Abstract
Objective
Cognitive control as well as stress reactivity is assumed to depend on prefrontal dopamine and decline with age. Because Ginkgo biloba extract EGb761® increases prefrontal dopamine in animals, we assessed its effects on cognitive functions related to prefrontal dopamine.
Methods
Effects of 240‐mg EGb761® daily on task‐set‐switching, response‐inhibition, delayed response, prospective‐memory, task‐related fMRI‐BOLD‐signals and the Trier Social Stress‐Test were explored in a randomized, placebo‐controlled, double‐blind pilot‐trial in 61 elderly volunteers with subjective memory impairment.†
Results
Baseline‐fMRI‐data showed BOLD‐responses in regions commonly activated by the specific tasks. Task‐switch‐costs decreased with EGb761® compared to placebo (ANOVA‐interaction: Group × Time × Switch‐Costs p = 0.018, multiple tests uncorrected), indicating improved cognitive flexibility. Go–NoGo‐task reaction‐times corrected for error‐rates indicated a trend for improved response inhibition. No treatment effects were found for the delayed response and prospective‐memory tasks and fMRI‐data. A non‐significant trend indicated a potentially accelerated endocrine stress‐recovery. EGb761® was safe and well tolerated.
Conclusion
We observed indications for improved cognitive flexibility without changes in brain activation, suggesting increased processing efficiency with EGb761®. Together with a trend for improved response inhibition results are compatible with mild enhancement of prefrontal dopamine. These conclusions on potential beneficial effect of EGb761® on prefrontal dopaminergic functions should be confirmed by direct measurements. © 2016 The Authors. Human Psychopharmacology: Clinical and Experimental published by John Wiley & Sons, Ltd.
Keywords: cognitive control, dopamine, fMRI, EGb 761, ginkgo, task switch
Introduction
Cognitive control functions, i.e. cognitive flexibility, goal maintenance, inhibition of habitual or impulsive responses or prospective memory, decline in older age (Cabeza and Dennis, 2013). The prefrontal cortex (PFC) plays a central role in cognitive control functions (Miller and Cohen, 2001; Banich, 2009; Munakata et al., 2011; Ruge et al., 2013; Sreenivasan et al., 2014). Pharmacological trials, genetic imaging studies and studies of neurodegenerative diseases affecting dopaminergic pathways indicate that dopamine in the PFC modulates cognitive control and thereby influences attention, impulse inhibition, prospective memory and cognitive flexibility (cf. Müller et al., 2007a; Cools, 2008; Costa et al., 2008; Durstewitz and Seamans, 2008; Robbins and Arnsten, 2009; van Schouwenburg et al., 2010; Arnsten et al., 2012; Floresco, 2013; Goschke and Bolte, 2014). Reduced prefrontal dopamine has been associated with impaired cognitive control (Cools and D'Esposito, 2011; Floresco, 2013). Because prefrontal dopaminergic innervation and associated cognitive functions decrease with advancing age (Cabeza and Dennis, 2013), interventions that improve prefrontal dopaminergic functions in the elderly are of interest.
In Europe, Ginkgo biloba leaf extract EGb 761® is a registered drug for the treatment of age‐related cognitive decline including memory and concentration problems. Clinical efficacy in cognitive decline and dementia has been confirmed by a series of randomized, double‐blind, placebo‐controlled clinical trials (Janssen et al., 2010; Weinmann et al., 2010; Gauthier and Schlaefke, 2014; Tan et al., 2015). Improved microcirculation, enhanced neuroplasticity and support of mitochondrial energy production have been discussed as underlying mechanisms of action (Spieß et al., 2014). However, these suggested modes of action are mainly based on animal and in‐vitro‐data and have not been verified in man.
Neuropsychological analysis of published placebo‐controlled data revealed that chronic administration improves not only memory, but also selective attention and some executive functions (Kaschel, 2009). These effects suggest that the compound might improve prefrontal dopaminergic function. Indeed, in animal models EGb 761® increases extracellular dopamine specifically in the PFC (Su et al., 2009; Yoshitake et al., 2010). This effect is probably based on mild inhibition of the norepinephrine transporter, the protein mediating synaptic re‐uptake of most dopamine in the PFC (Fehske et al., 2009; Heal et al., 2013).
Therefore, we were interested whether the clinical benefits of EGb 761® might partly be based on improved prefrontal dopaminergic functions. For this purpose we assessed effects of EGb 761® in elderly non‐demented volunteers with subjective memory impairment on a wider range of cognitive control functions that have been reported to be modulated by the prefrontal dopaminergic system: cognitive set‐switching; maintenance of task‐relevant information in the face of interfering stimuli; response inhibition and prospective memory.
Prior neuroimaging studies of normal aging have shown that behavioral performance can be preserved in the elderly while brain activation during cognitive tasks is enhanced (cf. Cabeza, 2001; Han et al., 2007; for review see Han et al., 2009). Such enhanced brain activation can be interpreted as compensatory effort to reach a performance target, and can already be perceived by the subject as mild cognitive impairment (Erk et al., 2011). Assessment of neuropsychological metrics alone might miss minor functional deficits that can still be compensated by increased brain activation. Therefore we combined cognitive tasks with fMRI to examine whether (i) treatment with EGb 761® improved cognitive performance in the context of a similar level of mobilization of neuronal resources, or whether (ii) it resulted in similar achievement of cognitive performance with significantly less brain activation.
The PFC is also involved in the central regulation of the acute stress response (Lucassen et al., 2014). Variations in prefrontal dopaminergic tone have been associated with differences in stress response (Arnsten, 2009; Hernaus et al., 2013). Subjects bearing a genotype related to lower cortical dopaminergic tone exhibited a lower cortisol and subjective response to acute stress (Armbruster et al., 2012; Hernaus et al., 2013), but also reduced stress resilience (Kang et al., 2013).
Therefore we also assessed potential effects of EGb 761® on the stress response using a standardized well‐established stress induction protocol with measurements of salivary cortisol as a well‐established biomarker.
Methods
The trial was a monocenter, randomized, double‐blind, placebo‐controlled two‐arm parallel group study, conducted at the Technische Universität Dresden, Germany, from October 2010 to April 2012.
Study population
Healthy male and female volunteers (50 to 65 years; with normal routine laboratory values), who had given written informed consent, were included. Subjective memory impairment was indicated by at least one item answered with “rather often” or “very often” or at least five questions answered “sometimes” in the Prospective and Retrospective Memory Questionnaire (PRMQ; Crawford et al., 2003, 2006; Kliegel and Jäger, 2006). Participants were selected for average or slightly below average cognitive performance: Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) IQ scale range ≥ 85 to ≤ 115; The Consortium to Establish a Registry for Alzheimer's Disease‐Neuropsychological Battery; revised German edition (CERAD‐PLUS, Aebi, 2002) in all subtests to age, gender and educational level adjusted z‐scores in the range −1 to +1, or for a maximum of three subtests age, gender and educational level adjusted z‐scores in the range of −2 to +1.
Exclusion criteria included: depression requiring antidepressive drug treatment within the last 12 months and/or Beck‐Depression‐Inventory revised edition 1996 (BDI‐II, Hautzinger et al., 2009) score of >18, or other psychotherapeutic/psychiatric treatment within the last 12 months before study treatment; cerebrovascular diseases including stroke and Alzheimer's disease or other dementia. The participants were not allowed to take concomitant CNS‐affecting medication.
Participants were characterized by baseline chronic stress burden (Trier Inventory for Chronic Stress; Schulz and Schlotz, 1999), action vs. state orientation (German version of the Action Control Scale, HAKEMP‐90; Kuhl, 1994) and impulsivity (Barrett Impulsivity Scale, BIS‐11: Preuss et al., 2008).
The sample size was confined to 60 subjects evaluable for efficacy. According to the experience in the field of fMRI trials, this number was regarded as sufficient to describe differences between the treatment groups. No formal sample size calculation was conducted, because no data about treatment effects on cognitive control functions were available to estimate effect sizes and variation. Drop outs were replaced until the intended number of evaluable subjects was reached. Subjects were considered to be evaluable if at least 80% of the study drug in the treatment period was taken, evaluable fMRI and stress‐test testing before and after treatment could be performed, and the end of study visit was performed.
Endpoints
All endpoints were measured baseline and after 56 ± 4 days of treatment.
Stress reactivity
During the Trier Social Stress Test (TSST; Kirschbaum et al., 1993) subjects had to give a short speech and to conduct mental arithmetic in front of an audience; salivary cortisol was determined before (−30, −1 min) and after (1, 10, 20, 30, 45 and 60 min) the task. The multidimensional mental state questionnaire (“Mehrdimensionaler Befindlichkeitsbogen” MDBF; Steyer et al., 1997) was answered directly before and after the test period of the TSST on both test days.
Cognitive tasks
For measurement of cognitive control functions a set of experimental computer‐aided paradigms was used, with reaction time (RT) and error rates as test parameters:
Task‐set switching (Meiran et al., 2000; Monsell, 2003; Dreisbach and Goschke, 2004; Müller et al., 2007b) was used as a measure of cognitive flexibility. On each trial, subjects had to respond to one of two different digits (randomly drawn from 1 to 8) presented simultaneously one above the other in different colors (red, blue or green). The target stimulus appeared in a specific color that was signaled by a task cue (the word “RED”, “BLUE” or “GREEN” in German language), which was presented for 200 ms at the beginning of each trial. After a cue‐target interval of either 0 or 600 ms, the imperative stimulus appeared and subjects had to indicate whether the target digit was odd or even by pressing a left or a right button (ITI: 1000 ms). It is noteworthy, that this paradigm constitutes a specific type of switching task as introduced by Dreisbach and Goschke (2004), with the actual task “respond to digit parity” remaining unchanged on every trial, but the target selection criterion varying (based on color). Hence, the paradigm used here for assessing cognitive flexibility is actually more an attention‐switching paradigm (even though the definition of the term “task” is clearly a matter of debate).
Moreover, as stimuli could be presented in one of three colors and each trial constituted two stimuli appearing in different colors, there were two different kinds of switch types: a (i) learned irrelevance/negative priming switch in which the former distractor color becomes the target color and a new color becomes the distractor color and (ii) a perseveration switch in which the former target color becomes the distractor color and a new color constitutes the target.
There were 320 trials in total; of which were 50% repeat trials (target color stays the same as on the preceding trial) and 50% switch trials (target color changes). Fifty percent of the switch trials were perseveration trials, and 50% were learned irrelevance switches, resulting in 80 trials per switch condition. Half of all trials were response–compatible (i.e. both digits were odd or even) or response‐incompatible (i.e. one digit was odd and the other was even). The difference between RT and error rates on switch versus repeat trials defined the “switch‐cost”.
A delayed‐response task (Cools et al., 2007) was used to assess the ability to maintain task‐relevant information in the face of distracting stimuli. On each trial a central task cue, which was surrounded by pictures of two faces and two scenes, was presented for 2 s. The task cue instructed subjects to memorize either the faces or the scenes. During a 10‐s delay, during which the stimuli were no longer visible, a to‐be‐ignored distractor (face or scene) or a scrambled picture of a face or scene was presented. After the delay a probe was presented and subjects had to indicate whether or not the probe matched one of the pictures from the task‐relevant memory‐set. On half of the trials, the to‐be‐memorized category switched from faces to scenes or vice versa, whereas on the remaining trials the relevant category stayed the same. There were 80 trials in total, of which 40 trials contained a real distractor and 40 trials a scrambled picture instead. In about 50% of all trials, the relevant category switched, whereas it was repeated in about the other 50% of the trials, resulting in an equal proportion of switch and repeat trials for the different distractor categories.
The Go–NoGo task assessed the ability to inhibit strong prepotent, but nonintended response. On each trial a single letter was presented for 150 ms at the center of the screen (either “M” or “W”, ITI: 1300 ms). Subjects had to respond as fast as possible by pressing a response key to the letter “M” (Go‐trials), but had to withhold a response to the letter W (NoGo‐trials). There were 300 trials in total, of which 60 trials were NoGo‐trials. Thus, the NoGo‐stimulus was presented on 20% randomly chosen trials.
To assess prospective memory (Goschke and Dreisbach, 2008) subjects performed an attention‐demanding ongoing spatial‐compatibility‐task—a task comparable to a nonverbal spatial stroop task—during which they had to notice rarely occurring prospective memory (PM) cues. Each trial started with an arrow presented to the left, to the right, above or below a fixation cross. Arrows could be facing up, down, left or right and were presented in one of 10 different colors (ITI: 1500 ms). Each arrow appeared equally often at each location; and location, direction and color of the arrows were determined at random, resulting in approx. 25% congruent and 75% incongruent trials. Participants moved a joystick with their right hand in the direction the arrow was facing. On congruent trials arrow direction and location were compatible; on incongruent trials the arrow pointed to the opposite direction relative its location. After a baseline‐block (150 trials) with the spatial‐compatibility‐task only, participants performed a PM‐block (210 trials), in which they were instructed to press a response button with the left index finger instead of moving the joystick whenever a red arrow appeared (the PM‐cue). PM‐trials (17% of the trials, i.e. 35 PM‐cue trials in the PM block) were randomly selected with the constraint that two consecutive PM‐trials were separated by at least three ongoing‐task‐trials.
To assess daily prospective memory, a postcard with address and stamp was handed to the subjects on the study visit and they were requested to send it back two days later after having written down a headline from a current daily newspaper as well as date and time of the day.
FMRI
The above described cognitive tasks were performed while subjects were positioned in an fMRI‐scanner (Siemens 3‐Tesla whole‐body Trio System, Erlangen, Germany) to measure task‐related changes of cerebral activity in terms of the BOLD (blood oxygenation level dependent) response as an indirect indicator of neuronal activity. Event‐related designs were applied, i.e. cerebral activity was measured in every single trial of each paradigm to assess differences in the BOLD‐response between different trial types (e.g. switch vs. repeat trials; NoGo vs. Go‐trials).
Safety
Adverse events (AEs) were analyzed as safety parameter. If abnormal laboratory values, abnormal vital signs or abnormalities of physical examination were considered clinically relevant by the investigator and were not present before the first application of the study medication they were captured as AE.
Treatment, randomization and allocation concealment
240 mg EGb 761® or placebo had to be taken once daily in the morning as film coated tablet for a time period of 56 ± 4 days. EGb 761® is a defined, quantified dry extract from dried leaves of Ginkgo biloba L. (Ginkgoaceae; maidenhair tree), manufactured by Schwabe Pharmaceuticals (Karlsruhe, Germany), drug‐extract‐ratio 35–67:1, primary extraction solvent: acetone 60% (w/w). The extract is adjusted to 22.0–27.0% ginkgo flavonoids, calculated as ginkgo flavone glycosides and 5.0–7.0% terpene lactones consisting of 2.8–3.4% ginkgolides A, B, C and 2.6–3.2% bilobalide and contains less than 5‐ppm ginkgolic acids. The 240‐mg dose was selected because efficacy of this dose has been established for dementia (Tan et al., 2015), mild (Gavrilova et al., 2014) and very mild (Grass‐Kapanke et al., 2011) cognitive impairment and in healthy elderly (Kaschel, 2011). Treatment for six to eight week has been sufficient to demonstrate effects in healthy elderly volunteers (Mix and Crews, 2002; Cieza et al., 2003; Kaschel et al., 2007; Kaschel, 2011). Placebo tablets were identical in taste, form, size and appearance of coating and tablet core.
For randomization of subjects in a 1:1 ratio, a biometrician of a Clinical Research Organization not further involved in trial conduct or data management generated a randomization list by means of PROC PLAN by SAS® with a balanced‐block design (block size of four). To reduce gender effects, at least 40% of the subjects were male and female, in each treatment group, respectively. Sequentially numbered medication containers were produced based on this randomization list. Any other personnel involved in conduct or management of the trial had no access to the information contained in the randomization list throughout the trial.
Investigators and subjects were blinded to treatment allocation and block size throughout conduct of the trial.
Objectives
Three efficacy endpoints were defined a‐priori: behavioral performance in the cognitive tasks, fMRI during cognitive task performance and TSST. In addition, tolerability and safety of EGb 761® in non‐demented elderly were assessed.
Statistical evaluation
Randomized subjects with at least one baseline and post‐treatment assessment of one of the efficacy endpoints form the intent‐to‐treat population on which the efficacy analysis was performed. The safety population included all enrolled subjects who have received at least one dose of study medication.
Cognitive testing
Statistical analyses was based on the general linear model and multifactorial ANOVA for mixed designs with the between‐subjects variables group (verum/placebo) and the within‐subjects variable time (pre‐treatment/post‐treatment), as well as additional within‐subjects variables specific for the cognitive tasks (e.g. switch vs. repeat trials in the task‐switching paradigm). Critical statistical hypotheses referred to the interaction of group and time with respect to the relevant dependent measure in the cognitive tasks. Provided this interaction was significant at the pre‐specified significance level α ≤ 5% for a given task, planned contrasts were computed to delineate the nature of the interaction. We predicted improved cognitive performance after treatment with EGb 761® versus placebo. As four independent psychological functions were assessed, no correction for multiple testing was applied in this trial.
fMRI‐data
Data was analyzed with SPM5 and SPM8 (Welcome Department of Cognitive Neurology, London, UK) based on MATLAB R2011b. Preprocessing included slice‐time correction to the reference slice 2, body movement correction, normalization by directly registering the mean functional image to the standard Montreal Neurological Institute (MNI) echo‐planar‐imaging template image provided by SPM5 (interpolated spatial resolution 3 × 3 × 3 mm) and smoothing of the functional images (Gaussian Kernel, full width half maximum = 8 mm).
After pre‐processing, the General Linear Model (GLM) approach (Friston et al., 1995) was used at first level for estimating model regressors capturing BOLD‐activation associated with different task conditions, both for pre‐ and post‐drug session (high‐pass filtered with a cutoff frequency of 1/128 Hz). Based on the resulting beta‐weight maps, the condition contrasts of interest were computed for each subject and cognitive task.
For statistical assessment of treatment effects, the resulting contrast maps of all subjects were entered into a second‐level GLM in two steps. First, in a whole brain analysis, regions of interest (ROIs) showing activation changes related to specific task parameters were identified based on pre‐treatment data. Task specific contrast analyses were corrected for multiple comparisons based on the Gaussian Random Field Theory for p < 0.05 (i.e. FWE cluster‐level and voxel‐level corrections as implemented in SPM 8) at an initial voxel‐level statistics threshold of p < 0.05 FWE whole brain corrected. In a second step, second‐level GLM analyses were computed inside those ROIs to identify treatment effects (small‐volume corrected for multiple comparisons based on the Gaussian Random Field Theory for p < 0.05, i.e. cluster‐level and voxel‐level corrections as implemented in SPM 8, initial voxel level statistics threshold: p < 0.05 uncorrected).
Stress reactivity
Sixteen cortisol values of each volunteer were analyzed in an ANOVA for repeated measurements (3‐factorial design; factors: treatment‐group; pre‐post treatment; specimen time). Cortisol values not normally distributed were log10 transformed. In case of violation of the sphericity assumption, the Greenhouse–Geisser and Huynh–Feldt estimates were to be applied as a correction factor. For proof of the hypothesis a significance level of α ≤ 5% was set for the interaction group × pre‐post treatment.
Results
Study population
Of 211 subjects screened, 136 did not meet the inclusion criteria and 75 were randomized, 43 to EGb 761® and 32 to placebo. Eight subjects (7 verum, 1 placebo) turned out not to be suited for MRI testing, 3 (2/1) were excluded because of upcoming surgery and two participants in the verum group for unspecified reasons. One subject taking verum dropped out because of poor compliance, so that the safety analysis groups consisted of 32/30 and the efficacy groups of 31/30, respectively (Supporting Information, Figure S1). Although the rather unequal dropout rate of 7:1 of participants not suitable for MRT testing appears to deviate from the expected 4:4 ratio, those drop outs still occurred totally at chance. For example, some participants arrived at the MRI scanner for the pre‐treatment testing, but could not go into the scanner as it was discovered that they had or might have unspecified metal inside their bodies. Others discovered while actually already lying inside the scanner, that they could not do the testing because of claustrophobic reasons or that their vision could not be corrected to normal by available MRI compatible glasses.
Treatment duration in the ITT population was similar in the treatment allocation EGb 761® (57.9 +/− 2.3 d) and placebo (58.0 +/− 3.6 d) with at least 99% compliance as determined by pill‐counting. Treatment groups were not significantly different in age, gender and BMI (Table 1).
Table 1.
Demographic characteristics, screening and baseline tests (ITT population)
| Item | Parameter | EGb 761® | Placebo |
|---|---|---|---|
| N | 31 | 30 | |
| Age | Years | 57.5 +/− 4.6 | 57.1 +/− 4.4 |
| Gender | (♀ : ♂) | 15 : 16 | 18 : 12 |
| BMI | kg/m2 | 25.1 +/− 3.0 | 25.1 +/− 3.5 |
| Screening tests | |||
| CERAD PLUS | z‐scores outside the range −1 to +1 | 1.6 +/− 1.2 | 1.2 +/− 1.3 |
| PRMQ | Sum raw score – prospective scale | 20.1 +/− 3.8 | 20.1 +/− 3.9 |
| Sum raw score – retrosp. scale | 20.3 +/− 3.0 | 19.4 +/− 3.3 | |
| BDI‐II* | Sum score | 6.9 +/− 3.6 | 4.8 +/− 5.3 |
| WASI | Full‐scale intelligence quotient | 105.1 +/− 6.3 | 104.3 +/− 7.9 |
| TICS | SSCS sum T‐score | 53.0 +/− 6.7 | 52.1 +/− 8.2 |
| Baseline only tests | |||
| HAKEMP 90 | Prospective state, sum score* | 6.2 +/− 2.7 | 7.9 +/− 2.6 |
| Failure‐related state, sum score | 4.7 +/− 3.3 | 4.8 +/− 3.8 | |
| Action‐related state, sum score | 9.7 +/− 2.1 | 9.6 +/− 2.1 | |
| BIS‐11 | Mean sum scores | 58.6 +/− 7.3 | 59.5 +/− 6.9 |
a: Wilcoxon rank sum test; b: Fisher's exact test; all two‐sided.
: significant group difference Wilcoxon rank‐sum test to the 5% alpha level.
Psychometric testing verified that all randomized subjects had average or slightly below average cognitive performance, did not suffer from significant depressive symptoms and had average perception of chronic stress. Treatment groups only differed in their BDI‐II mean score which was higher with 6.9 vs. 4.8 points (p < 0.02) in the verum group; both values are, however, well within the range of minimal depressive constitution (below 13). Another significant difference was found in the HAKEMP‐90 subscale prospective acting state with 6.2 vs. 7.9 points (p = 0.014) both representing medium degrees of readiness to act on a scale from 0 to 12 (Table 1).
Efficacy results
Cognitive paradigms
Results of the task‐set switching‐paradigm suggest that EGb 761® significantly improved task‐set switching performance (Figure 1A). A 2 × 2 × 2 repeated measures ANOVA with Time (pre/post‐treatment) and Trial‐Type (repeat/switch) as within‐subjects variables, Group (verum/placebo) as between‐subjects variable and mean RT as the dependent variable yielded highly significant effect of Time, F(1,57) = 39.05, p < 0.001, η 2 = 0.41, reflecting practice effects and Trial‐Type, F(1,57) = 92.55, p < 0.01, η 2 = 0.62, reflecting increased RT for task switch trials. Most importantly, the critical Time × Trial‐Type × Group interaction was also significant: F(1,57) = 5.99, p < 0.02, η 2 = 0.10. The interaction remained significant, when the cue‐target‐interval was added as factor (F(1,57) = 4.48, p = 0.039, η 2 = 0.073). Also, there was no interaction between CTI and the relevant Time × Trial‐Type × Group interaction (Time × Trial‐Type × Group × CTI: F(1,57) = 0.043, p = 0.836, η 2 = 0.001).
Figure 1.
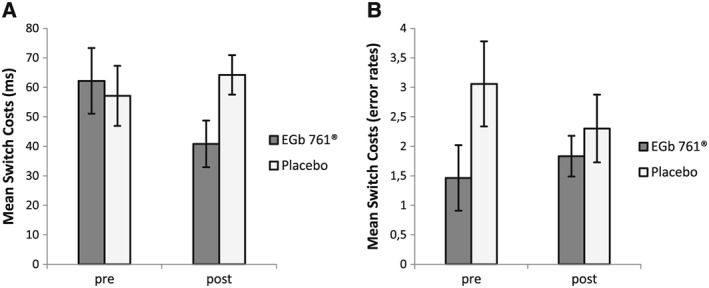
Task switching: mean switch‐costs (difference between switch and repeat trials, A: reaction time, B: error rates) in the pre‐ and post‐treatment sessions for the EGb 761® and placebo groups
Accounting for observed group differences in BDI scores during baseline testing as well as for the unbalanced gender ratio between the groups, an additional covariance analysis with BDI score as a covariate, gender as a between subject factor and otherwise equal factors (Time, Trial‐Type, Group) was conducted. Results showed no effect of BDI scores on task switch performance (interaction Time × Trial‐Type × BDI: F(1,54) = 0.61, p = 0.44, η 2 = 0.01) and no further effects of BDI (all p > 0.05). Gender had no impact on the critical three‐way interaction (Time × Trial‐Type × Group × Gender: F(1,54) = 0.69, p = 0.41, η 2 = 0.01) and there were also no other effects of gender (all p > 0.05). Most importantly, the critical Time × Trial‐Type × Group interaction remained significant (F(1,54) = 4.61, p = 0.036, η 2 = 0.08). Therefore, it is very unlikely, that differences in task switch performance resulted from differences in BDI scoring or were influenced by an unequal gender ratio among the groups.
Planned contrasts showed that switch‐costs did not significantly differ between the two groups in the pre‐treatment session, t(57) = .34, p > .70 (switch‐costs were 62 and 57 ms in the verum and placebo groups, respectively), whereas in the post‐treatment session the verum group showed significantly smaller switch‐costs (41 ms) than the placebo group (64 ms), t(57) = −2.22, p < .04. Moreover, whereas the verum group showed a significant reduction of the switch‐cost from the pre to the post‐session, t(28) = 2.29, p = .03, in the placebo group the switch‐cost did not differ between the pre and the post‐session, t(29) = −1.01, p > .30.
Moreover, for further investigation of the observed effect of EGb 761® on switch costs in regard to the different switch types (perseveration vs. learned irrelevance switch trial), an additional 2 × 2 × 2 repeated measures ANOVA with Time (pre/post‐treatment) and Switch‐Type (perseveration/learned irrelevance) as within‐subjects variables, Group (verum/placebo) as between‐subjects variable and switch‐cost RT (RT on switch trials − RT on repeat trials) as the dependent variable was conducted. Interestingly, the critical Time × Group interaction (F(1,57) = 6.12, p = 0.016, η 2 = 0.10) was further specified by a significant three way interaction between Time, Group and Switch‐Type (F(1,57) = 4.69, p = 0.035, η 2 = 0.08, Figure 2 A and B). Post‐hoc comparisons for the verum group showed a significant reduction (32 ms) of switch costs in the learned irrelevance condition from the pre to the post‐session (t(28) = 2.66, p = .013), but no difference in switch costs between pre to post‐session for the perseveration condition (10 ms, t(28) = 1.09, p = .29). Therefore, the observed effect of reduced switch costs in the verum group was mainly driven by learned irrelevance trials.
Figure 2.
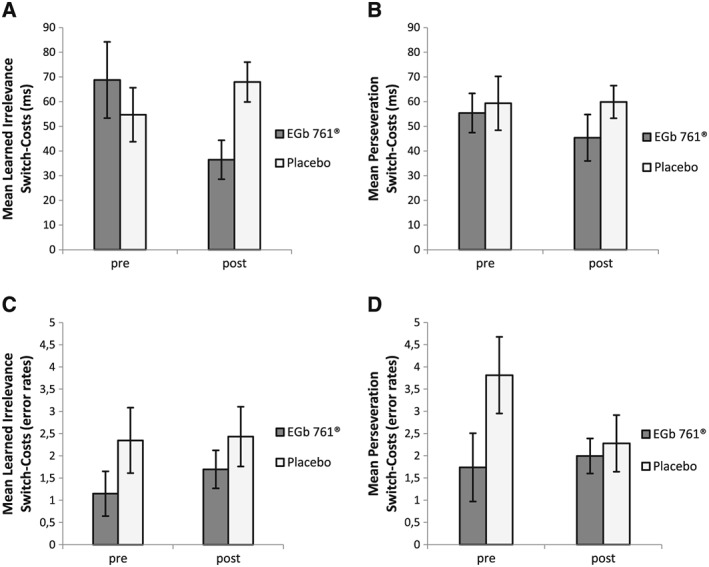
Task Switching – Switch Type Analysis. Mean switch‐costs (difference between switch and repeat trials) in the pre‐ and post‐treatment sessions for the EGb 761® and placebo groups, displayed separately for learned irrelevance switch (A,C) and perseveration switch condition (B, D). Shown are switch costs for reaction times (A,B) and error rates (C,D). Learned irrelevance switch: former distractor color of trial N − 1 becomes the target color of trial N + new distractor color. Perseveration switch: target color of trial N − 1 becomes distractor color of trial N + new target color
A 2 (Time) × 2 (Trial‐Type) × 2 (Group) ANOVA with error rates as the dependent variable yielded significant effects of Time (F(1,57) = 15.93, p < 0.01, η 2 = 0.22) and Trial‐Type (F(1,57) = 47.14, p < 0.01, η 2 = 0.45). Error rates were smaller in the post‐compared to the pre‐treatment session (reflecting unspecific practice effects), and error rates were higher on task‐switch than on task‐repeat trials, reflecting a commonly observed task switch effect on error rates. There was a significant main effect of group, F(1,57) = 5.72, p < 0.05, η 2 = 0.09, reflecting the fact that the placebo group made slightly more errors than the verum group (5.8% vs. 3.7%). Importantly, the critical Time × Trial‐Type × Group interaction was not significant (F(1,57) = 1.28, p = 0.26, η 2 = 0.02), and there were no significant interactions of Group with any other factor (all p > .05). However, one could argue that numerically, the pattern seems to contradict the observed RT pattern (Figure 1B). That is, for the verum group, the error‐switch costs increased by 0.37%, whereas it decreased for the control group by 0.75% (i.e. 1.12% interaction effect in the direction opposite to the RT interaction effect). Nevertheless, in line with the results from the ANOVA, post‐hoc comparisons of error switch‐costs between pre‐ and post‐treatment conducted separately for each group demonstrate, that this descriptive speed accuracy trade of is not significant (verum: t(28) = −.53, p = .60; placebo: t(29) = 1,07, p = .29). Also, as the verum induced reduction of RT switch‐cost occurred mainly in the learned irrelevance condition, it is important to look at a potential speed accuracy effect in respect to different switch‐type conditions (Figure 2 C and D). Therefore, a 2 × 2 × 2 repeated measures ANOVA with Time (pre/post‐treatment) and Switch‐Type (perseveration/learned irrelevance) as within‐subjects variables, Group (verum/placebo) as between‐subjects variable and switch‐cost error rates (percent errors on switch trials − percent errors on repeat trials) as the dependent variable was conducted. Results showed no significant effect of the critical three‐way interaction Time × Group × Switch‐Type (F(1,57) = 1.49, p = 0.23, η 2 = 0.03) nor any other significant effect (all p > 0.05). Post‐hoc comparisons of error switch‐costs between pre‐ and post‐treatment conducted separately for each group and switch‐type demonstrate no statistical difference (all p > 0.05). Only numerically, in the learned irrelevance condition, error‐switch costs increased by 0.55% in the verum group, but also by 0.08% for the control group. Therefore, when looking at the relevant switch condition driving the observed verum effect on switch‐cost RTs instead of switch trials per se, the numerical error‐rate interaction effect in the direction opposite to the RT interaction effect is reduced to only 0.46% (relative to 1.1%). Thus, the interaction of group and time with respect to the RT switch‐cost was not counteracted by error effects.
In the Go–NoGo task the drug and placebo groups differed with respect to the speed‐accuracy tradeoff between the pre‐ and post‐treatment sessions (Figure 3). An ANOVA on false alarm rates for NoGo‐trials in the pre‐treatment session with the factor Group (verum/placebo) and mean RT on Go‐trials as a covariate revealed a significant effect of Go‐trial RT on the NoGo false alarm rate, F(1,55) = 27.82, p < 0.001, η 2 = 0.336, but no significant effect of group (F(1,55) = 0.99, p = n.s., η 2 = 0.018), indicating no statistically significant group difference in false alarm rates when correcting for Go‐trial RTs. In contrast, the ANOVA on post‐treatment data did show both a significant effect of Go‐trial RT, F(1,55) = 8.77, p < .01, η 2 = 0.138, and a significant effect of group when controlling for Go‐trial RTs, F(1,55) = 4.10, p < .05, η 2 = 0.069, on false alarm rates for NoGo‐trials, indicating fewer false alarms for the verum‐group in the absence of a speed‐accuracy tradeoff. To control for group differences in BDI scoring and gender ratio, a second ANOVA on post‐treatment data was conducted with the additional between subject factor gender and BDI scores as a covariate. There were no significant effects of BDI (F(1,52) = 0.31, p = .58, η 2 = 0.006) or gender (interaction Group × Gender: F(1,52) = 0.32, p = .58, η 2 = 0.006; main effect gender: F(1,52) = 0.59, p = .45, η 2 = 0.011). However, the critical effect of Group on false alarm rates for NoGo‐trials during post‐treatment was reduced to F(1,52) = 3.92, p = .052, η 2 = 0.07. Thus, this finding has to be interpreted with caution, as the significance was not clearly below p = 0.05 when controlling for BDI, Gender and Go‐trial RT and also given the fact that no significant interaction between group and time was observed.
Figure 3.
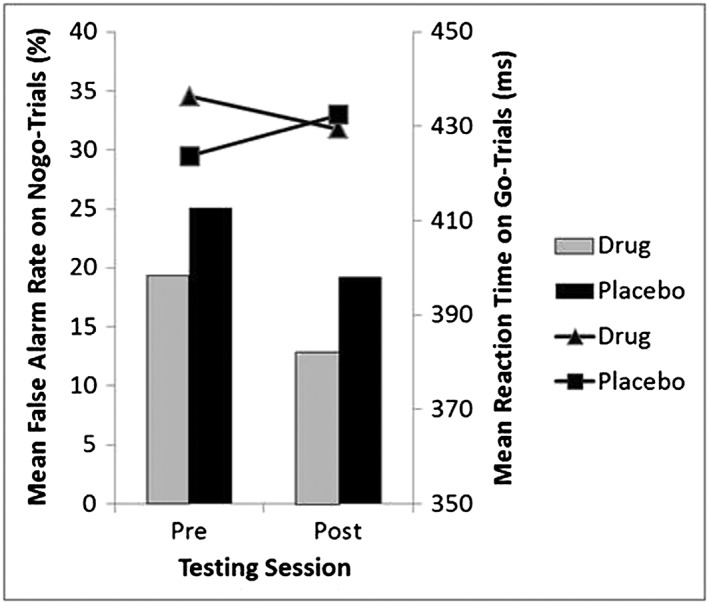
False alarm rates on NoGo‐trials (bars) and reaction times (lines) on Go trials in the pre‐ and post‐treatment sessions for the EGb 761® and placebo groups
No significant treatment effects could be detected in the delayed response task and the prospective memory task. Results of the cognitive tasks are shown in Table 2.
Table 2.
Results of cognitive and stress tests
| Test | Efficacy parameter | Independent parameters |
Dependent parameters |
Group N | Result baseline | Result post‐treatment | |
|---|---|---|---|---|---|---|---|
| Cognitive paradigms | Task‐set switching | Reaction times (RT) [ms] |
Time, group, switch |
Mean RT (switch − repeat = switch‐costs) | V 29 | 930 − 868 = 62 | 857 − 816 = 41 |
| P 30 | 932 − 875 = 57 | 881 − 817 = 64 | |||||
|
Error rates (ER) [%] |
Mean ER (switch − repeat = switch‐costs) | V 29 | 5.1 − 3.7 = 1.5 | 3.9 − 2.1 = 1.8 | |||
| P 30 | 8.9 − 5.8 = 3.1 | 5.4 − 3.1 = 2.3 | |||||
| Delayed response task | Reaction times (RT) [ms] | Time, group, switch, probe stimulus type | Mean RT, switch mode | V 26 | 1370 +/− 231 | 1358 +/− 230 | |
| P 30 | 1384 +/− 216 | 1356 +/− 198 | |||||
| Mean RT, repeat mode | V 26 | 1376 +/− 233 | 1380 +/− 223 | ||||
| P 30 | 1363 +/− 324 | 1380 +/− 203 | |||||
|
Error rates (ER) [%] |
Mean ER, switch mode | V 26 | 27.1 +/− 10.6 | 23.4 +/− 9.7 | |||
| P 30 | 31.2 +/− 11.9 | 28.5 +/− 12.9 | |||||
| Mean ER, repeat mode | V 26 | 30.2 +/− 11.3 | 26.3 +/− 9.8 | ||||
| P 30 | 32.3 +/− 10.6 | 28.3 +/− 10.8 | |||||
| Prospective memory (PM) task |
Reaction times (RT) [ms] and error rates (ER) [%] |
Time, group | Mean RT | V 28 | 672 +/− 99 | 643 +/− 94 | |
| P 30 | 699 +/− 160 | 642 +/− 85 | |||||
| Mean ER | V 28 | 1.2 +/− 2.3 | 0.3 +/− 0.9 | ||||
| P 30 | 2.3 +/− 4.0 | 0.4 +/− 1.0 | |||||
|
Time, group, block *
(ongoing task) |
Mean RT PM − baseline = costs | V 28 | 738 − 733 = 5 | 718 − 705 = 13 | |||
| P 30 | 779 − 737 = 42 | 711 − 705 = 6 | |||||
| Mean ER PM − baseline = costs | V 28 | 3.3 − 1.5 = 1.8 | 1.2 − 0.7 = 0.5 | ||||
| P 30 | 3.9 − 2.9 = 1.0 | 1.5 − 0.9 = 0.6 | |||||
| Time, group, congruency** | Mean RT incongr − congr = costs | V 28 | 748 − 699 = 49 | 723 − 689 = 34 | |||
| P 30 | 784 − 726 = 58 | 718 − 683 = 35 | |||||
| Mean ER incongr − congr = costs | V 28 | 2.9 − 2.3 = 0.6 | 1.3 − 0.6 = 0.7 | ||||
| P 30 | 3.5 − 4.0 = −0.5 | 1.5 − 0.8 = 0.7 | |||||
| Go–NoGo‐task | Reaction times (RT) [ms] | Time, group | Mean RT Go trials | V 28 | 436 +/− 61 | 429 +/− 51 | |
| P 30 | 424 +/− 36 | 432 +/− 40 | |||||
| False alarm rates (ER) [%] | Mean ER in NoGo trials | V 28 | 19.4 +/− 14.3 | 12.9 +/− 8.0 | |||
| P 30 | 25.1 +/− 16.7 | 19.2 +/− 17.0 | |||||
| Reaction to psychosocial stress | TSST | Salivary cortisol levels | Time, group, specimen time | Salivary cortisol levels | V 30 | separate results | separate results |
| P 29 | |||||||
| MDBF | MDBF subscale good/bad mood sum score | Time, group, specimen time |
Subscale sum scores pre‐TSST |
V 31 | 10.7 +/− 1.3 | 10.8 +/− 1.4 | |
| P 30 | 10.7 +/− 1.9 | 10.9 +/− 1.6 | |||||
|
Subscale sum scores post‐TSST |
V 31 | 10.9 +/− 1.2 | 10.8 +/− 1.3 | ||||
| P 30 | 10.6 +/− 2.2 | 10.6 +/− 1.4 | |||||
| MDBF subscale alertness/fatigue sum score |
Subscale sum scores pre‐TSST |
V 31 | 11.1 +/− 1.9 | 11.1 +/− 1.4 | |||
| P 30 | 10.8 +/− 1.9 | 10.6 +/− 1.5 | |||||
|
Subscale sum scores post‐TSST |
V 31 | 11.5 +/− 1.6 | 11.3 +/− 1.8 | ||||
| P 30 | 10.6 +/− 1.4 | 10.3 +/− 2.0 | |||||
| MDBF subscale calmness/restlessness sum score |
Subscale sum scores pre‐TSST |
V 31 | 11.4 +/− 2.2 | 11.0 +/− 2.1 | |||
| P 30 | 11.3 +/− 2.0 | 11.4 +/− 2.0 | |||||
|
Subscale sum scores post‐TSST |
V 31 | 12.3 +/− 1.5 | 11.8 +/− 2.3 | ||||
| P 30 | 11.7 +/− 1.7 | 10.8 +/− 1.9 | |||||
Baseline vs. prospective memory block.
Response‐congruent vs. response‐incongruent trials.
fMRI
Whole brain analyses on pre‐treatment data identified BOLD‐activity in ROIs associated with cognitive tasks that were generally in congruence with previous reports: frontal and parietal cortex for task‐set switching (Ruge et al., 2013), midcingulate cortex/pre‐SMA and inferior frontal gyrus for the Go–NoGo task (Aron et al., 2007). In the delayed response task, posterior cingulate cortex, fusiform gyrus and hippocampus, regions commonly engaged in episodic memory retrieval and memory consolidation (Marshall and Born, 2007; Summerfield et al., 2009), were differentially active in switch minus repeat trials. Interestingly, these results differ from commonly observed effects in task‐switch paradigms probably reflecting different, more memory focused processes occurring on switch trials in this delayed response paradigm. Among others, lateral PFC was defined as critical for distractor vs. scrambled picture differentiation in the delayed response task as expected from a previous study using this paradigm which found that a dopamine (D2) receptor agonist modulated activation specifically in lateral PFC when task‐relevant information had to be maintained and shielded from distracting stimuli (Cools et al., 2007). In the prospective memory task, event‐related activations to PM‐cues were observed in a widespread but more differentiable network including temporal cortex, anterior cingulate cortex, precuneus and ventral parietal cortex that are commonly reported by studies on event‐based PM (Burgess et al., 2011; Beck et al., 2014). See Supporting Information for a complete list of pre‐treatment fMRI results.
Although some differences related to group or training effects could be confirmed, none of the critical analyses related to treatment effects was significant (all p > .05) in any of the four implemented tasks.
Postcards
The majority of participants remembered to send back the postcard in time. Pre‐treatment six participants (19%) of the EGb 761® group and two (7%) of the placebo group forgot, post‐treatment four participants (13%) of each group. Numerically this indicates improved prospective memory performance in the drug group, but given the small overall number of subjects who forgot to send back the postcard, no further statistical analyses were performed.
Stress reactivity
EGb 761® had no significant effect on salivary cortisol concentrations nor on subjective measures of affect during the TSST (Table 2). However, a statistically non‐significant effect was numerically observable, indicating a potentially enhanced endocrine stress recovery in the EGb 761® treatment group (Figure 4).
Figure 4.
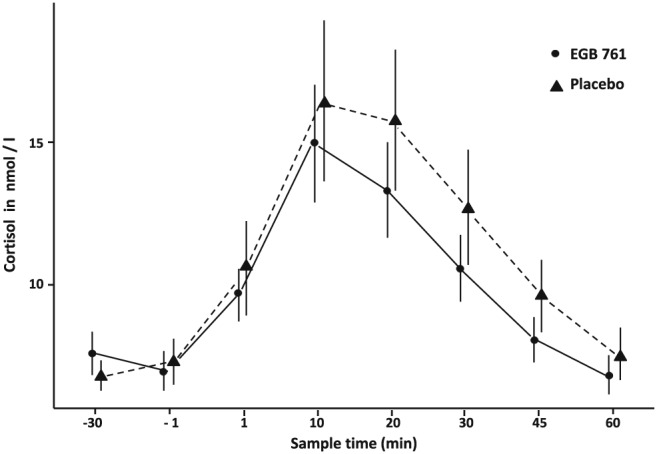
Mean salivary cortisol (SEM) during TSST after 8 weeks of treatment
Safety results
EGb 761® administered in a dose of 240 mg once daily in healthy subjects for about 8 weeks was safe and well tolerated. Although the proportion of subjects who reported at least one AE was higher in subjects allocated to EGb 761® (19 = 59.4%) than in the placebo group (12 = 40%), the vast majority of AEs were of mild severity, only two in each group being of moderate severity. Severe or serious AEs were not reported; no subject terminated drug intake because of an AE.
The safety profile was consistent with the known safety profile for EGb 761®. The most frequently reported AE in this study was headache, a listed adverse drug reaction of EGb 761®, reported overall in eight subjects, six being allocated to EGb 761®. In most cases headache was mild, only one subject reported headache of moderate severity.
No relevant changes of laboratory tests or vital signs were detected in the two treatment groups.
Discussion
Our results indicate that EGb 761® treatment potentially improved cognitive flexibility as assessed in a task‐switching paradigm. Reduced task‐switch costs suggesting improved cognitive flexibility after EGb 761® treatment were observed in the absence of significant changes in brain activation, indicating that subjects' improved ability to switch between cognitive sets did not incur a cost in terms of increased recruitment of neural systems and/or resources. This result stands in line with the assumption that the improved switching performance after EGb 761® treatment group was because of an increase in cognitive processing efficiency.
Pre‐treatment data of all four tasks showed a pattern of behavioral performance as well as a pattern of brain activity consistent with what had been expected on the basis of previous research. Thus, the method was working, and the absence of treatment‐related BOLD effects may not be explained by technical factors.
In addition, we observed a positive effect of EGb 761® on the speed‐accuracy tradeoff in the Go/NoGo task, a descriptive tendency for an accelerated endocrine stress recovery and no effects on our measures of response delay and prospective memory.
Cognitive flexibility as assessed by task switching has been found to be under dopaminergic influence (Klanker et al., 2013). Subjects with high dopamine synthesis capacity had better performance in object feature shifting (Dang et al., 2012), and the dopamine D2 receptor agonist bromocriptine reduced task switch costs, an effect that was prevented by the D2 receptor antagonist sulpirid (van Holstein et al., 2011).
Dopaminergic influence on impulse inhibition as measured by the Go/NoGo paradigm is suggested by the general positive effect of drugs used in ADHD on impulsivity, reduced NoGo accuracy in response to the D2/D3 receptor antagonist haloperidol (Luijten et al., 2013) and increased error rate in a rewarded Go/NoGo task by acute phenylalanin/tyrosin depletion diet that was restored by L‐Dopa (Leyton et al., 2007). More recent studies did not reveal relations between genetic variants that influence dopaminergic pathways on Go/NoGo accuracy in healthy subjects, but on RT variability (Gurvich and Rossell, 2014; Mulligan et al., 2014). The D2 receptor antagonist cabergoline had no effect on Go/NoGo error rates, but improved error awareness (Nandam et al., 2013). Therefore our finding of improved speed‐accuracy tradeoff without a significant main effect on error rate is compatible with improved D2 receptor mediated function.
Response accuracy and PFC activation during response delay in a working memory task had been reported to correlate with dopamine synthesis capacity (Landau et al., 2009). In a recent trial, single dose bromocriptine had no net effect on delayed response accuracy, but impaired performance after face relative to scene distraction during a delayed match‐to‐sample task with face stimuli (Bloemendaal et al., 2015). We did not observe impaired delayed response with congruent distractors by EGb 761®, indicating that the Ginkgo extract is no direct D2 receptor agonist.
Dopaminergic influence on prospective memory was suggested by animal data (Goto and Grace, 2008), and the observation that L‐Dopa improved executing an action after 10‐min delay in patients with Parkinson's disease (Costa et al., 2008). However, more recently significant effects of nicotine on prospective memory have been consistently reported (Rusted et al., 2011; Evans et al., 2013), highlighting the preponderance of the cholinergic systems for this task.
As regards stress responsivity, EGb 761® treatment exhibited no significant influence on endocrine, as well as subjective reactivity and recovery to acute psychosocial stress. A nonsignificant trend indicated a potentially accelerated endocrine stress recovery. In line with this trend Jezova et al. (2002) reported EGb 761® to inhibit blood pressure increase in response to a memory test with concomitant physical handgrip exercise; increase of cortisol release was observed in male participants and inhibited by EGb 761®. No other studies assessed the effects of pharmacological manipulation of the dopaminergic system onto the acute stress response so far.
Taken together our results are compatible with an EGb 761®‐induced mild prefrontal dopaminergic enhancement. Recent reports from animal models of dopamine sensitive learning (Moeller et al., 2009), sexual function (Yeh et al., 2011) and Parkinson's disease (Rojas et al., 2012) as well as clinical findings in ADHD (Klement et al., 2011), tardive dyskinesia (Zhang et al., 2011) and negative symptoms in schizophrenia (Doruk et al., 2008) contribute to the hypothesis of mild prefrontal dopaminergic action of this specific ginkgo extract. Dopaminergic effects in the PFC could be explained by mild inhibition of the norepinephrine transporter that has been reported for ginkgo flavone glycosides (Fehske et al., 2009). Because of the paucity of dopamine transporters in the human PFC, re‐uptake of dopamine from the synaptic cleft is largely mediated by the norepinephrine transporter in this part of the brain (Heal et al., 2013).
However, alternative explanations for our findings need to be considered. Inhibition of the norepinephrine transporter increases both, dopamine and norepinephrine concentrations (Heal et al., 2013), and chronic administration of EGb 761® has been found to increase PFC concentrations of both neurotransmitters in animals (Yoshitake et al., 2010). A U‐shaped relation between noradrenergic stimulation and set shifting has been deduced from animal data (Chamberlain and Robbins, 2013; Logue and Gould, 2014). In human trials pharmacologic manipulations of the noradrenergic system yielded no or inconsistent effects on cognitive flexibility. Some data indicate that noradrenergic overstimulation might impair set shifting (Chamberlain and Robbins, 2013). Alpha receptor antagonists had no effect on response inhibition, and the effects reported for the selective norepinephrine transporter inhibitor atomoxetine cannot directly be linked to norepinephrine, as atomoxetine also inhibits dopamine reuptake in the PFC. Therefore it is unlikely that modulation of the noradrenergic system explain our results of reduced task‐switch costs and improved speed‐accuracy tradeoff.
Effects of EGb 761® on neuroplasticity could be another explanation for our finding. Cognitive flexibility has been linked to synaptic plasticity (Stokes et al., 2013), and EGb 761® has been shown in several preclinical models to increase nearly all aspects of impaired neuroplasticity (long‐term potentiation, spine density, neuritogenesis, neurogenesis; Müller et al., 2012). As we studied elderly volunteers with subjective memory impairment, impaired synaptic plasticity is likely to have been present in many of them.
Our data add to recent findings that EGb 761® improves cognitive functions in elderly healthy volunteers or in patients with mild cognitive impairment when an adequate dosage of 240 mg/day and a treatment duration of eight weeks or longer is used (Grass‐Kapanke et al., 2011; Kaschel, 2011; Gavrilova et al., 2014). Moreover, the compound has been demonstrated to improve cognition and activities of daily living in demented patients (Brondino et al., 2013). Conflicting results have been reported with shorter treatment periods, lower dosages and different ginkgo preparations (Moulton et al., 2001; Nathan et al., 2002; Solomon et al., 2002; Persson et al., 2004; Laws et al., 2012). Therefore our findings cannot be extrapolated to the variety of ginkgo products with variable quality and dosing, which are popular food supplements in many countries.
However, our trial has some limitations. As it was a small pilot trial, the results need to be replicated in a larger confirmatory study. Although the effect of EGb 761® on cognitive flexibility had been predicted a‐priori, this finding should nevertheless be interpreted with caution given that no correction for multiple comparisons was conducted across the four different cognitive tasks used to assess cognitive control.
Also, one should mention, that although not statistically significant, the numerical pattern of error rates in the task switch paradigm contradicted the observed effect on RTs. That is, for the verum group, the error‐switch costs slightly increased, whereas it decreased for the control group. However, looking at error rates only in the critical switch‐condition mainly driving the verum effect on RT switch‐cost reduction, a different pattern was observed. That is, in the learned irrelevance condition, error rates increased from pre‐ to post‐treatment in both the verum and placebo group, although slightly stronger in the verum group. Thus, although one might argue that descriptively a speed accuracy trade off was observed for switch trials per se, this effect is no longer that obvious when looking at the specific switch type condition actually driving the RT effect. Additionally, all error effects were only numerically present and not significant. There are also “integrated” measures of RT and accuracy, such as “inverse efficiency scores” (i.e. RT divided by percent correct); however, it is not entirely clear whether this would actually simplify the interpretation of the present tradeoff. Therefore, it is rather unlikely, that the observed effect of EGb 761® in terms of reduced RT differences between switch and repeat trials resulted from an increase in error rates.
Moreover, one might argue, that the observed baseline differences in BDI scores, i.e. significantly higher scores for the verum group, might explain some of the positive development in task‐switch performance. However, there are several aspects suggesting that this was rather not the case. First, although impairment of cognitive control in depression has been extensively discussed, and deficits in task set switching were observed by some studies (Merriam et al., 1999; Lo and Allen, 2011; Whitmer and Gotlib, 2012), scores observed in our sample are classified as “no depression” or “minimal depression” by the manual and thus were below any clinical relevance or the cutoff (BDI = 18) for subclinical symptoms of depression (Beck et al., 1996; Arnau et al., 2001; Hautzinger et al., 2009). In those studies reporting effects of depression on cognitive functions, effects were found in individuals with a full diagnosis of major depressive disorder as assessed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID; First et al., 1995). This was not the case in our sample, a BDI score > 18 or the clinical diagnosis of depression that required any antidepressive drug treatment within the last 12 months were explicit exclusion criteria.
Second, for excluding the possibility that differences in even very low BDI scores might nevertheless have impacted the observed reduction in task‐switch costs, a covariance analysis using BDI scores as a covariate was conducted. Results showed no effects of BDI on task parameters and although controlling for BDI scores the critical three‐way interaction of Group × Time × Switch remained significant. Therefore, it is very unlikely, that differences in BDI scoring accounted for the observed effect.
Third, consistent with the finding, that individuals diagnosed with major depression disorder show high levels of perseveration (Waford and Lewine, 2010) recent studies suggest, that rumination and perseveration underlie impaired task set switching in depression (Merriam et al., 1999; Whitmer and Banich, 2007; Meiran et al., 2011; Whitmer and Gotlib, 2012). These findings would predict an effect of depression symptoms on the perseveration switch condition of the paradigm used in the current study, a condition requiring participants to respond to a new target color, although the old target color is still present as the distractors color. However, additional switch‐type analysis showed that the observed verum effect on RT switch‐cost reduction was mainly driven by the learned irrelevance condition and not the perseveration condition. Thus, it is very unlikely, that depression related changes in cognitive processing such as increased perseveration underlie the observed switch effect. Rather, on speculative terms, the verum might have improved the ability to update a no longer relevant former inhibition of task response to a certain color as it was required for correct responding in the learned irrelevance condition.
Furthermore, it has been argued, that the mere size of switch costs are rather difficult to interpret and it is the “preparatory reduction of switch costs” that is a valid marker of cognitive control (e.g. Monsell, 2003). In the current study, the cue target interval (CTI) was also varied as for investigating preparatory processes. However, results show no interaction between the observed verum effect (Group × Time × Switch interaction) and the CTI variation. Hence, we did not find evidence for the fact that the observed verum effect on switch costs constitutes a preparatory reduction of switch costs. The current results rather suggest an impact of the drug on processes occurring at the time of stimulus presentation.
Another important issue that should be highlighted is the fact, that effects on stress reactivity were only numerically present but not statistically significant. Therefore, a replication of our findings is required before improvement of cognitive flexibility and stress reactivity in the healthy elderly by EGb 761® can definitely be concluded.
The prospective‐memory postcard‐task had a ceiling effect and was not sensitive enough to capture treatment effects while the computer task only captured prospective memory for seconds because of the fMRI context. As deficits in prospective memory are frequently expressed in the elderly, future trials should use validated tasks that capture the function of remembering to do things regularly or after a number of days.
Moreover, recent findings revealed that the relation between dopaminergic tone in the PFC and cognitive control functions is far more complex. For example, the positive effect of D2 receptor stimulation on cognitive flexibility is limited to subjects with low basal dopaminergic tone and might be opposite in those with high dopamine synthesis capacity (Klanker et al., 2013). Furthermore, cognitive control functions are modified by complex interactions between the dopamine, norepinephrine, serotonin and cholinergic systems (Logue and Gould, 2014). Therefore, although investigating effects of EGb 761® on behavior and BOLD activation in cognitive tasks assumed to involve the dopaminergic system can constitute valuable suggestions about neurotransmission systems underlying treatment effects, inference from psychological effects onto the mesocortical dopaminergic system is not straightforward. Thus, future human trials should more directly assess effects of EGb 761® on dopaminergic systems.
Conflict of Interest
The authors have declared no conflict of interest with the exception of T.G. who received a honorarium as a lecturer and consultant for Dr. Willmar Schwabe GmbH & Co. KG.
Ethical Standards
The authors assert that all procedures contributing to this work comply with ethical principles of Good Clinical Practice complying with the Declaration of Helsinki of 1975, as revised in 2008, the ICH‐GCP‐Guidelines and national regulations governing the conduct of clinical studies. The protocol and informed consent documents were approved by the competent federal authority (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM; EudraCT‐Nr.: 2009‐017851‐90) and given favorable opinion by the Ethics Committee of the Medical Faculty of the Technical University Dresden.
Source of Funding
MB is employee of Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany, manufacturer of EGb 761® and sponsor of this trial. Funding of the project by Dr. Willmar Schwabe GmbH & Co. KG included consumables, participant payment, salaries for student assistance and PhD students.
Supporting information
Figure S1: Subject disposition
Table S1. Task set switching fMRI results: Results from the whole brain analysis of pre‐treatment data for task switch related activation [Switch_Pre − Repeat_Pre], initial voxel level threshold of p < 0.05 FWE whole brain corrected.
Table S2. Go–Nogo task fMRI results: Results from the whole brain analysis of pre‐treatment data for response inhibition related activation [NoGo_Pre − Go_Pre], initial voxel level threshold of p < 0.001 FWE whole brain corrected.
Table S3.1 Switch effects delayed response task (fMRI results): Results from the whole brain analysis of pre‐treatment data for Switch related activation during the Delayed Response task [Switch_Pre − Repeat_Pre], initial voxel level threshold of p < 0.05 FWE whole brain corrected.
Table S3.2 Distractor effects delayed response task (fMRI results): Results from the whole brain analysis of pre‐treatment data for distractor related activation from the Delayed Response task [Distractor_Pre − Scrambled Picture_Pre], initial voxel level threshold of p < 0.05 FWE whole brain corrected.
Table S4. Prospective memory task fMRI results: Results from whole brain analysis of pre‐treatment data for event‐related activations during PM cue presentation [PM block (PM cue − Ongoing trail) − baseline block (red arrow/later PM cue − Ongoing trial)], initial voxel level threshold of p < 0.001 FWE corrected. PM = Prospective Memory.
Supporting info item
ACKNOWLEDGEMENTS
We thank Bianca Fricke for outstanding support with participant recruitment, psychometric‐testing and organizational issues.
Beck, S. M. , Ruge, H. , Schindler, C. , Burkart, M. , Miller, R. , Kirschbaum, C. , and Goschke, T. (2016) Effects of Ginkgo biloba extract EGb 761® on cognitive control functions, mental activity of the prefrontal cortex and stress reactivity in elderly adults with subjective memory impairment – a randomized double‐blind placebo‐controlled trial. Hum. Psychopharmacol Clin Exp, 31: 227–242. doi: 10.1002/hup.2534.
German Clinical Trials Registry DRKS00000423
References
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. 2007. Converging evidence for a fronto‐basal‐ganglia network for inhibitory control of action and cognition. The Journal of Cognitive Neuroscience 27(44): 11860–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi C. (2002). Validierung der neuropsychologischen Testbatterie CERAD‐NP. Eine Multi‐Center Studie. [Validation of the neuro‐psychological test battery CERAD‐NP. A multicenter study.]. PhD Thesis, Philosophical Faculty, University of Basel, Switzerland.
- Arnau RC, Meagher MW, Norris MP, Bramson R. 2001. Psychometric evaluation of the Beck Depression Inventory‐II with primary care medical patients. Health Psychol 20(2): 112–119. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. 2009. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10(6): 410–422. DOI:10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster D, Mueller A, Strobel A, Lesch KP, Brocke B, Kirschbaum C. 2012. Children under stress—COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. Int J Neuropsychopharmacol 15: 1229–1239. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Wang MJ, Paspalas CD. 2012. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76(1): 223–239. DOI:10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. 2009. Executive function. The search for an integrated account. Curr Dir Psychol Sci 18: 89–94. [Google Scholar]
- Beck SM, Ruge H, Walser M, Goschke T. 2014. The functional neuroanatomy of spontaneous retrieval and strategic monitoring of delayed intentions. Neuropsychologia 52: 37–50. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory‐II. Psychological Corporation: San Antonio, TX. [Google Scholar]
- Bloemendaal M, van Schouwenburg MR, Miyakawa A, Aarts E, D'Esposito M, Cools R. 2015. Dopaminergic modulation of distracter‐resistance and prefrontal delay period signal. Psychopharmacology (Berl) 232: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Brondino N, De Silvestri A, Re S, et al. 2013. A systematic review and meta‐analysis of Ginkgo biloba in neuropsychiatric disorders: from ancient tradition to modern‐day medicine. Evid Based Complement Alternat Med 2013: 915691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Gonen‐Yaacovi G, Volle E. 2011. Functional neuroimaging studies of prospective memory: what have we learnt so far? Neuropsychologia 49: 2246–2257. [DOI] [PubMed] [Google Scholar]
- Cabeza R. 2001. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol 42: 277–286. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA. 2013. Frontal lobes and aging: deterioration and compensation In Principles of Frontal Lobe Function, 2nd edn, Stuss DT, Knight RT. (eds). Oxford University Press: New York. [Google Scholar]
- Chamberlain SR, Robbins TW. 2013. Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol 27: 694–718. [DOI] [PubMed] [Google Scholar]
- Cieza A, Maier P, Poppel E. 2003. Effects of Ginkgo biloba on mental functioning in healthy volunteers. Arch Med Res 34: 373–381. [DOI] [PubMed] [Google Scholar]
- Cools R. 2008. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist 14(4): 381–395. DOI:10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. 2011. Inverted‐U‐shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69(12): e113–125. DOI:10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. 2007. Impulsive personality predicts dopamine‐dependent changes in frontostriatal activity during component processes of working memory. J Neurosci 27: 5506–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Peppe A, Brusa L, Caltagirone C, Gatto I, Carlesimo GA. 2008. Levodopa improves time‐based prospective memory in Parkinson's disease. J Int Neuropsychol Soc 14: 601–610. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Smith G, Maylor EA, Della Sala S, Logie RH. 2003. The Prospective and Retrospective Memory Questionnaire (PRMQ): normative data and latent structure in a large non‐clinical sample. Memory 11: 261–275. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD, Ward AL, Blake J. 2006. The Prospective and Retrospective Memory Questionnaire (PRMQ): latent structure, normative data and discrepancy analysis. Br J Clin Psychol 45: 83–104. [DOI] [PubMed] [Google Scholar]
- Dang LC, Donde A, Madison C, O'Neil JP, Jagust WJ. 2012. Striatal dopamine influences the default mode network to affect shifting between object features. J Cogn Neurosci 24: 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doruk A, Uzun O, Ozsahin A. 2008. A placebo‐controlled study of extract of ginkgo biloba added to clozapine in patients with treatment‐resistant schizophrenia. Int Clin Psychopharmacol 23: 223–227. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. 2004. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cognit 30(2): 343–353. DOI:10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. 2008. The dual‐state theory of prefrontal cortex dopamine function with relevance to catechol‐o‐methyltransferase genotypes and schizophrenia. Biol Psychiatry 64(9): 739–749. DOI:10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. 2011. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry 68: 845–852. [DOI] [PubMed] [Google Scholar]
- Evans S, Gray MA, Dowell NG, et al. 2013. APOE E4 carriers show prospective memory enhancement under nicotine, and evidence for specialisation within medial BA10. Neuropsychopharmacology 38: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehske CJ, Leuner K, Mueller WE. 2009. Ginkgo biloba extract (EGb 761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol Res 60: 68–73. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 1995. The Structured Clinical Interview for DSM–III–R Personality Disorders (SCID–II). Part I: description. J Pers Disord 9: 83–91. DOI:10.1521/pedi.1995.9.2.83. [Google Scholar]
- Floresco SB. 2013. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted‐U” toward a family of functions. Front Neurosci 7: 62 DOI:10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. 1995. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 2(2): 166–172. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Schlaefke S. 2014. Efficacy and tolerability of Ginkgo biloba extract EGb 761(R) in dementia: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Clin Interv Aging 9: 2065–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova SI, Preuss UW, Wong JWM, et al. 2014. Efficacy and safety of Ginkgo biloba extract EGb 761® in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo‐controlled, double‐blind, multi‐center trial. Int J Geriatr Psychiatr 29(10): 1087–1095. [DOI] [PubMed] [Google Scholar]
- Goschke T, Bolte A. 2014. Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia 62: 403–423. DOI:10.1016/j.neuropsychologia.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Goschke T, Dreisbach G. 2008. Conflict‐triggered goal shielding: response conflicts attenuate background monitoring for concurrent prospective memory cues. Psychol Sci 19: 25–32. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. 2008. Dopamine modulation of hippocampal‐prefrontal cortical interaction drives memory‐guided behavior. Cereb Cortex 18: 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass‐Kapanke B, Busmane A, Lasmanis A, Hoerr R, Kaschel R. 2011. Effects of Ginkgo Biloba Special Extract EGb 761® in Very Mild Cognitive Impairment (vMCI). Neurosci Med 2: 48–56. [Google Scholar]
- Gurvich CT, Rossell SL. 2014. Genetic variations in dopamine and inhibitory control: lack of influence on action restraint. Behav Brain Res 267: 12–16. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, et al. 2007. Verbal paired‐associate learning by APOE genotype in non‐demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging 28: 238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Bangen K, Bondi MW. 2009. Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Alzheimer's disease: review and recommendations. Dement Geriatr Cogn Disord 27: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M, Keller F, Kühner C. 2009. BDI‐II. Beck‐Depressions‐Inventar. Revision, 2nd. edn. Pearson Assessment: Frankfurt on Main, Germany. [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ. 2013. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol 27: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaus D, Collip D, Lataster J, et al. 2013. COMT Val158Met genotype selectively alters prefrontal [18 F]fallypride displacement and subjective feelings of stress in response to a psychosocial stress challenge. PLoS One 8: e65662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen IM, Sturtz S, Skipka G, Zentner A, Velasco Garrido M, Busse R. 2010. Ginkgo biloba in Alzheimer's disease: a systematic review. Wiener Medizinische Wochenschrift 160: 539–46. [DOI] [PubMed] [Google Scholar]
- Jezova D, Duncko R, Lassanova M, Kriska M, Moncek F. 2002. Reduction of rise in blood pressure and cortisol release during stress by Ginkgo biloba extract (EGb 761) in healthy volunteers. J Physiol Pharmacol 53: 337–48. [PubMed] [Google Scholar]
- Kang JI, Kim SJ, Song YY, Namkoong K, An SK. 2013. Genetic influence of COMT and BDNF gene polymorphisms on resilience in healthy college students. Neuropsychobiology 68: 174–180. [DOI] [PubMed] [Google Scholar]
- Kaschel R, Hörr R, Kresimon J, Rychlik R. 2007. Einfluss von Ginkgo‐Spezialextrakt EGn 761® auf die Leistungsfähigkeit bei gesunden Probanden am Bildschirmarbeitsplatz—offene klinische Studie im prä‐post‐Design mit Kontrollgruppe. J Pharmakol Ther 16: 3–9. [Google Scholar]
- Kaschel R. 2009. Ginkgo biloba: specificity of neuropsychological improvement—a selective review in search of differential effects. Hum Psychopharmacol 24: 345–70. [DOI] [PubMed] [Google Scholar]
- Kaschel R. 2011. Specific memory effects of Ginkgo biloba extract EGb 761 in middle‐aged healthy volunteers. Phytomedicine 18: 1202–7. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. 1993. The ‘Trier Social Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychology 28: 76–81. [DOI] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D. 2013. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci 7: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement S, Bock N, Rothenberger A. (2011). Ginkgo biloba extract EGb 761® is effective and safe in the treatment of ADHD in children. World Federation on ADHD, 3rd International Congress on ADHD—From Childhood to Adult Disease. 26 – 29 May 2011 Berlin, Germany, P‐35‐06.
- Kliegel M, Jäger T. 2006. Can the Prospective and Retrospective Memory Questionnaire (PRMQ) predict actual prospective memory performance? Curr Psychol 25: 182–191. [Google Scholar]
- Kuhl J. 1994. Action and state orientation: psychometric properties of the action control scales (ACS‐90) In Volition and Personality: Action Versus State Orientation, Kuhl J, Beckmann J. (eds). Hogrefe: Göttingen, Germany; 47–59. [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. 2009. Striatal dopamine and working memory. Cereb Cortex 19: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws KR, Sweetnam H, Kondel TK. 2012. Is Ginkgo biloba a cognitive enhancer in healthy individuals? A meta‐analysis. Hum Psychopharmacol 27: 527–33. [DOI] [PubMed] [Google Scholar]
- Leyton M, aan het RM, Booij L, Baker GB, Young SN, Benkelfat C. 2007. Mood‐elevating effects of d‐amphetamine and incentive salience: the effect of acute dopamine precursor depletion. J Psychiatry Neurosci 32: 129–136. [PMC free article] [PubMed] [Google Scholar]
- Lo BCY, Allen NB. 2011. Affective bias in internal attentional shifting among depressed youth. Psychiatry Res 187: 125–129. DOI:10.1016/j.psychres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Logue SF, Gould TJ. 2014. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav 123: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, et al. 2014. Neuropathology of stress. Acta Neuropathol 127: 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, Hester R, et al. 2013. The role of dopamine in inhibitory control in smokers and non‐smokers: a pharmacological fMRI study. Eur Neuropsychopharmacol 23: 1247–1256. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. 2007. The contribution of sleep to hippocampus‐dependent memory consolidation. Trends Cogn Sci 11: 442–450. [DOI] [PubMed] [Google Scholar]
- Meiran N, Diamond GM, Toder D, Nemets B. 2011. Cognitive rigidity in unipolar depression and obsessive compulsive disorder: examination of task switching, Stroop, working memory updating and post‐conflict adaptation. Psychiatry Res 185(1‐2): 149–156. DOI:10.1016/j.psychres.2010.04.044. [DOI] [PubMed] [Google Scholar]
- Meiran N, Chorez Z, Sapir A. 2000. Component processes in task switching. Cogn Psychol 41: 211–253. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. 1999. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatr 156: 257–289. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Mix JA, Crews WD, Jr . 2002. A double‐blind, placebo‐controlled, randomized trial of Ginkgo biloba extract EGb 761 in a sample of cognitively intact older adults: neuropsychological findings. Hum Psychopharmacol 17: 267–277. [DOI] [PubMed] [Google Scholar]
- Moeller CK, Kurt S, Scheich H, Schulze H. 2009. Improvement of auditory discrimination learning by Ginkgo biloba extract EGb 761. Neurosci Lett 463: 219–22. [DOI] [PubMed] [Google Scholar]
- Monsell S. 2003. Task switching. Trends Cogn Sci 7: 134–140. [DOI] [PubMed] [Google Scholar]
- Moulton PL, Boyko LN, Fitzpatrick JL, Petros TV. 2001. The effect of Ginkgo biloba on memory in healthy male volunteers. Physiol Behav 73: 659–65. [DOI] [PubMed] [Google Scholar]
- Müller J, Dreisbach G, Brocke B, Lesch KP, Strobel A, Goschke T. 2007a. Dopamine and cognitive control: The influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set‐shifting. Brain Res 1131(1): 155–162. DOI:10.1016/j.brainres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Müller J, Dreisbach G, Goschke T, Hensch T, Lesch K‐P, Brocke B. 2007b. Dopamine and cognitive control: the prospect of monetary gains influences the balance between flexibility and stability in a set‐shifting paradigm. Eur J Neurosci 26(12): 3661–3668. DOI:10.1111/j.1460-9568.2007.05790.x. [DOI] [PubMed] [Google Scholar]
- Müller WE, Heiser J, Leuner K. 2012. Effects of the standardized Ginkgo biloba extract EGb 761® on neuroplasticity. Int Psychogeriatr 24(Suppl 1): S21–4. [DOI] [PubMed] [Google Scholar]
- Mulligan RC, Kristjansson SD, Reiersen AM, Parra AS, Anokhin AP. 2014. Neural correlates of inhibitory control and functional genetic variation in the dopamine D4 receptor gene. Neuropsychologia 62: 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. 2011. A unified framework for inhibitory control. Trends Cogn Sci 15(10): 453–459. DOI:10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, et al. 2013. Dopamine D(2) receptor modulation of human response inhibition and error awareness. J Cogn Neurosci 25: 649–656. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Ricketts E, Wesnes K, Mrazek L, Greville W, Stough C. 2002. The acute nootropic effects of Ginkgo biloba in healthy older human subjects: a preliminary investigation. Hum Psychopharmacol 17: 45–9. [DOI] [PubMed] [Google Scholar]
- Persson J, Bringlöv E, Nilsson LG, Nyberg L. 2004. The memory‐enhancing effects of Ginseng and Ginkgo biloba in healthy volunteers. Psychopharmacology (Berlin) 172: 430–4. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Rujescu D, Giegling I, et al. 2008. Psychometrische Evaluation der deutschsprachigen Version der Barratt‐Impulsivness‐Skala. [Psychometric evaluation of the German version of the Barratt Impulsiveness Scale]. Nervenarzt 79: 305–319. [DOI] [PubMed] [Google Scholar]
- Rojas P, Ruiz‐Sanchez E, Rojas C, Ogren SO. 2012. Ginkgo biloba extract (EGb 761) modulates the expression of dopamine‐related genes in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐induced Parkinsonism in mice. Neuroscience 223: 246–257. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. 2009. The neuropsychopharmacology of fronto‐executive function: monoaminergic modulation. Annu Rev Neurosci 32: 267–287. DOI:10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Jamadar S, Zimmermann U, Karayanidis F. 2013. The many faces of preparatory control in task switching: reviewing a decade of fMRI research. Hum Brain Mapp 34(1): 12–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted J, Ruest T, Gray MA. 2011. Acute effects of nicotine administration during prospective memory, an event related fMRI study. Neuropsychologia 49: 2362–2368. [DOI] [PubMed] [Google Scholar]
- Schulz P, Schlotz W. 1999. Das Trierer Inventar zur Erfassung von chronischem Streß (TICS): Skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. Diagnostica 45: 8–19. [Google Scholar]
- Solomon PR, Adams F, Silver A, Zimmer J, DeVeaux R. 2002. Ginkgo for memory enhancement: a randomized controlled trial. JAMA 288: 835–40. [DOI] [PubMed] [Google Scholar]
- Spieß E, Juretzek W, Schulz V. 2014. Ginkgo‐biloba‐Blätter In: Blaschek W, Ebel S, Hilgenfeldt U, Jiang T, Reichling J. Hagers Enzyklopädie der Arzneistoffe und Drogen. http://www.drugbase.de/de/datenbanken/hagers‐enzyklopaedie.html. Accessed 16.03.2015. Stuttgart, Wissenschaftliche Verlagsgesellschaft. [Google Scholar]
- Sreenivasan KK, Curtis CE, D'Esposito M. 2014. Revisiting the role of persistent neural activity during working memory. Trends Cogn Sci 18(2): 82–89. DOI:10.1016/j.tics.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. 1997. Der Mehrdimensionale Befindlichkeitsbogen (MDBF). Hogrefe: Göttingen, Germany. [Google Scholar]
- Stokes MG, Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. 2013. Dynamic coding for cognitive control in prefrontal cortex. Neuron 78: 364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SY, Hsieh CL, Wu SL, et al. 2009. Transcriptomic analysis of EGb761‐regulated neuroactive receptor pathway in vivo. J Ethnopharmacol 123: 68–73. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. 2009. Cortical midline involvement in autobiographical memory. Neuroimage 44: 1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MS, Yu JT, Tan CC, et al. 2015. Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta‐analysis. J Alzheimers Dis 43: 589–603. [DOI] [PubMed] [Google Scholar]
- van Holstein HM, Aarts E, van der Schaaf ME, et al. 2011. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl) 218: 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg MR, Aarts E, Cools R. 2010. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and basal ganglia. Curr Pharml Des 16(18): 2026–2032. [DOI] [PubMed] [Google Scholar]
- Waford RN, Lewine R. 2010. Is perseveration uniquely characteristic of schizophrenia? Schizophr Res 118: 128–133. DOI:10.1016/j.schres.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence™ and Wasi™ (WASI™) In The Psychological Corporation®. San Antonio, Tx: Pearson. [Google Scholar]
- Weinmann S, Roll S, Schwarzbach C, Vauth C, Willich SN. (2010). Effects of Ginkgo biloba in dementia: systematic review and meta‐analysis. BMC Geriatr 10:14. Published online 2010 March 17. doi: 10.1186/1471-2318-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer AJ, Banich MT. 2007. Inhibition versus switching deficits in different forms of rumination. Psychol Sci 18(6): 545–553. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, Gotlib IH. 2012. Switching and backward inhibition in major depressive disorder: the role of rumination. J Abnorm Psychol 121(3): 570–8. DOI:10.1037/a0027474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KY, Wu CH, Tsai YF. 2011. Ginkgo biloba treatment increases copulation but not nNOS activity in the medial preoptic area in male rats. Neurosci Lett 18(500): 182–6. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Kehr J. 2010. The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br J Pharmacol 159: 659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WF, Tan YL, Zhang XY, Chan RC, Wu HR, Zhou DF. 2011. Extract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: a randomized, double‐blind, placebo‐controlled trial. J Clin Psychiatr 72: 615–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Subject disposition
Table S1. Task set switching fMRI results: Results from the whole brain analysis of pre‐treatment data for task switch related activation [Switch_Pre − Repeat_Pre], initial voxel level threshold of p < 0.05 FWE whole brain corrected.
Table S2. Go–Nogo task fMRI results: Results from the whole brain analysis of pre‐treatment data for response inhibition related activation [NoGo_Pre − Go_Pre], initial voxel level threshold of p < 0.001 FWE whole brain corrected.
Table S3.1 Switch effects delayed response task (fMRI results): Results from the whole brain analysis of pre‐treatment data for Switch related activation during the Delayed Response task [Switch_Pre − Repeat_Pre], initial voxel level threshold of p < 0.05 FWE whole brain corrected.
Table S3.2 Distractor effects delayed response task (fMRI results): Results from the whole brain analysis of pre‐treatment data for distractor related activation from the Delayed Response task [Distractor_Pre − Scrambled Picture_Pre], initial voxel level threshold of p < 0.05 FWE whole brain corrected.
Table S4. Prospective memory task fMRI results: Results from whole brain analysis of pre‐treatment data for event‐related activations during PM cue presentation [PM block (PM cue − Ongoing trail) − baseline block (red arrow/later PM cue − Ongoing trial)], initial voxel level threshold of p < 0.001 FWE corrected. PM = Prospective Memory.
Supporting info item


