Abstract
Hyperglycaemia is commonly observed on admission and during hospitalization for medical illness, traumatic injury, burn and surgical intervention. This transient hyperglycaemia is referred to as stress‐induced hyperglycaemia (SIH) and frequently occurs in individuals without a history of diabetes. SIH has many of the same underlying hormonal disturbances as diabetes mellitus, specifically absolute or relative insulin deficiency and glucagon excess. SIH has the added features of elevated blood levels of catecholamines and cortisol, which are not typically present in people with diabetes who are not acutely ill. The seriousness of SIH is highlighted by its greater morbidity and mortality rates compared with those of hospitalized patients with normal glucose levels, and this increased risk is particularly high in those without pre‐existing diabetes. Insulin is the treatment standard for SIH, but new therapies that reduce glucose variability and hypoglycaemia are desired. In the present review, we focus on the key role of glucagon in SIH and discuss the potential use of glucagon receptor blockers and glucagon‐like peptide‐1 receptor agonists in SIH to achieve target glucose control.
Keywords: critical illness, glucagon, ICU, insulin, stress‐induced hyperglycaemia
Introduction
Under normal physiological conditions, glucagon produced in the α cells of the pancreas acts primarily on the liver to increase hepatic glucose output to maintain an adequate supply of fuel to the brain and other vital organs 1, 2. In uncontrolled type 1 diabetes mellitus (T1D) and type 2 diabetes mellitus (T2D), hyperglucagonaemia is universally present, suggesting aberrant glucagon secretion 3, 4, 5. Several lines of evidence indicate that the hyperglucagonaemia of diabetes is the direct result of loss of insulin‐induced suppression of pancreatic α‐cell glucagon secretion 6, 7, 8. Glucagon‐induced hepatic glucose output has been implicated as a major cause of uncontrolled diabetes. In subjects with T1D, suppression of glucagon secretion by somatostatin without changing insulin levels ameliorates hyperglycaemia 9, 10. In patients with T2D, glucagon receptor blockers decrease fasting and postprandial glucose 11, 12, 13. Preclinical studies in glucagon receptor knockout mice have demonstrated protection from diabetes after complete β‐cell destruction, providing support for the hypothesis that excess glucagon secretion is directly responsible for many of the metabolic perturbations of diabetes 14, 15, 16.
Relative insulin deficiency, insulin resistance and concomitant increases in the counter‐regulatory hormones (i.e. glucagon, epinephrine and cortisol) are present in medically ill patients with hyperglycaemia and, under experimental conditions, administration of this hormonal cocktail to normal healthy subjects produces metabolic changes resembling stress‐induced hyperglycaemia (SIH) 17, 18, 19, 20, 21. Although the individual effects of insulin, glucagon, cortisol and epinephrine on normal glucose metabolism are well described, the contribution of each to metabolic changes in the setting of medical illness is more difficult to define. In this review, we will focus on what is known about hyperglucagonaemia in the context of the complex hormonal milieu of SIH. We will also discuss the potential for glucagon receptor blockers and glucagon‐like peptide‐1 (GLP‐1) receptor agonists to treat SIH, with the goal of causing less glucose variability and hypoglycaemia than with insulin, the standard of care.
Stress‐induced Hyperglycaemia
Stress‐induced hyperglycaemia, also referred to as stress hyperglycaemia, hospital hyperglycaemia or hyperglycaemia of critical illness is a serious and common condition where blood glucose levels >140 mg/dl occur during hospitalization for traumatic injury, burn, surgery and critical or acute medical illness 22, 23, 24, 25, 26, 27, 28, 29. SIH typically resolves on recovery from the acute medical insult and before discharge from the hospital. Some restrict the use of the term SIH to those patients without a history of diabetes, while others include all patients irrespective of their baseline diabetes status. In the present review, patients previously diagnosed with diabetes and those with no medical history of diabetes will be discussed together. SIH typically occurs in 35–40% of all hospitalized patients when 140 mg/dl is used as the threshold 23. In a more recent study assessing almost 50 million point‐of‐care glucose values from over 3.4 million patients, the prevalence of hyperglycaemia (>180 mg/dl) was 32.2% in patients in intensive care units (ICUs) and 32.0% in non‐ICU patients 30. Approximately 70–80% of patients with SIH admitted to the ICU have no history of diabetes 23, 31. With over 38 million US hospital discharges per year in 2011 and the high prevalence of SIH, this condition is estimated to affect millions annually and has a substantial impact on healthcare costs 32, 33, 34.
Glucose levels that are well above normal are believed to be maladaptive in SIH and exert an array of negative effects, primarily through immune dysfunction and oxidative stress 35, 36, 37, 38. These adverse effects contribute to the morbidity and mortality associated with SIH, where substantial increases in infections, need for kidney dialysis, blood transfusions, polyneuropathy and up to an 18‐fold increased risk of death have been described 23, 25, 26, 39, 40.
The underlying cause of SIH is thought to be a combination of insufficient insulin secretion to overcome the hyperglycaemic effects of counter‐regulatory hormones and insulin resistance in the later stage of illnesses that have significant amounts of tissue injury (Figure 1) 21, 41, 42, 43. One study, designed to determine the effect of trauma on insulin secretion, used graded glucose infusions to induce hyperglycaemia and found that insulin secretion was impaired in patients with major and minor trauma compared with normal individuals 44. In subjects with major trauma, the impairment in insulin secretion persisted for at least 5 days, while insulin secretion returned to normal sooner in patients with minor trauma. Other studies have shown decreased insulin secretion during the shock phase of burns 19 or in the early hours after myocardial infarction 17. Taken together, these data indicate that diminished insulin secretion during the early phase of multiple types of illnesses and injuries is a key contributor to the onset and persistence of SIH. In contrast, insulin resistance appears more prominently during the established or recovery phase of SIH, particularly in situations of severe tissue injury 19.
Figure 1.
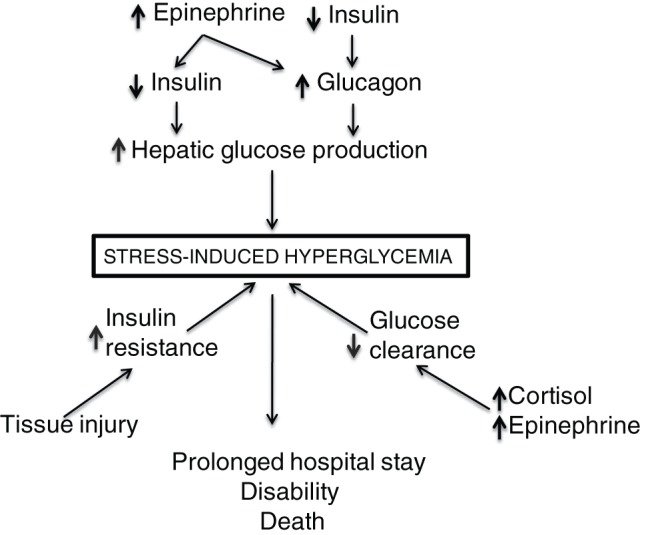
Diagram of hormonal mechanisms of stress‐induced hyperglycaemia.
Studies in lean and obese healthy human volunteers have advanced the concept that simultaneous intravenous infusion of glucagon, epinephrine and cortisol, without the addition of exogenous insulin or experimental alteration in insulin secretion is sufficient to replicate the metabolic effects of SIH 21, 45, 46. Insulin secretion is typically reduced relative to the level of hyperglycaemia and is not able to compensate for the combined effects of the counter‐regulatory hormones 17, 19, 44. Additional neuro‐hormonal factors and cytokines may play a modulatory or secondary role in SIH 47, 48, 49, 50.
Treatment of patients with SIH is limited primarily to insulin administration, irrespective of diabetes status and baseline pancreatic β‐cell reserves. In critically ill ICU patients, intravenous insulin infusion is typically used, while basal and supplemental subcutaneous insulin is preferred in those who are not critically ill. Most current treatment guidelines recommend maintaining glucose in the 140–180 mg/dl range for patients in the ICU 22, 51, 52. A pivotal single‐site study demonstrated in critically ill patients that intensive insulin therapy to reduce glucose to a target range of 80–110 mg/dl improved outcome and decreased length of ICU and hospital stay 39. Tight glycaemic control was then reported to translate into significant healthcare costs savings 29, 53, 54. However, a subsequent large multicentre trial determined that a glucose target of <180 mg/dl resulted in lower mortality than glucose targets of 81–108 mg/dl. This landmark study moved the standard of care away from tight glycaemic control with intensive insulin therapy in critically ill patients 55.
The prevalence of insulin‐induced hypoglycaemia in SIH clinical trials can exceed 6%, even when more conservative glucose levels are targeted 56, 57. Of greater concern is that in clinical practice, hypoglycaemia rates have been reported of up to 20% in ICU patients with SIH 58. When hypoglycaemia is severe it can cause death from cardiovascular or neural events 56.
In addition to targeting mean glucose levels and avoiding hypoglycaemia, glucose variability has emerged as a key variable in predicting outcome in critically ill patients. One retrospective observational study of >7000 patients in four ICUs in Australia determined that glucose variability measured by the blood gas analyser and targeting glucose values of 6 and 10 mM, with no specific insulin protocol, was a significant and independent predictor of ICU and hospital mortality 59. Glucose variability was a stronger predictor of ICU mortality than mean glucose concentration. In that study, ICU mortality was 12% and hospital mortality 22%. Studies in other ICU populations have corroborated these results 60, 61. More recent studies have determined that glucose variability is a stronger predictor of mortality in ICU patients without a history of diabetes compared with those with a history of diabetes 62.
Lastly, insulin administration requires a significant amount of the healthcare provider's time to adjust the insulin dose to achieve target glucose control. Although insulin itself is relatively inexpensive, monitoring requirements contribute to the increase in healthcare costs 34, 53. Thus, novel therapies are needed that are easy to administer and are able to achieve optimum glucose control while avoiding hypoglycaemia and glucose variability 63.
Regulation of Glucagon Secretion in Stress‐induced Hyperglycaemia
Elevated blood glucagon levels in relatively young healthy patients with traumatic injuries were described >40 years ago 64, 65. Soon thereafter, it was discovered that people admitted for a variety of medical illnesses, including acute myocardial infarction 42, 66, burns 67, 68 and sepsis 41, also had elevated glucagon levels of up to five times the normal level. A common finding across the studies was that the degree of glucagon elevation was positively correlated with the severity of the medical illness. Glucagon levels typically did not return to baseline until the patient had recovered from the illness or injury. These data, along with others, have led to the notion that glucagon is a stress response hormone, potentially with effects beyond glucose homeostasis 69
Under normal physiological conditions, glucagon secretion from pancreatic α cells is regulated by fluctuations in plasma glucose, either directly or indirectly through the autonomic nervous system, circulating hormones, GLP‐1, and secretory products from other islet cells 70. Factors known to suppress glucagon secretion, including insulin, may be absent or low in medical illness when a patient is often not eating normal amounts of food and when stress levels of catecholamines, which suppress insulin secretion, are very high 6, 42, 71. There is evidence that most of the inhibitory effect of insulin on glucagon secretion is mediated by paracrine effects within the pancreatic islets 8, 72, 73. Studies in α‐cell‐specific insulin receptor knockout mice have confirmed that insulin decreases glucagon secretion through direct effects on α cells 7. In hyperinsulinaemic–euglycaemic clamp studies in subjects with T1D, insulin administration to attain blood levels of ∼500 µU/ml, lowered plasma glucagon levels by 20–30%, confirming the suppressive effect of insulin on glucagon secretion 6; however, using supraphysiological insulin levels to suppress glucagon secretion to this degree in hyperglucagonaemic patients with SIH will probably not be sufficient to suppress glucagon to normal levels.
Epinephrine has been shown to directly stimulate glucagon secretion 2, 74. In non‐diabetic individuals; epinephrine stimulates glucagon secretion primarily through β‐adrenergic receptors with α‐adrenergic receptor stimulation, accounting for no more than 20% of the effect 75. In healthy subjects, epinephrine infusion causes only a modest 19% increase from baseline in glucagon levels 76. This effect of epinephrine on glucagon levels is relatively small compared with the three‐to‐fivefold increase in glucagon levels seen in patients with medical illness. Under extreme stress conditions, however, e.g. after cardiac arrest, epinephrine levels can increase 1000‐fold from normal levels of <0.05 ng/ml and may thus have a greater effect on glucagon secretion in the critically ill patient 77.
Endogenous cortisol was not found to alter glucagon secretion in healthy volunteers given adrenocorticotropic hormone to stimulate cortisol production 78. However, earlier studies found that exogenous glucocorticoids given for 3 days increased blood glucagon levels by 55% in non‐diabetic lean and 110% in non‐diabetic obese individuals 79. Furthermore, a recent cross‐sectional prospective study found that 0.6% (6 out of 813) patients with T2D in diabetes clinics who had no overt hypercortisolism had Cushing's syndrome 80. The diabetes was cured in four of the six patients after treatment of their Cushing's syndrome and normalization of cortisol.
Other factors may influence glucagon secretion in SIH, but no studies have conclusively identified the dominant inducer of hyperglucagonaemia. The preponderance of evidence suggests that intra‐islet insulin deficiency or insulin resistance, epinephrine excess or a combination of these factors, drive most of the excessive glucagon secretion in SIH.
Novel Treatment Approaches to Stress‐induced Hyperglycaemia
Glucagon‐like Peptide‐1 Receptor Agonists
Dysregulated GLP‐1 secretion or GLP‐1 deficiency is not a well‐defined feature of SIH, but ∼50% of the glucose‐lowering effect of GLP‐1 receptor agonists, approved for the treatment of T2D, has been attributed to suppression of glucagon secretion, making this class of agents a plausible treatment option in patients with SIH 81. There have been several clinical trials of GLP‐1 receptor agonists in hospitalized patients admitted to the coronary care unit 82, on total parenteral nutrition 83, and in other situations of critical illness 84. In general, GLP‐1 receptor agonists have been shown to be effective in glucose‐lowering, to reduce glycaemic variability 85, and to result in equal or less hypoglycaemia compared with insulin, but with increased nausea and vomiting in the hospital setting. One study in nine patients with hyperglycaemia, receiving total parenteral nutrition infused with GLP‐1, showed an ∼50‐mg/dl glucose‐lowering effect, as well as an increase in plasma insulin and C‐peptide, and a trend towards a reduction in glucagon and free fatty acids 83. The glucagon‐lowering effects of GLP‐1 infusion were also seen in a study of critically ill surgical patients 85. Patients who have undergone coronary artery bypass grafting, with preserved left ventricular function, who were treated with continuous infusion of a GLP‐1 receptor agonist peri‐operatively had better glycaemic control with less insulin administration and fewer arrhythmias requiring antiarrhythmic agents compared with a control group 86. Thus, GLP‐1 receptor agonists are potential alternatives to insulin for the treatment of SIH, but nausea and vomiting are potential undesirable effects.
Glucagon Receptor Antagonism
As discussed previously in this review, hyperglucagonemia is common in SIH and has the potential to initiate or worsen hyperglycaemia. Thus, glucagon receptor blockers may be a reasonable alternative or adjunct to insulin therapy to treat SIH during the hospital admission. An early study in patients with burns showed that infusion of somatostatin, an inhibitor of glucagon and insulin secretion, for 30 min, significantly reduced the rate of glucose production 87, 88. Somatostatin administration as a therapeutic tool in SIH, however, would require concomitant administration of insulin to counter the suppressive effect on insulin secretion. In addition, somatostatin analogues that are approved to treat other conditions, such as acromegaly, have been shown to cause biliary tract abnormalities including; gallstones, sludge without stones, and biliary duct dilatation in a high percentage of patients 89. For these reasons, somatostatin analogues are not likely to be treatment options to decrease hyperglucagonemia and control glucose in patients with SIH.
Studies investigating glucagon receptor antagonist for the treatment of SIH have not been reported. Based on clinical trials of small‐molecule glucagon receptor antagonists in patients with T2D, these agents may provide an effective alternative to insulin therapy, but with a lower incidence of hypoglycaemia and potentially reduced glucose variability 11, 13, 90; however, reversible adverse effects of increased LDL cholesterol and liver enzymes have been reported in early‐phase T2D trials with small‐molecule glucagon receptor blockers 11, 13, 90. Preclinical studies suggest the pharmacokinetic and pharmacodynamic properties of anti‐glucagon receptor antibodies may also be an effective approach to treat SIH. One study in diabetic monkeys of a human monoclonal blocking antibody to the glucagon receptor showed that a single dose had a rapid onset of action within hours, with sustained glucose‐lowering over a 7‐day period 91. Studies with another antibody in obese hyperglucagonaemic mice showed a decrease in hepatic glucose output, the main culprit of SIH 92. Future studies will determine whether glucagon receptor antagonists that have a rapid onset of action offer a safe and effective treatment option for SIH.
Conclusions
Stress‐induced hyperglycaemia is a major medical problem requiring prompt treatment of the hyperglycaemia to decrease morbidity and mortality. The complex interplay between relative insulin deficiency in early stages of severe illness, combined with evidence that the elevated levels of glucagon, epinephrine and cortisol sustain hyperglycaemia, suggest that insulin moderates only some of the hormonal dysregulation observed in SIH. Thus, insulin alone may not be an optimum treatment strategy for SIH and may in fact contribute to increased morbidity and mortality by causing hypoglycaemia and increased glucose variability. GLP‐1 and GLP‐1 receptor agonists approved for the treatment of T2D have shown some promise in hospitalized patients with hyperglycaemia when infused continuously. Given that glucagon receptor blockers are in development for T2D, consideration of these agents for SIH deserves exploration in future clinical trials as they may address the hyperglycaemia, while resulting in less glucose variability and insignificant hypoglycaemia, and requiring less intensive glucose monitoring. It remains to be seen whether the transient increase in liver enzymes and other adverse effects will stall further development of this class of agents.
References
- 1. Unger RH, Orci L. Glucagon and the A cell: physiology and pathophysiology (second of two parts). N Engl J Med 1981; 304: 1575–1580. [DOI] [PubMed] [Google Scholar]
- 2. Unger RH, Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med 1981; 304: 1518–1524. [DOI] [PubMed] [Google Scholar]
- 3. Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin‐dependent diabetes mellitus. J Clin Endocrinol Metab 1987; 64: 106–110. [DOI] [PubMed] [Google Scholar]
- 4. Unger RH, Aguilar‐Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 1970; 49: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muller WA, Faloona GR, Aguilar‐Parada E, Unger RH. Abnormal alpha‐cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970; 283: 109–115. [DOI] [PubMed] [Google Scholar]
- 6. Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 2010; 59: 2936–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawamori D, Kurpad AJ, Hu J et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009; 9: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A 2010; 107: 16009–16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raskin P, Unger RH. Hyperglucagonemia and its supression: importance in the control of diabetes. N Engl J Med 1978; 299: 433–436. [DOI] [PubMed] [Google Scholar]
- 10. Gerich JE, Lorenzi M, Schneider V et al. Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N Engl J Med 1974; 291: 544–547. [DOI] [PubMed] [Google Scholar]
- 11. Kelly RP, Garhyan P, Raddad E et al. Short‐term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes Metab 2015; 17: 414–422. [DOI] [PubMed] [Google Scholar]
- 12. van Dongen MG, Geerts BF, Morgan ES et al. First proof of pharmacology in humans of a novel glucagon receptor antisense drug. J Clin Pharmacol 2014; 55: 298–306. [DOI] [PubMed] [Google Scholar]
- 13. Kazda CM, Garhyan P, Kelly RP et al. A randomized, double‐blind, placebo‐controlled phase 2 study of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes. Diabetes Care 2015: dc151643. DOI: 10.2337/dc15-1643 [Epub ahead of print]. [Google Scholar]
- 14. Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin‐deficient type 1 diabetes in mice. Diabetes 2011; 60: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y, Berglund ED, Wang MY et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci U S A 2012; 109: 14972–14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012; 122: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allison SP, Chamberlain MJ, Hinton P. Intravenous glucose tolerance, insulin, glucose, and free fatty acid levels after myocardial infarction. Br Med J 1969; 4: 776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allison SP, Chamberlain MJ, Miller JE et al. Effects of propranolol on blood sugar, insulin and free fatty acids. Diabetologia 1969; 5: 339–342. [DOI] [PubMed] [Google Scholar]
- 19. Allison SP, Hinton P, Chamberlain MJ. Intravenous glucose‐tolerance, insulin, and free‐fatty‐acid levels in burned patients. Lancet 1968; 2: 1113–1116. [DOI] [PubMed] [Google Scholar]
- 20. Bessey PQ, Lowe KA. Early hormonal changes affect the catabolic response to trauma. Ann Surg 1993; 218: 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shamoon H, Hendler R, Sherwin RS. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab 1981; 52: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 22. Umpierrez GE, Hellman R, Korytkowski MT et al. Management of hyperglycemia in hospitalized patients in non‐critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 16–38. [DOI] [PubMed] [Google Scholar]
- 23. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87: 978–982. [DOI] [PubMed] [Google Scholar]
- 24. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009; 373: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773–778. [DOI] [PubMed] [Google Scholar]
- 26. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 27. Kosiborod M, Inzucchi SE, Krumholz HM et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med 2009; 169: 438–446. [DOI] [PubMed] [Google Scholar]
- 28. Kosiborod M, Inzucchi SE, Spertus JA et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation 2009; 119: 1899–1907. [DOI] [PubMed] [Google Scholar]
- 29. Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one‐half years experience at a university‐affiliated community hospital. Semin Thorac Cardiovasc Surg 2006; 18: 317–325. [DOI] [PubMed] [Google Scholar]
- 30. Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract 2011; 17: 853–861. [DOI] [PubMed] [Google Scholar]
- 31. Plummer MP, Bellomo R, Cousins CE et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med 2014; 40: 973–980. [DOI] [PubMed] [Google Scholar]
- 32. Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest 2006; 129: 644–650. [DOI] [PubMed] [Google Scholar]
- 33. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013; 36: 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krinsley JS. Is glycemic control of the critically ill cost‐effective? Hosp Pract 2014; 42: 53–58. [DOI] [PubMed] [Google Scholar]
- 35. Clement S, Braithwaite SS, Magee MF et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27: 553–591. [DOI] [PubMed] [Google Scholar]
- 36. Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol 2014; 2014: 486403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stegenga ME, van der Crabben SN, Blumer RM et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood 2008; 112: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stegenga ME, van der Crabben SN, Dessing MC et al. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med 2008; 25: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Berghe G, Wouters P, Weekers F et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 40. Wahl WL, Taddonio M, Maggio PM, Arbabi S, Hemmila MR. Mean glucose values predict trauma patient mortality. J Trauma 2008; 65: 42–47. [DOI] [PubMed] [Google Scholar]
- 41. Rocha DM, Santeusanio F, Faloona GR, Unger RH. Abnormal pancreatic alpha‐cell function in bacterial infections. N Engl J Med 1973; 288: 700–703. [DOI] [PubMed] [Google Scholar]
- 42. Willerson JT, Hutcheson DR, Leshin SJ, Faloona GR, Unger RH. Serum glucagon and insulin levels and their relationship to blood glucose values in patients with acute myocardial infarction and acute coronary insufficiency. Am J Med 1974; 57: 747–752. [DOI] [PubMed] [Google Scholar]
- 43. Christensen NJ, Videbaek J. Plasma catecholamines and carbohydrate metabolism in patients with acute myocardial infarction. J Clin Invest 1974; 54: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meguid MM, Aun F, Soeldner JS. Temporal characteristics of insulin: glucose ratio after varying degrees of stress and trauma in man. J Surg Res 1978; 25: 389–393. [DOI] [PubMed] [Google Scholar]
- 45. Gelfand RA, Matthews DE, Bier DM, Sherwin RS. Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest 1984; 74: 2238–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bessey PQ, Watters JM, Aoki TT, Wilmore DW. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg 1984; 200: 264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barnes TM, Otero YF, Elliott AD et al. Interleukin‐6 amplifies glucagon secretion: coordinated control via the brain and pancreas. Am J Physiol Endocrinol Metab 2014; 307: E896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krogh‐Madsen R, Moller K, Dela F, Kronborg G, Jauffred S, Pedersen BK. Effect of hyperglycemia and hyperinsulinemia on the response of IL‐6, TNF‐alpha, and FFAs to low‐dose endotoxemia in humans. Am J Physiol Endocrinol Metab 2004; 286: E766–E772. [DOI] [PubMed] [Google Scholar]
- 49. Soop M, Duxbury H, Agwunobi AO et al. Euglycemic hyperinsulinemia augments the cytokine and endocrine responses to endotoxin in humans. Am J Physiol Endocrinol Metab 2002; 282: E1276–E1285. [DOI] [PubMed] [Google Scholar]
- 50. Khan AS, Gibson JM, Carlson GL, Rooyackers O, New JP, Soop M. Protein kinetics in human endotoxaemia and their temporal relation to metabolic, endocrine and proinflammatory cytokine responses. Br J Surg 2015; 102: 767–775. [DOI] [PubMed] [Google Scholar]
- 51. Jacobi J, Bircher N, Krinsley J et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012; 40: 3251–3276. [DOI] [PubMed] [Google Scholar]
- 52. Moghissi ES, Korytkowski MT, DiNardo M et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32: 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med 2006; 34: 612–616. [DOI] [PubMed] [Google Scholar]
- 54. Sadhu AR, Ang AC, Ingram‐Drake LA, Martinez DS, Hsueh WA, Ettner SL. Economic benefits of intensive insulin therapy in critically Ill patients: the targeted insulin therapy to improve hospital outcomes (TRIUMPH) project. Diabetes Care 2008; 31: 1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finfer S, Chittock DR, Su SY et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–1297. [DOI] [PubMed] [Google Scholar]
- 56. Finfer S, Liu B, Chittock DR et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 57. Lazar HL. How important is glycemic control during coronary artery bypass? Adv Surg 2012; 46: 219–235. [DOI] [PubMed] [Google Scholar]
- 58. Krinsley JS, Schultz MJ, Spronk PE et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care 2011; 15: R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short‐term mortality in critically ill patients. Anesthesiology 2006; 105: 244–252. [DOI] [PubMed] [Google Scholar]
- 60. Ali NA, O'Brien JM Jr, Dungan K et al. Glucose variability and mortality in patients with sepsis. Crit Care Med 2008; 36: 2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM Jr, May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg 2008; 74: 679–685. [DOI] [PubMed] [Google Scholar]
- 62. Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol 2009; 3: 1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Normoglycaemia in Intensive Care Evaluation Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) STUDY Protocol 293201 (January 14, 2008) . Available from URL: https://studies.thegeorgeinstitute.org/nice/. Accessed date: 15 February 2012.
- 64. Meguid MM, Brennan MF, Muller WA, Aoki TT. Glucagon and trauma. Lancet 1972; 2: 1145. [DOI] [PubMed] [Google Scholar]
- 65. Lindsey A, Santeusanio F, Braaten J, Faloona GR, Unger RH. Pancreatic alpha‐cell function in trauma. JAMA 1974; 227: 757–761. [PubMed] [Google Scholar]
- 66. Laniado S, Segal P, Esrig B. The role of glucagon hypersecretion in the pathogenesis of hyperglycemia following acute myocardial infarction. Circulation 1973; XLVIII: 797–800. [Google Scholar]
- 67. Vaughan GM, Becker RA, Unger RH et al. Nonthyroidal control of metabolism after burn injury: possible role of glucagon. Metabolism 1985; 34: 637–641. [DOI] [PubMed] [Google Scholar]
- 68. Wilmore DW, Lindsey CA, Moyland JA, Faloona GR, Pruitt BA, Unger RH. Hyperglucagonaemia after burns. Lancet 1974; 1: 73–75. [DOI] [PubMed] [Google Scholar]
- 69. Jones BJ, Tan T, Bloom SR. Minireview: glucagon in stress and energy homeostasis. Endocrinology 2012; 153: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gromada J, Franklin I, Wollheim CB. Alpha‐cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007; 28: 84–116. [DOI] [PubMed] [Google Scholar]
- 71. Young A. Inhibition of glucagon secretion. Adv Pharmacol 2005; 52: 151–171. [DOI] [PubMed] [Google Scholar]
- 72. Samols E, Stagner JI, Ewart RB, Marks V. The order of islet microvascular cellular perfusion is B––A––D in the perfused rat pancreas. J Clin Invest 1988; 82: 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002; 51: 958–965. [DOI] [PubMed] [Google Scholar]
- 74. Taborsky GJ Jr. The physiology of glucagon. J Diabetes Sci Technol 2010; 4: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha‐ and beta‐adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin‐induced hypoglycemia. J Clin Invest 1979; 64: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sacca L, Vigorito C, Cicala M, Corso G, Sherwin RS. Role of gluconeogenesis in epinephrine‐stimulated hepatic glucose production in humans. Am J Physiol 1983; 245: E294–E302. [DOI] [PubMed] [Google Scholar]
- 77. Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 2009; 24: 293–316. [DOI] [PubMed] [Google Scholar]
- 78. McGregor VP, Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am J Physiol Endocrinol Metab 2002; 282: E770–E777. [DOI] [PubMed] [Google Scholar]
- 79. Wise JK, Hendler R, Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest 1973; 52: 2774–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Terzolo M, Reimondo G, Chiodini I et al. Screening of cushing's syndrome in outpatients with type 2 diabetes: results of a prospective multicentric study in Italy. J Clin Endocrinol Metab 2012; 97: 3467–3475. [DOI] [PubMed] [Google Scholar]
- 81. Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon‐like peptide 1 contribute equally to its glucose‐lowering action. Diabetes 2010; 59: 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abuannadi M, Kosiborod M, Riggs L et al. Management of hyperglycemia with the administration of intravenous exenatide to patients in the cardiac intensive care unit. Endocr Pract 2013; 19: 81–90. [DOI] [PubMed] [Google Scholar]
- 83. Nauck MA, Walberg J, Vethacke A et al. Blood glucose control in healthy subject and patients receiving intravenous glucose infusion or total parenteral nutrition using glucagon‐like peptide 1. Regul Pept 2004; 118: 89–97. [DOI] [PubMed] [Google Scholar]
- 84. Pinelli NR, Jones MC, Monday LM, Smith Z, Rhoney DH. Exogenous glucagon‐like peptide‐1 for hyperglycemia in critically ill patients. Ann Pharmacother 2012; 46: 124–129. [DOI] [PubMed] [Google Scholar]
- 85. Galiatsatos P, Gibson BR, Rabiee A et al. The glucoregulatory benefits of glucagon‐like peptide‐1 (7‐36) amide infusion during intensive insulin therapy in critically ill surgical patients: a pilot study. Crit Care Med 2014; 42: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sokos GG, Bolukoglu H, German J et al. Effect of glucagon‐like peptide‐1 (GLP‐1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol 2007; 100: 824–829. [DOI] [PubMed] [Google Scholar]
- 87. Jahoor F, Herndon DN, Wolfe RR. Role of insulin and glucagon in the response of glucose and alanine kinetics in burn‐injured patients. J Clin Invest 1986; 78: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wolfe RR, Burke JF. Somatostatin infusion inhibits glucose production in burn patients. Circ Shock 1982; 9: 521–527. [PubMed] [Google Scholar]
- 89. Sandostatin® LAR Depot [package insert] Sandoz GmbH , Schaftenau, Austria 2011.
- 90. Guan HP, Yang X, Lu K et al. Glucagon receptor antagonism induces increased cholesterol absorption. J Lipid Res 2015; 56: 2183–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Okamoto H, Kim J, Aglione J et al. Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 2015; 156: 2781–2794. [DOI] [PubMed] [Google Scholar]
- 92. Kim WD, Lee YH, Kim MH et al. Human monoclonal antibodies against glucagon receptor improve glucose homeostasis by suppression of hepatic glucose output in diet‐induced obese mice. PLoS One 2012; 7: e50954. [DOI] [PMC free article] [PubMed] [Google Scholar]


