosteroids | 5 | 3.53 (1.05–11.81) | 0 | |
| Severe CHD | Combination treatments | 21 | 1.97 (1.12–3.49) | 36 |
| Aortic valve atresia/stenosis | Combination treatments | 5 | 4.21 (1.29–13.7) | 67 |
| AVSD | Any asthma medications | 10 | 3.15 (1.32–7.51) | 0 |
| Combination treatments | 6 | 6.05 (2.04–17.97) | 30 | |
| Anal atresia/stenosisb | Inhaled corticosteroids | 6 | 3.40 (1.15–10.04) | 0 |
| Renal dysplasia | Any asthma medications | 18 | 2.16 (1.14–4.09) | 0 |
| Inhaled beta‐2‐agonists | 16 | 2.30 (1.17–4.52) | 0 | |
| Short‐acting beta‐2‐agonists | 16 | 2.37 (1.20–4.67) | 0 | |
| Limb reduction | Any asthma medications | 20 | 1.97 (1.08–3.6) | 56 |
| Inhaled beta‐2‐agonists | 18 | 2.22 (1.18–4.20) | 74 | |
| Short‐acting beta‐2‐agonists | 18 | 2.32 (1.23–4.38) | 76 | |
| Systemic corticosteroids | 3 | 4.93 (1.10–22.14) | 0 | |
| Club foot | Any asthma medications | 44 | 1.52 (1.01–2.27) | 0 |
| Systemic corticosteroids | 6 | 3.49 (1.21–10.06) | 71 | |
| Syndactyly | Any asthma medications | 20 | 1.85 (1.02–3.38) | 59 |
| Short‐acting beta‐2‐agonists | 16 | 2.00 (1.02–3.90) | 78 |
Adjusted for maternal age and socio‐economic position.
Found in previous literature (Lin et al.20).
The association of anal atresia/stenosis with exposure to inhaled steroids was significant at the 1% level (OR 3.4; 99% CI 1.15–10.04). There was no further significant associations found in the literature at 5% level for anomaly‐exposure signals.5
Despite significant associations between use of inhaled beta‐2‐agonists and cleft palate and gastroschisis in a previous analysis in an overlapping study population with different exposure data source,5 we found no significant associations here: the odds ratio was 1.05 (99% CI 0.44–2.51, n = 9) for cleft palate and 1.08 (99% CI 0.37–3.15, n = 6) for gastroschisis.
Sensitivity analysis with a more strict exposure definition reduced total exposure by approximately 50–80%. For cataract and aortic valve atresia/stenosis, there were too few exposed cases to analyse. For club foot ORs were still increased (1.57), but the statistical significance disappeared. For the associations common AV canal and combination treatment, anal atresia and inhaled steroids and renal dysplasia and SABA, results were still significant and ORs were higher. For the association severe CHD and combination treatment, the OR was increased, but not significantly (OR 2.63, 99% CI 0.98–7.05) (Table S2).
Discussion
Main findings
Our cohort study found that exposure to different types of asthma medications was associated with similar increased risks of a major congenital anomaly (OR close to 1.2 for all medication types), but only few significant associations for specific groups of congenital anomalies. This may be explained by the maternal asthma being causal, rather than the fetal exposure to asthma medications being causal. We found very small differences between OR and adjusted OR for all major congenital anomalies, showing that there is little if any confounding by maternal age or socioeconomic position.
Strengths and limitations
The strength of this study is the population‐based design with congenital anomaly registries linked to prescription databases. Furthermore, the medication data were collected prospectively by the databases and therefore maternal recall bias is not a problem. It is a limitation that we do not know whether the pregnant women took the prescribed medications. Inhaled beta‐2‐agonists may not be taken regularly and could be prescribed for occasional use. For this reason, the inhaler may also have been prescribed outside the defined exposure period, so that women who actually did use their inhalers during the first trimester were misclassified. Even with a cohort of 519 242, the number of exposed cases for specific anomalies was often very small, leading to large uncertainty of the estimates, particularly as data could not be pooled and a meta‐analysis approach had to be applied. Bias is unlikely to occur due to the different GA at inclusion in Wales (24 instead of 20 weeks), as the likelihood of being born and diagnosed with a malformation is not higher at GA 20–23 weeks than at 24 weeks and the proportion of missed cases and controls is very small (0.15%). As several asthma medications are often used together, it is hard to disentangle the possible effects of the individual asthma drugs. Adjustment for socioeconomic status was crude due to the limited availability of information and the small number of cases in some anomaly subgroups, and may not have been sufficient to account for the complex effect of socioeconomic position on risk of congenital anomaly. The study is not able to distinguish between the potential teratogenic effect of the maternal asthma and the potential teratogenic effects of asthma medications. As asthma has been linked to environmental toxins, the link to the congenital anomalies could be the toxins and not the disease or the medication. The large number of comparisons made means that significant associations must be interpreted with caution, particularly where not confirmed by other studies.
Interpretation
Two other cohort studies have found almost the same increased odds of congenital anomaly in general as in our study. A study in Canada, including 13 280 pregnancies with at least one diagnosis of asthma and one prescription for asthma medications, found an adjOR of 1.34 (95% CI 1.22–1.47) for any major congenital anomaly.19 A study from the Swedish Medical Birth registry included women who reported use of anti‐asthmatic medication in the first trimester of pregnancy.4 Here adjOR for any major congenital anomaly was 1.09 (95% CI 1.03–1.12). Both studies included live‐ and stillbirths, but not TOPFA, as in our study.
Our study showed an association of anal atresia and inhaled corticosteroids (OR 3.4, 99% CI 1.15–10.04). A case–control study found an increased odds of isolated anal atresia with an OR of 2.12 (95% CI 1.09–4.12) for exposure to anti‐inflammatory asthma medications including inhaled and systemic corticosteroids and adjusted for other asthma medications.20 A cohort study from Sweden in 1995–2004 found an OR of 1.69 (95% CI 1.11–2.56) after exposure to any asthma medication,21 but in the next publication from the same population for the years 1996–2011 the OR for anal atresia was reduced (OR 1.21, 95% CI 0.78–1.87).4 There is limited information in the literature about the risk of specific congenital anomalies after exposure to inhaled corticosteroids. An increased risk for hypospadias has been described (OR 1.8, 95% CI 1.1–3.0) after exposure to inhaled and nasal corticosteroids.22 The EUROmediCAT case‐malformed control study did not find increased odds for any specific congenital anomaly after exposure to inhaled corticosteroids,5 although the odds ratios of exposure to inhaled corticosteroids for anal atresia and adjusted for exposure to other asthma medications was non‐significantly increased (OR 1.49, 95% CI 0.74–3.02).
There are few studies on the risk of congenital anomalies after exposure to LABA and these have a limited number of exposed cases. A Canadian study found an increased odds of CHD based on seven exposed cases (OR 2.38, 95% CI 1.11–5.10).23 Our study found an increased odds of severe CHD after exposure to the combined treatment with LABA and inhaled steroids, and also a significantly increased odds for aortic valve atresia/stenosis and common AV canal separately. These associations may be caused by the medication exposure, but they may also be explained by the asthma severity with the need for daily use of long‐acting beta‐2‐agonists. The EUROmediCAT case‐malformed control study found an association between renal dysplasia and the combined treatment.5 We are not aware of other published studies evaluating the risk of the combined treatment with LABA and inhaled steroids and congenital anomalies.
The associations between SABA and renal dysplasia, limb reduction defects and syndactyly have not been described previously. The ORs were from 2.00 to 2.37, but decreased and statistical significance disappeared when we restricted exposure to at least two prescriptions, where we could be more confident that exposure had indeed occurred to these intermittently taken medications. However, these findings need future attention and possible confirmation in other populations.
Significant results from other studies include exposure to beta‐2‐agonists and increased odds of cleft palate, cleft lip and palate, esophageal atresia, omphalocele and gastroschisis.20, 24, 25 None of these beta‐2‐agonist exposure–congenital anomaly combinations was significant in our study at the 5% level (no confirmation of previous literature signals). The EUROmediCAT case‐malformed control study confirmed two of these signals (cleft palate and gastroschisis) using a different data source for exposure (medical records) and a different methodology comparing the signal anomaly cases with malformed controls.26
The association of congenital cataract and exposure to inhaled steroids was based on five cases from Wales with no exposed cases in Norway and Funen, Denmark. The finding is of interest as cataract has been described as a rare adverse effect of inhaled steroids in children and adults.27 The prevalence of cataract in the EUROCAT registry in Wales is higher than in the two other registries, probably explained by better ascertainment of congenital eye anomalies in general.
Exposure to systemic steroids may be a marker of disease severity. For the all‐anomaly group, the association with systemic steroids was associated with higher OR than for exposure to inhaled medications (OR 1.51, 99% CI 1.03–2.22). This may also reflect greater bioavailability. However, the low number of pregnancies exposed to systemic steroid limited our possibilities for finding associations with specific congenital anomalies. Furthermore, systemic steroids may have been taken during hospital admissions and these exposures are not covered by the prescription databases.
The higher exposure rate to asthma medications in Wales may be explained in several ways. The Wales database captured prescriptions issued to the woman, whereas the databases in Norway and Denmark captured prescriptions dispensed at the pharmacy. To the extent that this explains the higher exposure rate, it will also cause heterogeneity in the estimates, as exposure misclassification is higher in Wales. We note, however, that for the significant associations with high heterogeneity, the heterogeneity did not generally take the form of a lower OR in Wales. Another explanation for a higher prevalence of asthma in Wales is the socio‐demographic differences described in Table S1 (maternal age, smoking, body mass index, socio‐economic position). The differences in asthma prevalence and prescribing of asthma medication during pregnancy between Wales and Scandinavia has been described in other studies.28
Conclusion
Most of the specific congenital anomalies have prevalence rates between 1 in 1000 and 1 in 10 000 births. Even with an OR after exposure to beta‐2‐agonists of between 2 or 3, the risk for the individual pregnant woman is low. Maternal asthma exacerbation during the first trimester of pregnancy has been reported to be associated with an increased risk of congenital anomalies29 and exacerbation during pregnancy may be associated with other unfavourable pregnancy outcomes such as preterm birth and pre‐eclampsia.30 The general consensus is that uncontrolled asthma increases perinatal risks, whereas well‐controlled asthma reduces these risks.3, 31 Therefore, pregnant women should continue to use the prescribed inhaled corticosteroids, where we find little evidence of teratogenic risk, for optimal asthma control and to reduce the need for beta‐2‐agonists and systemic corticosteroids, which may be associated with greater teratogenic risk.
Funding
Financial support for this study was provided by the European Union under the Seventh Framework Programme (grant agreement HEALTH‐F5‐2011‐260598). The funding source had no involvement in the study.
Disclosure of interests
Full disclosure of interests available to view online as supporting information.
Contribution to authorship
HD and EG contributed to the design of the work. All authors attended meetings to discuss details in the design of the study and later for discussion of results and conclusions. Data acquisition and analysis were carried out by KK and AE (Norway), SJ, DST, GD and DT (Wales), and AH, AA and EG (Funen, Denmark). AH and JM carried out the meta‐analysis. EG wrote the first draft of the paper. All authors were involved in the next versions and approved the final version of the paper before submission.
Details of ethics approval
Wales: The EUROmediCAT project received approval from the SAIL/HIRU Information Governance Review Panel (IGFRP) on 24 March 2011. Norway: The EUROmediCAT project received approval from the Norwegian Data Inspectorate on 12 February 2013 (12/00617‐4/EOL), and from the Ethical Committee for Research on 5 June 2012 and 7 July 2015 (2012/757/REK nord). Funen, Denmark: Linkage of databases for the EUROmediCAT project was approved by the Danish Data Inspection Agency on 27 May 2011 (2011‐231‐0098).
Supporting information
Table S1. Socio‐demographic characteristics within the three registry areas.
Table S2. Comparison of results with different exposure definitions (at least one prescription as described in Tables 2 and 3, or more than one prescription).
Acknowledgements
This study used anonymised data held in the Secure Anonymised Information Linkage (SAIL) system, which is part of the national e‐health record research infrastructure for Wales. We would like to acknowledge all the data providers who make anonymised data available for research.
Garne E, Vinkel Hansen A, Morris J, Jordan S, Klungsøyr K, Engeland A, Tucker D, Thayer DS, Davies GI, Nybo Andersen A‐M, Dolk H. Risk of congenital anomalies after exposure to asthma medication in the first trimester of pregnancy – a cohort linkage study. BJOG 2016;123: 1609–1618.
Linked article This article is commented on by CV Ananth and AM Friedman, p. 1619 in this issue. To view this mini commentary visit http://dx.doi.org/10.1111/1471-0528.14130.
References
- 1. Charlton RA, Hutchison A, Davis KJ, de Vries CS. Asthma management in pregnancy. PLoS One 2013;8:e60247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. British Thoracic Society/Scottish Intercollegiate Guidelines Network . British guidelines on asthma management: a national clinical guideline. Thorax 2008;63(Suppl. 4):12147–58. [Google Scholar]
- 3. Murphy VE, Wang G, Namazy JA, Powell H, Gibson PG, Chambers C, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalization among pregnant women with asthma: a systematic review and meta‐analysis. BJOG 2014;121:812–22. [DOI] [PubMed] [Google Scholar]
- 4. Källen B. Maternal asthma and use of antiasthmatic drugs in early pregnancy and congenital malformations in the offspring. J Pulm Respir Med 2014;4:1. [Google Scholar]
- 5. Garne E, Hansen AV, Morris J, Zaupper L, Addor M‐C, Barisic I, et al. Use of asthma medication during pregnancy and risk of specific congenital anomalies—a European case‐malformed control study. J Allergy Clin Immunol 2015;136:1496–502. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Morrison‐Carpenter T, Holt JB, Callahan DB. Trends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000–2009. BMC Public Health 2013;13:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy VE, Gibson PG. Asthma in pregnancy. Clin Chest Med 2011;32:93–110. [DOI] [PubMed] [Google Scholar]
- 8. Greenlees R, Neville A, Addor M‐C, Amar E, Arriola L, Bakker M, et al. EUROCAT member registries: organization and activitites. Birth Defects Res A Clin Mol Teratol (Part A) 2011;91:S51–100. [DOI] [PubMed] [Google Scholar]
- 9. Boyd P, Haussler M, Barisic I, Loane M, Garne E, Dolk H. Paper 1: The EUROCAT Network – organisation and processes. Birth Defects Res A Clin Mol Teratol (Part A) 2011;91:S2–15. [DOI] [PubMed] [Google Scholar]
- 10. Charlton R, Neville A, Jordan S, Pierini A, Damase‐Michel C, Klungsøyr K, et al. Healthcare databases in Europe for studying medicine use and safety during pregnancy. Pharmacoepidemiol Drug Saf 2014;23:506–94. [DOI] [PubMed] [Google Scholar]
- 11. Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 2000;79:435–9. [PubMed] [Google Scholar]
- 12. Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol 2010;106:86–94. [DOI] [PubMed] [Google Scholar]
- 13. Ford DV, Jons KH, Verplancke JP, Lyons RA, John G, Brown G, et al. The SAIL Databank: building a national architecture for e‐health research and evaluation. BMC Health Serv Res 2009;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyons R, Jones K, John G, Brooks CJ, Verplancke JP, Ford DV, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak 2009;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. London: Routledge, 1988. [Google Scholar]
- 16. Thygesen LC, Daasnes C, Thaulow I, Brønnum‐Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 2011;39:12–6. [DOI] [PubMed] [Google Scholar]
- 17. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 18. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med 2004;23:1351–75. [DOI] [PubMed] [Google Scholar]
- 19. Blais L, Kettani F‐Z, Elftouh N, Forget A. Effect of maternal asthma on the risk of specific congenital malformations: a population‐based cohort study. Birth Defects Res (Part A) 2010;88:216–22. [DOI] [PubMed] [Google Scholar]
- 20. Lin S, Munsie JPW, Herdt‐Losavio ML, Druschel CM, Campbell K, Browne ML, et al. Maternal asthma medication use and the risk of selected birth defects. Pediatrics 2012;129:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Källen B, Oalusson PO. Use of anti‐asthmatic drugs during pregnancy. 3. Congenital malformations in the fetus. Eur J Clin Pharmacol 2007;63:383–8. [DOI] [PubMed] [Google Scholar]
- 22. Carmichael SL, Ma C, Werler MM, Olney RS, Shaw GM. Maternal corticosteroid use and hypospadias. J Pediatr 2009;155:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eltonsy S, Forget A, Blais L. Beta‐2‐agonists use during pregnancy and the risk of congenital malformations. Birth Defects Res Part A Clin Mol Teratol 2011;91:937–47. [DOI] [PubMed] [Google Scholar]
- 24. Lin S, Munsie JP, Herft‐Losavio ML, Druschel C, Romitti PA, Olney R, et al. Maternal asthma medication and the risk of gastroschisis. Am J Med Genet 2008;168:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munsie JPW, Lin S, Browne ML, Browne ML, Campbell KA, Caton AR, et al. Maternal bronchodilator use and the risk of orofacial clefts. Human Reprod 2011;26:3147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hook EB. What kind of controls to use in case‐control studies of malformed infants: recall bias versus ‘teratogen nonspecificity’ bias. Teratology 2000;61:325–6. [DOI] [PubMed] [Google Scholar]
- 27. Weatherall M, Clay J, James K, Perrin K, Shirtcliffe P, Beasley R. Dose‐response relationship of inhaled corticosteroids and cataracts: a systematic review and meta‐analysis. Respirology 2009;14:983–90. [DOI] [PubMed] [Google Scholar]
- 28. Blais L, Forget A. Asthma exacerbations during first trimester of pregnancy and the risk of congenital malformations among asthmatic women. J Allergy Clin Immunol 2008;121:1379–84. [DOI] [PubMed] [Google Scholar]
- 29. Vatti RR, Teuber SS. Asthma and pregnancy. Clin Rev Allergy Immunol 2012;43:45–56. [DOI] [PubMed] [Google Scholar]
- 30. Rocklin RE. Asthma, asthma medications and their effect on maternal/fetal outcomes during pregnancy. Reprod Toxicol 2011;32:89–97. [DOI] [PubMed] [Google Scholar]
- 31. Charlton R, Perini A, Klungsøyr K, Neville A, Jordan S, de Jong‐van den Berg LTW, et al. Asthma medication prescribing before, during and after pregnancy: a study in seven European regions. BMJ Open 2016;6:e009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Socio‐demographic characteristics within the three registry areas.
Table S2. Comparison of results with different exposure definitions (at least one prescription as described in Tables 2 and 3, or more than one prescription).


