Abstract
Background
Systemic hypertension and proteinuria are established adverse effects of tyrosine kinase inhibitor treatment in people.
Objective
The objective of this study was to investigate changes in systolic blood pressure and the incidence of proteinuria secondary to treatment with toceranib phosphate in dogs with cancer.
Animals
Twenty‐six control dogs and 30 dogs with cancer were evaluated for the first part of the study (baseline characteristics). For the second part (effect of toceranib phosphate treatment), 48 client‐owned dogs were evaluated, including 20 control dogs and 28 dogs with various types of neoplasia.
Methods
Prospective cohort study. Client‐owned healthy control dogs and dogs with cancer were enrolled. Blood pressure and urine protein:creatinine ratios were measured before treatment and 2 weeks after initiation of toceranib phosphate treatment.
Results
Systolic blood pressure was significantly (P = 0.0013) higher in previously normotensive treatment dogs after initiation of treatment with toceranib phosphate (152 mmHg ± 19) compared to baseline (136 mmHg ± 14). 37% of treated dogs developed SBP ≥ 160 mmHg. The prevalence of systemic hypertension (37%) and proteinuria (21%) at baseline in treatment dogs did not differ from that of age‐matched healthy controls (15% [P = 0.13] and 0% [P = 0.069], respectively).
Conclusions and Clinical Importance
Toceranib phosphate treatment might result in increased systolic blood pressures in dogs. Systemic hypertension should be considered a potential adverse effect of this drug in dogs. Systemic hypertension and proteinuria were detected at clinically relevant frequencies in the dogs with cancer before antineoplastic therapies suggesting that monitoring of these variables might be warranted in this population.
Keywords: Angiogenesis inhibitors, Chemotherapy, Systemic hypertension, Tyrosine kinase inhibitors
Abbreviations
- ACEi
angiotensin‐converting enzyme inhibitor
- HT
systemic hypertension
- NO
nitric oxide
- NSAID
nonsteroidal anti‐inflammatory drugs
- SBP
systolic blood pressure
- TKI
tyrosine kinase inhibitors
- UPC
urine protein to creatinine ratio
- VEGF
vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) plays a key role in tumor neoangiogenesis, which is essential for tumor growth and metastasis.1 Tyrosine kinase inhibitors (TKI) have been developed as antineoplastic therapies in humans and dogs, with some TKI being capable of inhibiting the VEGF pathway.2, 3 Toceranib phosphate1 is a TKI that was approved by the FDA for use in the treatment of mast cell tumors in dogs in 2009 and has subsequently been found to have activity against other tumor types.2
Systemic hypertension (HT) has emerged as a frequent adverse effect in people treated with TKI.3, 4 Hypertension occurs in 16% to 47% of human patients treated with sunitinib or sorafenib.5, 6, 7, 8, 9 The exact mechanism of TKI‐induced HT is not fully understood, but decreased nitric oxide (NO) bioavailability appears to be an essential component.3 VEGF signaling results in increased production and release of NO by endothelial cells.10 NO is a potent vasodilator that plays an important role in maintaining normal vascular tone.11 Decreased NO production mediated by VEGF pathway inhibitors like TKI results in increased vasoconstriction and subsequently increased vascular resistance leading to HT.12 Other mechanisms might also contribute to TKI‐induced HT and include arterial rarefaction, increased arterial stiffness and renal thrombotic microangiopathy.13, 14, 15, 16
In addition to HT, TKI might result in other adverse effects in people including proteinuria, thrombosis, and hemorrhage.17 The pathogenesis of TKI‐induced proteinuria is not fully understood. VEGF has been shown to play a role in podocyte survival as well as maintaining the integrity of the renal endothelial cell lining.18, 19 In mice, decreased VEGF concentrations result in a glomerulopathy associated with proteinuria, endotheliosis, and hyaline deposits.20 Hypertension might also contribute to the proteinuria observed in people taking TKI.
Human oncology patients are screened regularly for potential complications during treatment with TKI. Screening for proteinuria and HT is recommended before the administration of TKI and blood pressure is monitored regularly while patients are treated with these agents. Most patients that develop TKI‐induced HT are responsive to antihypertensive agents.21 Patients with severe or persistent HT that fail to respond to antihypertensive treatment might require a temporary or permanent discontinuation of treatment with TKI to stabilize their blood pressure.
Although multiple studies have found the use of TKI in people to be associated with HT, minimal information exists on this potential relationship in companion animals. To date, the reported adverse effects of toceranib phosphate in dogs include diarrhea, anorexia, vomiting, musculoskeletal pain, weakness, weight loss, occult blood in stool, lethargy, neutropenia and dermal abnormalities.2 We recently showed that the use of toceranib phosphate was associated with increases in systolic blood pressure (SBP) in the majority of dogs in a small preliminary retrospective study, and that 40% of the dogs with cancer in the retrospective study had previously undiagnosed HT at enrollment.2 The primary objective of this study was to investigate the effect of toceranib phosphate on systolic blood pressure and incidence of proteinuria prospectively in dogs with cancer. Secondary objectives included comparison of the prevalence of systemic HT and proteinuria in the treatment population of dogs with cancer before treatment to prevalence of systemic HT and proteinuria in age‐matched healthy control dogs.
Materials and Methods
Dogs
Client‐owned dogs were prospectively enrolled by the UW Veterinary Care cardiology and oncology services between February 2014 and July 2015. All owners signed an informed consent form giving permission to participate in the study. The study was approved by the University of Wisconsin Animal Care and Use Committee. Control dogs ≥8 years of age were recruited to act as a control group. Exclusion criteria included known renal disease or systemic hypertension measured at the time of screening, clinically relevant systemic disease, evidence of urinary tract infection on baseline screening or current treatment with any vasoactive medications, including but not limited to vasodilators or phenylpropanolamine. Dogs that were to receive toceranib phosphate for treatment of various neoplastic diseases were recruited for screening. Exclusion criteria for prospective treatment dogs included evidence of urinary tract infection on baseline screening urinalysis or current treatment with any vasoactive medications. Dogs that were enrolled but did not receive toceranib phosphate consistently between the two visits were excluded from treatment analyses.
Study Design
This study was designed as a prospective cohort study to investigate changes in SBP and the incidence of proteinuria in dogs treated with toceranib phosphate compared to healthy age‐matched controls at Day 0 and Day 14. A baseline evaluation was performed on all dogs on Day 0. Control dogs had history and physical examination results recorded to exclude the presence of overt systemic disease. Treatment dogs were evaluated by the veterinary oncologist in charge of each dog's clinical care. Day 0 study evaluation included blood pressure assessment, urinalysis, and urine protein to creatinine ratio (UPC) assessment. Blood pressure was measured using oscillometric or Doppler techniques as previously described.22, 23 At each assessment event, 6 measurement repetitions were recorded; the initial SBP reading was discarded and the SBP recorded as representative for that event was the mean of the last 5 values obtained. Hypertension was defined as SBP ≥ 160 mmHg and proteinuria was defined as UPC ≥ 0.5, as previously recommended by ACVIM consensus statements.24, 25 Blood pressure assessment was repeated within 6 hours in patients with initial SBP ≥ 160 mmHg. If the SBP remained elevated further evaluation was recommended to screen for causative disease and hypertensive injury, including fundic examination.
At the time of screening, apparently healthy dogs that were diagnosed with HT were excluded from further analysis (n = 4). Dogs scheduled to received toceranib phosphate treatment were divided into 2 groups based on SBP recorded at screening: dogs that were normotensive were assigned to Group 2a and dogs diagnosed with HT were assigned to Group 2b. After baseline evaluation, dogs enrolled in Group 2a received toceranib phosphate at a standard dose as previously published.2 Group 2b dogs received toceranib phosphate as described, but were also treated with standard‐of‐care treatment for HT, including amlodipine besylate (0.2–0.4 mg/kg PO q 24 hours), enalapril maleate (0.5 mg/kg PO q 12 hours), or both, initiated at the discretion of the attending veterinarian. Some dogs were treated with additional medications, including corticosteroids, at the discretion of the oncologist. All dogs were re‐evaluated on Day 14 (±3 days) at which time a UPC and blood pressure measurements were performed.
Statistical Analysis
All statistical analyses were performed using commercially available software.3 P values ≤0.05 were considered significant. Weight and age on Day 0 and SBP and UPC on Day 0 and Day 14 were tested for normal distribution (D'Agostino & Pearson omnibus normality test). SBP values were normally distributed but normal distribution could not be assumed for UPC data. Baseline data for all dogs weres compared between control dogs and treatment dogs. Treatment dogs were then divided into two groups based on initial SBP, and subsequent analyses included 3 groups, Group 1 (control dogs), Group 2a (normotensive treatment dogs), and Group 2b (hypertensive treatment dogs). When comparing control and treatment groups at baseline, a paired Student's t‐test or Wilcoxon signed rank test was used to compare mean or median values for paired data as appropriate. For unpaired data comparisons, a Student's t‐test or Mann‐Whitney U‐test was used. Proportional comparisons between control and treatment groups were evaluated with Fisher's exact test. Day 0 and Day 14 SBP and UPC values were compared in each group using a paired Student's t‐test or Wilcoxon signed rank test. One‐way ANOVA or Kruskal‐Wallis testing was used to compare means or medians among Group 1, Group 2a and Group 2b dogs, at Day 1 and Day 14. When differences among the groups were detected, Tukey's multiple comparison test was used as a posthoc analysis to identify groups that differed significantly (P < 0.05).
Results
Enrollment
Twenty‐six control dogs were initially enrolled; 6 dogs were excluded due to HT detected at first visit (n = 4), presence of a UTI (n = 1) and 1 dog was lost to follow‐up, resulting in 20 control dogs available for analysis (Group 1). Thirty dogs with cancer were enrolled in the initial treatment group (Groups 2a and 2b). One dog each in Group 2a and Group 2b had their data excluded from analysis because they did not receive toceranib phosphate consistently between visit 1 and visit 2. Of the 28 remaining treatment dogs screened, 18 were normotensive (Group 2a) at baseline and 10 were hypertensive (Group 2b) at baseline. The UPC data from 6 of the 10 dogs in Group 2b were censored from analysis because a UPC was not performed at visit 1 (n = 1) or visit 2 (n = 3) or because a urinary tract infection was detected at visit 1 (n = 2). Due to an incomplete data set, UPC data for dogs in Group 2b at baseline were not analyzed.
Population at Screening
Descriptive statistics for the control dogs (n = 26) and treatment dogs (n = 28) at screening are presented in Table 1. The neoplasia type for the 28 treatment dogs was carcinoma (n = 9), mast cell tumor (n = 6), hemangiosarcoma (n = 5), osteosarcoma (n = 4), neuroblastoma, melanoma, soft tissue sarcoma, and large cell lymphoma (n = 1 each). Four dogs in total were receiving prednisone at the time of enrollment and during the study period; 3 dogs from Group 2a and 1 dog from Group 2b. There was no difference in the gender distribution or median age between control dogs and treatment dogs. Median weight of the control dogs was significantly lower than that of treatment dogs (P = 0.012). The number of days elapsed between visit 1 and visit 2 was not statistically different (P = 0.314) between control dogs (n = 20) and treatment dogs (n = 28).
Table 1.
Screening visit information in healthy dogs (“Control”) and dogs with cancer (“Treatment”)
| Controla (n = 26) | Treatmentb (n = 30) | P value | |
|---|---|---|---|
| Sex | |||
| Male | n = 13 | n = 15 | 1.0 |
| Female | n = 13 | n = 15 | |
| Age (months) | 314 (±53) | 293 (±76) | 0.24 |
| Weight (kg) | 20.4 (±10.4) | 28.6 (±13.0) | 0.012 |
| SBP 1 (mmHg) | 143 (±17) | 153 (±25) | 0.16 |
| HR 1 (bpm) | 109 (±23) | 118 (±29) | 0.17 |
| UPC 1 | 0.04 [0.01–0.33] | 0.05 [0.01–12.9] | 0.066 |
| % Systolic HT | 15% | 37% | 0.13 |
| % Proteinuric | 0% | 21% | 0.069 |
Systolic HT: SBP ≥ 160 mmHg, Proteinuria: UPC ≥ 0.5.
Parametric data are expressed as mean (±SD); nonparametric data are expressed as median [range].
Controls, healthy dogs screened for control group.
Treatment, dogs with cancer screened before treatment with toceranib phosphate.
At screening, the mean SBP and median UPC did not differ between control and treatment groups. The frequency of proteinuria (UPC > 0.5) in the control group (0/20 dogs, 0%) did not differ from that in the treatment group (6/28 dogs, 21%, P = 0.069). Four of 26 control dogs (15%) were diagnosed with HT versus 11/30 (37%) dogs in the treatment group (P = 0.13).
Time Point Comparisons
Mean SBP and median UPC did not differ in Group 1 dogs between visit 1 and visit 2 (Table 2). In Group 2a dogs, mean SBP was significantly higher at visit 2 (P = 0.0013), but median UPC was unchanged (P = 0.51). In Group 2a, 12/18 (67%) dogs experienced an increase in SBP > 10 mmHg, and 6/18 (33%) had a > 20 mmHg increase in SBP. In Group 2b dogs, mean SBP was unchanged between visit 1 and visit 2; there were insufficient data to compare UPC in this group. In Group 2b, 3/10 (30%) dogs had a > 10 mmHg increase in SBP and 2/10 (20%) had a > 20 mmHg increase. The frequency of abnormal UPC in Group 2a was 2/18 (11%) at visit 1 and 4/18 (22%) at visit 2 (P = 0.66).
Table 2.
Comparison of visit 1 versus. visit 2 for three study groups
| Group 1 (n = 20) | Group 2a (n = 18) | Group 2b (n = 10)* | P value | |
|---|---|---|---|---|
| SBP (mmHg) | ||||
| Visit 1 | 138 (±13)* | 136 (±14)* | 177 (±12)∧ | <0.0001 |
| Visit 2 | 142 (±20)* | 152 (±19)*∧ | 171 (±27)∧ | 0.0036 |
| P value (SBP) | 0.53 | 0.0013 | 0.44 | |
| HR (bpm) | ||||
| Visit 1 | 104 (±23) | 109 (±27) | 126 (±28) | 0.094 |
| Visit 2 | 97 (±20) | 103 (±26) | 112 (±29) | 0.28 |
| P value (HR) | 0.094 | 0.14 | 0.18 | |
| % Sys HT Visit 2† | 20% | 28% | NA∧ | 0.709 |
| Chg BP# (mmHg) | +4 (±25)*∧ | +16 (±17)* | −6 (±25)∧ | 0.048 |
| UPC | ||||
| Visit 1 | 0.03 [0.01–0.33] | 0.07 [0.02–12.90] | Insufficient data | 0.042 |
| Visit 2 | 0.03 [0.01–0.41] | 0.06 [0.01–19.23] | Insufficient data | 0.014 |
| P value (UPC) | 0.53 | 0.51 | NA | |
| %Abn UPC | ||||
| Visit 1 | 0% | 11% | NA | 0.22 |
| Visit 2 | 0% | 22% | NA | 0.042 |
| P value (Abn UPC) | NA | 0.66 | NA | |
*n = 7 for the UPC data for Group 2b at visit 1. Three dogs were excluded from this analysis. See text for further information. ∧not analyzed, all dogs in category HT by design, †percent of dogs normotensive at previous visit that had SBP ≥ 160 mmHg at visit 2, #Mean (SD) difference in SBP between visit 1 and visit 2. SBP, systolic blood pressure; HR, heart rate; UPC, urine protein:creatinine ratio; NS, no significant difference (significance defined as P ≤ 0.05); NA, not applicable. Parametric data are expressed as mean (±SD); nonparametric data are expressed as median [range]. Groups with differing superscripts are significantly different at the P value indicated. HT, percentage of dogs with systemic hypertension: ≥160 mmHg; Abn UPC: UPC ≥ 0.5.
Group Comparisons
SBP at baseline was significantly higher in Group 2b dogs compared to both Group 1 and Group 2a dogs (P < 0.0001, Table 2, Fig 1). SBP at Day 14 was significantly higher in Group 2b dogs compared to Group 1 dogs (P = 0.004), but there was no difference between Group 2a and Group 2b (Table 2, Fig 1). Four of 20 (20%) Group 1 dogs had SBP ≥ 160 mmHg at visit 2 compared to 5/18 (28%) of Group 2a dogs (P = 0.709). The mean (SD) change in SBP between visits 1 and 2 did not differ between Group 1 and Group 2a, but the mean change in SBP in Group 2b (−6 mmHg) differed significantly from Group 2a (+16 mmHg, P = 0.048), but not Group 1 (+4 mmHg). Median UPC1 and UPC2 were compared between Group 1 and Group 2a dogs only; Group 2b had an incomplete data set. Group 2a dogs had UPC1 (P = 0.042) and UPC2 (P = 0.014) that were significantly higher than Group 1 dogs, but there was no difference detected in median UPC between visit 1 and visit 2 in Group 2a dogs (P = 0.51). No dogs in Group 1 had abnormal UPCs at visit 1 or visit 2 and there was no difference between visit 1 and visit 2 (P = 0.53). In Group 2a, 2/18 (11%) had UPC1 ≥ 0.5 (2.27 dog 1, 12.9 dog 2); the abnormal values in these dogs increased at visit 2 (5.71 dog 1, 19.23 dog 2). Two Group 2a dogs with a normal UPC at baseline had an abnormal UPC at visit 2. The UPC was abnormal in 3/7 (43%) Group 2b dogs that had UPC recorded at baseline. There was no difference in the percent abnormal UPC at baseline between Group 1 dogs (0%) and Group 2a dogs (11%, P = 0.22).
Figure 1.
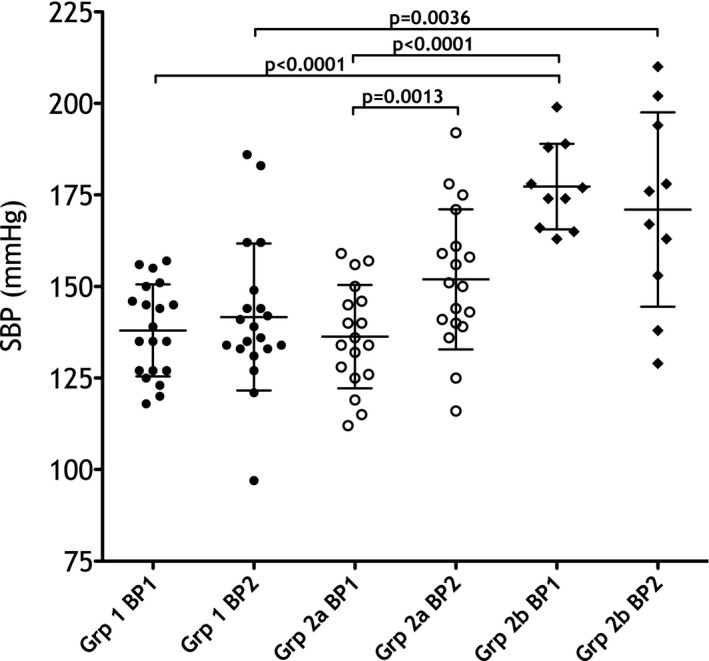
Mean (SD) systolic blood pressure (SBP, mmHg) at visit 1 (BP1) and visit 2 (BP2) in all groups. Significant differences are indicated with P values.
In Group 2b, 4 dogs received no treatment (SBP1 range: 163–174 mmHg). Of these 4, SBP normalized at visit 2 in 2 dogs and remained abnormal in 2 dogs. Six dogs received antihypertensive treatment; 4 dogs (SBP1 174–189 mmHg) received enalapril as a monotherapy (~0.5 mg/kg q 12 hours) and 2 dogs (SBP1 174 and 199 mmHg) received a similar dose of enalapril paired with amlodipine (0.2–0.25 mg/kg q 24 hours). No dog treated with enalapril as monotherapy showed a decrease in SBP between visits (range of change +1 mmHg to +28 mmHg), while the 2 dogs treated with both drugs showed changes in SBP of −40 mmHg and −36 mmHg, respectively.
Discussion
This study was designed to generate an age‐matched control group and resulted in study groups that were well‐matched in terms of age and gender. The median weight of the control dogs was lower than that of treatment dogs but although SBP is higher in some breeds of dogs, body weight (overweight, underweight) appears to have minimal effect.26 The mean SBP did not significantly differ between groups. In a recent study of dogs with lymphoma, affected dogs had significantly higher SBP than control dogs.27 The control dogs and the affected dogs did not differ in age in that report, but the control dogs were slightly younger than the control dogs of this study. Our older control group might have narrowed the difference between control and treatment dogs as BP increases with age in dogs.26
There was no statistical difference in the frequency of HT in the treatment group compared to the control group. The frequency of confirmed HT in the control group (15%) is comparable to the prevalence of HT in clinically normal Shetland sheep dogs in a recent study (13%)28, and is similar to the estimated population prevalence of HT previously published for dogs (10–22%).29 The prevalence of HT in dogs with neoplasia has not been well‐studied, although in the study of dogs with lymphoma previously cited, over half of the lymphoma dogs had SBP ≥ 150 mmHg at screening.27 The frequency of HT at baseline in our small population was 37%, which is similar to the prevalence of HT as a comorbidity in adult people with various types of neoplasia (32–48%).30, 31 These findings suggest that screening for HT in all patients with neoplasia might be warranted.
There was no statistical difference in the median UPC of control dogs and treatment dogs at baseline. The prevalence of proteinuria in treatment dogs tended to be higher than the control dogs and despite the range of neoplasia types represented in this study, was comparable to the prevalence of proteinuria in dogs with lymphoma or osteosarcoma previously reported (9–25%).4 , 32
Group 2a dogs treated with toceranib phosphate had significantly higher SBP after the initiation of TKI treatment, which appears consistent with previous reports in both dogs2 and people.3, 4, 5, 6, 7, 8, 9 In a study of 20 human patients receiving sorafenib, mean SBP increased from 131 to 151 mmHg, with 75% of patients experiencing an increase of >10 mmHg and 60% increasing >20 mmHg. In our study, the change in mean SBP was similar (136 to 152 mmHg) with 67% of dogs experiencing an increase in SBP > 10 mmHg, and 33% increasing >20 mmHg.15 These findings suggest that the HT response of dogs receiving toceranib phosphate might be similar to those of people receiving similar drugs. The exact mechanism for TKI‐associated HT in dogs remains unknown and was not the focus of this study. Human studies suggest that both normotensive and hypertensive patients experience an increase in SBP in response to angiogenesis‐inhibiting medications.5, 33 In our study, the use of antihypertensive medication in many Group 2b dogs makes the SBP response to toceranib phosphate treatment difficult to discern in that group. Nonetheless, the >20 mmHg increase in SBP seen in 33% of Group 2a dogs suggests that monitoring of SBP during toceranib phosphate treatment is indicated.
Group 2b dogs were hypertensive at baseline and remained hypertensive despite concurrent antihypertensive treatment in the majority of these dogs. This study was not designed to evaluate antihypertensive treatment in dogs receiving toceranib phosphate treatment and no conclusions regarding concurrent use of toceranib phosphate and antihypertensive treatment in hypertensive oncology patients can be drawn. Nevertheless, Group 2b dogs provide a small amount of pilot data that suggests that more investigation is needed. No attempt was made in this study to standardize treatment and antihypertensive medications were prescribed by the primary clinician for the dog. In this group, dogs with minor elevations in SBP at visit 1 did not receive antihypertensive medications and SBP at visit 2 remained mildly elevated in 2 of these dogs and normalized in the remaining 2 dogs. Enalapril monotherapy had minimal effects on SBP in the 4 dogs in which it was prescribed and a combination of enalapril and amlodipine seemed to have greater SBP‐lowering effects. In people, some studies have indicated that renin and aldosterone concentrations do not show changes after treatment with toceranib‐like medications15, suggesting that changes in these humoral factors are not playing a role in the development of HT in these patients and might indicate why monotherapy with enalapril appeared to be less effective in lowering blood pressure than enalapril combined with amlodipine besylate. A similar therapeutic effect of amlodipine has been demonstrated in rats.34 In that study, amlodipine treatment significantly attenuated a rise in SBP when given concurrently with the TKI sunitinib, in comparison to rats treated with sunitinib alone. HT was not prevented in rats when treated with sunitinib and the ACEi captopril.34 Current recommendations suggest that people with angiogenesis‐inhibitor related HT should be treated using standard protocols.33 Our uncontrolled results suggest that further investigation of therapeutic efficacy of antihypertensive medications in these veterinary patients is warranted.
Group 2a dogs had significantly higher UPC values at both time points; this finding suggests that dogs with neoplastic processes might be more likely to have pre‐existing or concurrent proteinuria before treatment with TKI. Dogs in Group 2a were more likely to have an abnormal UPC1 and UPC2 while none of the control dogs had an abnormal UPC at either time point. While conclusions regarding proteinuric effects of TKI in this small group of dogs cannot be established with certainty, TKI‐associated proteinuria is a well‐documented adverse effect in people. The exact mechanism of this effect remains unclear. Interestingly, ACEi prevented TKI‐induced proteinuria in rats while amlodipine did not, suggesting a separate mechanism of action of these deleterious effects.34 The increased number of abnormal UPC values documented at either visit suggests that UPC screening might be valuable in characterizing these patients before and during treatment.
Limitations
The primary limitation of this prospective study was the low number of study animals enrolled, which might have limited the power of some analyses. Although the increase in SBP was significant for Group 2a patients, the power of analyses to detect differing proportions of patients HT or proteinuric after toceranib treatment using the number of dogs enrolled was approximately 50%, increasing the likelihood of failing to identify a true effect should it exist. The proportions of dogs affected are similar to people receiving similar drugs, but not every dog had increases in SBP and some decreased. Some relatively small recorded changes might be due to the variability in the reliability of noninvasive BP measurement techniques in dogs.22, 24, 35 We attempted to minimize variability by confirming abnormal readings with a second measurement and using a standard measurement protocol with the same operator for BP measurements, and used a control group of normal dogs to control for week‐to‐week variability in measured BP values.23 The BP measurement techniques used are standard for our clinical patients and reflect commonly available and commonly used techniques.
The relatively small number of dogs might have not only obscured real changes in UPC values after administration of the medication but it is also possible that UPC was unaffected by toceranib treatment and changes noted were random or a consequence of the dogs’ underlying neoplasia. Although UPC is considered a valid assessment of proteinuria in dogs, day to day variability should be taken into effect when evaluating this test.36 Urine cultures were not performed in all dogs to exclude UTI.
The duration of this study (14 days) might have limited the degree of change in SBP and UPC noted. In people treated with angiogenesis inhibitors, progressive increases in BP might be seen up to 3–4 weeks after initiation of treatment.5, 15 The timing of our second evaluation was based on other clinical factors related to the patient's chemotherapy schedule; results from human studies suggest that increases in SBP might have been more pronounced had the dogs been followed further.
Both NSAID and corticosteroids are commonly used in this population and might result in retention of sodium and water through either inhibition of renal prostaglandins (NSAID) or as a consequence of elevated blood cortisol (corticosteroids).37 The effect on SBP and proteinuria might be variable and depend on dose and dosing schedule. Healthy dogs treated with hydrocortisone do not appear to have an increase in SBP or UPC after 5 days but do show a mild increase after 28 days in both variables, although this increase did not result in systemic hypertension or proteinuria (UPC > 0.5) in one study.38 In another study, healthy dogs treated with high doses of prednisone (2 mg/kg q12 hour) develop clinically significant proteinuria (UPC > 0.5) by 14 days of treatment.39 The dose and dosing frequency of these medications were not controlled in this study and were highly variable depending on the needs of the dog, but only 1 of the 4 dogs receiving prednisone at baseline was hypertensive and proteinuric, with normal SBP and UPC values in the remaining 3 dogs. Some influence of prednisone on the SBP or UPC in this single dog cannot be excluded.
Last, this study did not involve a group of dogs treated with other chemotherapeutic agents, so the baseline SBP and UPC characteristics, as well as the response of these values to chemotherapy, remains unknown.
In summary, toceranib phosphate treatment was associated with significant increases in SBP in dogs with varying tumor types in this study, and 37% of treated dogs experienced development of SBP ≥ 160 mmHg, a range associated with increased risk for target organ damage and warranting antihypertensive treatment. Furthermore, our baseline findings suggest that proteinuria and HT are present at clinically relevant prevalences in the oncology population before antineoplastic therapies. These findings suggest that screening for these comorbidities might be reasonable, even if treatment with agents other than toceranib phosphate is contemplated.
Acknowledgment
The authors acknowledge Michele Dolson for her assistance in blood pressure measurements.
Conflict of Interest Declaration: This study was supported by an unrestricted grant from Zoetis Inc. (Florham Park, NJ). The authors disclose no other potential conflicts of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was done at the Department of Medical Sciences, School of Veterinary Medicine, University of Wisconsin, Madison WI 53706.
Footnotes
Palladia, Zoetis Inc., Florham Park, NJ.
Markovic LE, Stepien RL. Development of systemic hypertension after administration of toceranib phosphate (Palladia®) in dogs (2010–2012). J Vet Intern Med 2013;27:637 (abstract).
GraphPad Prism 5.0b, GraphPad Software Inc, San Diego, CA.
Pressler BM, Proulx DA, Williams LE, et al. Urine albumin concentration is increased in dogs with lymphoma or osteosarcoma. J Vet Intern Med 2003;17:404 (abstract).
References
- 1. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:101–1027. [DOI] [PubMed] [Google Scholar]
- 2. London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet Comp Oncol 2012;10:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kruzliak P, Novak J, Novak M. Vascular endothelial growth factor inhibition‐induced hypertension: From pathophysiology to prevention and treatment based on long‐acting nitric oxide donors. Am J Hypertens 2014;27:3–12. [DOI] [PubMed] [Google Scholar]
- 4. Bhargava P. VEGF kinase inhibitors: How do they cause hypertension? Am J Physiol Regul Integr Comp Physiol 2009;297:R1–R5. [DOI] [PubMed] [Google Scholar]
- 5. Azizi M, Chedid A, Oudard S. Home blood‐pressure monitoring in patients receiving sunitinib. N Engl J Med 2008;358:95–97. [DOI] [PubMed] [Google Scholar]
- 6. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006;368:1329–1338. [DOI] [PubMed] [Google Scholar]
- 7. Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maki RG, Fletcher JA, Heinrich MC, et al. Results from a continuation trial of SU11248 in patients (pts) with imatinib (IM)‐resistant gastrointestinal stromal tumor (GIST). J Clin Oncol 2005;23:S9011. [Google Scholar]
- 9. Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 2006;12:7271–7278. [DOI] [PubMed] [Google Scholar]
- 10. Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol Heart Circ Physiol 1998;274:H1054–H1058. [DOI] [PubMed] [Google Scholar]
- 11. Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol 1996;27:838–844. [DOI] [PubMed] [Google Scholar]
- 12. Facemire CS, Nixon AB, Griffiths R, et al. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009;54:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inai T, Mancuso M, Hashizume H, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 2004;165:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 2006;290:H547–H559. [DOI] [PubMed] [Google Scholar]
- 15. Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol 2006;24:1363–1369. [DOI] [PubMed] [Google Scholar]
- 16. Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 2007;7:475–485. [DOI] [PubMed] [Google Scholar]
- 18. Hara A, Wada T, Furuichi K, et al. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int 2006;69:1986–1995. [DOI] [PubMed] [Google Scholar]
- 19. Guan F, Villegas G, Teichman J, et al. Autocrine VEGF‐A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol 2006;291:F422–F428. [DOI] [PubMed] [Google Scholar]
- 20. Eremina V, Sood M, Haigh J, et al. Glomerular‐specific alterations of VEGF‐A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003;111:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta‐analysis. Lancet Oncol 2008;9:117–123. [DOI] [PubMed] [Google Scholar]
- 22. Stepien RL, Rapoport GS. Clinical comparisons of three methods to measure blood pressure in nonsedated dogs. J Am Vet Med Assoc 1999;215:1623–1628. [PubMed] [Google Scholar]
- 23. Henik RA, Dolson MK, Wenholz LJ. How to obtain a blood pressure measurement. Clin Tech Small Anim Pract 2005;20:144–150. [DOI] [PubMed] [Google Scholar]
- 24. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 25. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J Vet Intern Med 2005;19:377–385. [DOI] [PubMed] [Google Scholar]
- 26. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract 1996;37:116–125. [DOI] [PubMed] [Google Scholar]
- 27. Fine DM, Selting K, Backus RC, et al. Hemodynamic and biochemical alterations in dogs with lymphoma after induction of chemotherapy. J Vet Intern Med 2014;28:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scansen BA, Vitt J, Chew DJ, et al. Comparison of forelimb and hindlimb systolic blood pressures and proteinuria in healthy Shetland sheepdogs. J Vet Intern Med 2014;28:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Remillard RL, Ross JN, Eddy JB. Variance of indirect blood pressure measurements and prevalence of hypertension in clinically normal dogs. Am J Vet Res 1991;52:561–565. [PubMed] [Google Scholar]
- 30. Prout GR, Wesley MN, Yancik R, et al. Age and comorbidity impact surgical treatment in older bladder carcinoma patients. Cancer 2005;104:1638–1647. [DOI] [PubMed] [Google Scholar]
- 31. Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital‐based cancer registry. J Am Med Assoc 2004;291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 32. Di Bella A, Maurella C, Cauvin A. Proteinuria in canine patients with lymphoma. J Small Anim Pract 2013;54:28–32. [DOI] [PubMed] [Google Scholar]
- 33. Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor‐treated patients. Ann Oncol 2009;20:807–815. [DOI] [PubMed] [Google Scholar]
- 34. Lankhorst S, Kappers MHW, van Esch JHM, et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: Preclinical study. Hypertension 2014;64:1282–1289. [DOI] [PubMed] [Google Scholar]
- 35. Wernick MB, Hopfner RM, Francey T. Comparison of arterial blood pressure measurements and hypertension scores obtained by use of three indirect measurement devices in hospitalized dogs. J Vet Intern Med 2012;240:962–968. [DOI] [PubMed] [Google Scholar]
- 36. Littmann MP, Daminet S, Grauer GF. Consensus recommendations for the diagnostic investigation of dogs with suspected glomerular disease. J Vet Cardiol 2013;27:S19–S26. [DOI] [PubMed] [Google Scholar]
- 37. Souza VB, Silva EN, Ribeiro ML, Martins Wde A. Hypertension in patients with cancer. Arq Bras Cardiol 2015;104:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schellenberg S, Mettler M, Gentilini F, et al. The effects of hydrocortisone on systemic arterial blood pressure and urinary protein excretion in dogs. J Vet Intern Med 2008;22:273–281. [DOI] [PubMed] [Google Scholar]
- 39. Waters CB, Adams LG, Scott‐Moncrieff JC, et al. Effects of glucocorticoid treatment on urine protein‐to‐creatinine ratios and renal morphology in dogs. J Vet Intern Med 1997;11:172–177. [DOI] [PubMed] [Google Scholar]


