Abstract
Objective
To assess the rates and predictors of traversing steps essential to good medical care for chronic migraine, including: (1) medical consultation, (2) accurate diagnosis, and (3) minimal pharmacologic treatment. Candidate predictors included socioeconomic, demographic, and headache‐specific variables.
Background
Previous research has established that barriers to effective management for episodic migraine include the absence of health insurance, lack of appropriate medical consultation, failure to receive an accurate diagnosis, and not being offered a regimen with acute and preventive treatments.
Methods/Design
The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, a longitudinal web‐based panel study of migraine, included a cross‐sectional module focused on patterns of and barriers to medical care. Participants eligible for this analysis met the study criteria for chronic migraine, had evidence of headache‐related disability, and provided data on health insurance status. The main outcomes in the current analysis included the proportion of respondents who sought consultation for headache with a designated healthcare professional, self‐reported receiving a diagnosis of chronic or transformed migraine, and received minimal pharmacologic treatment for headache with a focus on prescribed acute and preventive treatments.
Results
In the CaMEO Study, 80,783 respondents provided study data, 16,789 (20.8% of respondents) met criteria for migraine, and 1476 (8.8% of those with migraine) met chronic migraine criteria. In total, 1254 participants (85.0% of those with chronic migraine) met inclusion criteria for this analysis. Of those, 512 respondents (40.8%) reported currently consulting with a healthcare professional for headache. Odds of consulting increased with increasing age (OR 1.02; 95% CI 1.01–1.03), body mass index (BMI) (OR 1.01; 95% CI 1.00–1.03), migraine‐related disability (OR 1.02; 95% CI 1.00–1.04), and migraine severity (OR 1.16; 95% CI 1.11–1.22) and presence of health insurance (OR 4.61; 95% CI 3.05–6.96). Among those consulting a healthcare professional, 126 (24.6%) received an accurate diagnosis and 56 of those with a correct diagnosis (44.4%) received both acute and preventive pharmacologic treatments; odds of a CM diagnosis were higher for women (OR 1.93; 95% CI 1.03–3.61), those with greater migraine severity (OR 1.25; 95% CI 1.14–1.37), and those currently consulting a specialist (OR 2.38; 95% CI 1.54–3.69). No predictors of receiving appropriate treatment were identified among those currently consulting. Among our sample of people with chronic migraine, only 56 (4.5%) individuals successfully traversed the series of 3 barriers to successful chronic migraine care (ie, consulted a healthcare professional for migraine, received an accurate diagnosis, and were prescribed minimal acute and preventive pharmacologic treatments).
Conclusion
Our findings suggest that <5% of persons with chronic migraine traversed 3 barriers to receiving care for headache (consultation, diagnosis, and treatment), representing a large unmet need for improving care in this population. Predictors of consulting a healthcare professional included age, having health insurance, greater migraine‐related disability, and greater migraine symptom severity. Among those consulting, predictors of an appropriate diagnosis included consulting a specialist, female sex, and greater migraine severity. Public health efforts are needed to improve outcomes for patients with chronic migraine by a range of interventions and educational efforts aimed at improving consultation rates, diagnostic accuracy, and adherence to minimal pharmacologic treatment.
Keywords: migraine, chronic migraine, barrier to care, headache‐related disability, acute medication, preventive medication
Abbreviations
- AMPP
American Migraine Prevalence and Prevention Study
- AMS
American Migraine Study
- BMI
body mass index
- CaMEO
Chronic Migraine Epidemiology & Outcomes
- CM
chronic migraine
- DSM‐IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- EM
episodic migraine
- GAD‐7
7‐item Generalized Anxiety Disorder scale
- HCP
healthcare professional
- ICHD‐3b
International Classification of Headache Disorders, 3rd edition, beta version
- ID‐CM
Identify Chronic Migraine
- MIDAS
Migraine Disability Assessment Scale
- MSSS
Migraine Symptom Severity Score
- NSAID
nonsteroidal anti‐inflammatory drug
- OR
odds ratio
- PHQ‐9
9‐item PRIME‐MD® Patient Health Questionnaire
- RR
rate ratio
- TM
transformed migraine
BACKGROUND
Migraine is a chronic neurological disorder broadly classified into episodic migraine (EM) and chronic migraine (CM) based on the monthly number of headache days.1, 2, 3 EM is characterized by migraine with headache attacks that occur <15 days per month, whereas CM is generally characterized by ≥15 headache days per month with migrainous features ≥8 days per month.1 Within the US migraine population, the majority (93.3%) are classified as having EM, but those who have CM (7.7%)4 are recognized as the segment suffering most from the disorder. Supported by clinical experience, data have demonstrated that compared with people with EM, those with CM experience substantially greater headache impact on daily activities,5, 6 higher direct medical costs,7, 8, 9 greater healthcare resource utilization,5 reduced health‐related quality of life,5, 10 and higher rates of comorbidities.11, 12
In both the EM and CM populations, migraine attacks are frequently unpredictable and disabling. Since migraine‐specific treatments are available only by prescription in the US, management requires physician or other prescribing healthcare professional (HCP) direction. Good care for migraine, particularly CM, requires that a person seeks care, obtains a diagnosis to gain an understanding of their disorder, and receives an individualized treatment plan that consists of FDA‐ and guideline‐appropriate acute and preventive treatment (including pharmacologic, behavioral, and/or interventional treatments) approaches. Furthermore, poorly optimized treatment is associated with an increased risk of transformation from EM to CM.13 Previous research has established that barriers to effective migraine management include male sex, lack of health insurance, lack of appropriate medical consultation, failure to receive an accurate diagnosis, and not being offered a minimal pharmacologic treatment regimen.14, 15, 16 An analysis from the American Migraine Prevalence and Prevention (AMPP) Study determined the proportion of the population with EM that overcame all 3 established barriers to reach minimal pharmacologic management was low (26.3%),15 but that analysis did not explore barriers among people with CM. The AMPP Study showed that the majority of individuals with CM had not received a correct diagnosis (79.8%) nor specific acute (68.4%) or preventive (60.0%) medications. These data suggest that most patients with CM are not crossing the 3‐step series of barriers and thus are not effectively managed.4, 17
Developed from key learnings from the AMPP Study, the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study collected cross‐sectional and longitudinal epidemiologic data using multiple web‐based survey modules. The objectives of this study were to assess the rates and predictors of traversing a series of 3 steps judged to be essential to good care: (1) medical consultation, (2) accurate diagnosis, and (3) a minimal pharmacologic strategy that includes acute and preventive treatments. We also sought to identify socioeconomic, demographic, and headache‐specific variables associated with each step.
METHODS
Study Design
The CaMEO Study design and methodology have been reported in detail elsewhere.18 Briefly, the CaMEO Study was conducted over 15 months (September 2012–November 2013), and was approved by the Albert Einstein College of Medicine Institutional Review Board. The CaMEO Study employed unique cross‐sectional modules embedded in a longitudinal design and assessed headache frequency, headache severity and symptoms, headache‐related disability, healthcare utilization for headache, headache medication use, comorbid health problems, and family‐related burden associated with migraine. During the first (ie, baseline) of 3 stages in CaMEO, respondents completed the Screening, Core, and Barriers to Care modules, one immediately after the other. The current analysis details the results from the Barriers to Care Module in the population within CaMEO that met the study criteria for CM.
Study Population
Participants with migraine were identified from a web‐based panel (Research Now, Plano, TX, USA), which has 2.4 million members nationwide, and has a demographic composition broadly similar to that of the US population.19 A total of 489,537 persons in the panel were invited to participate, 80,783 (16.5% of invitees) responded to baseline modules (Screening, Core, and Barriers to Care modules), and 58,418 provided valid returns (11.9% of invitees) (Fig. 1).
Figure 1.
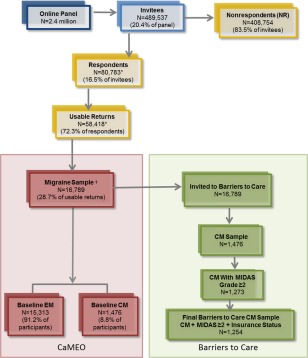
Defining the analysis sample. In the CaMEO Study, a total of 16,789 respondents met the study criteria and comprised the migraine sample. Of these, 1476 were classified as having chronic migraine per the CaMEO Study criteria. From the 1476 respondents classified as having chronic migraine, 1273 had chronic migraine and a MIDAS grade 2, 3, or 4, indicating that these subjects experienced substantial disability caused by their migraines. Because health insurance coverage is an important predictor of consultation, we restricted the sample to those who provided information on health insurance status (yes or no to having health insurance); thus our final sample included 1254 respondents who met the study criteria for chronic migraine, had a MIDAS grade ≥2, and reported insurance status. *N=22,365 respondents either (1) abandoned the survey, (2) were over‐quota, or (3) had invalid (unusable) data and were removed during data cleaning. †Met Inclusion Criteria: Agreed to participate, screened positive for modified ICHD‐3b migraine, were ≥18 years old, and had ≥1 headache in previous 12 months. CM=chronic migraine; EM=episodic migraine; ICHD‐3b=International Classification of Headache Disorders, 3rd edition, beta version; MIDAS=Migraine Disability Assessment.
The American Migraine Study (AMS)/AMPP diagnostic module was employed to obtain lifetime recall of migraine symptoms associated with the respondents' most severe headache (eg, unilateral, pulsating, moderate/severe pain intensity, routine activity exacerbation of headache pain, nausea, phonophobia, photophobia). Participants included in the analysis met AMS/AMPP diagnostic module criteria for migraine.20, 21 This was a modification of the International Classification of Headache Disorders, 3rd edition, beta version (ICHD‐3b) migraine criteria.1 The diagnostic module did not assess whether there were ≥5 lifetime migraine events (criterion A) and if the duration of an untreated attack was from 4 to 72 hours (criterion B). CM classification was derived from Silberstein–Lipton criteria22, 23 and ICHD‐3b criteria for CM. CM respondents were defined as those with ≥15 headache days per month averaged over the past 3 months, but did not include assessment of ICHD‐3b CM criterion C (ie, ≥8 days per month fulfilled migraine criteria), because it is difficult to evaluate in a large, self‐report data‐collection paradigm and requires the use of a diary and physician interview to accurately determine. The diagnostic module employed in this study has been demonstrated to have a sensitivity of 100% and specificity of 82% for the diagnosis of migraine,24 and sensitivity of 91% and specificity of 80% for the diagnosis of CM25 based on modified ICHD‐2 criteria. No significant changes occurred between ICHD‐2 and ICHD‐3b that are related to the criteria used in this study. Inclusion criteria required active panel membership, age ≥18 years, meeting modified ICHD‐3b migraine symptom criteria, and ≥1 headache within the past 12 months. A total of 16,789 respondents (3.4% of invitees) met inclusion criteria and comprise the CaMEO Study sample, of whom 1476 respondents met the criteria for CM (8.8%) and 15,313 met the criteria for EM (91.2%). The current analysis from the Barriers to Care Module included only those respondents who (1) met study criteria for CM, (2) reported mild or greater headache‐related disability (Migraine Disability Assessment Scale [MIDAS] disability grade ≥2, score ≥6) and (3) provided information regarding their health insurance status (Fig. 1).
Barriers to Care Cross‐Sectional Module
The CaMEO Study modules included validated instruments as well as pertinent survey questions developed based on the results of focus groups, expert opinion, item analysis, and preliminary survey trials. The Barriers to Care Module was based on the AMPP Study barriers to care items and analysis.15 This baseline module had a total of 65 questions that were generally divided into knowledge about headache and medical care and consulting behavior. The CaMEO Study was a web‐based survey, which allowed branching logic to direct respondents to appropriate questions based on their responses.
Questions were divided into domains that included: accuracy of diagnosis among those currently consulting any HCP; satisfaction with current treatments; and resources, awareness, outlook, and behaviors that possibly contributed to the lack of medical consultation among migraineurs. For respondents currently consulting an HCP and diagnosed with migraine, the survey investigated the motivation and modes of healthcare consultation, accuracy of diagnosis, and medications prescribed (both acute and preventive). Nonconsulters and lapsed consulters answered questions about their motivation for discontinuing or never seeking treatment, and about their headache‐related healthcare experiences. The median duration for a participant to complete this module was 20 minutes.
Outcomes
The main outcomes in the current analysis included the proportion of respondents who traversed each barrier to care, including: (1) number of respondents with CM who sought consultation for headache with a prescribing HCP, as defined by a list of providers; (2) number of respondents with CM who self‐reported receiving a diagnosis of CM or transformed migraine (TM; subsequently referred to as CM) from an HCP; and (3) number of respondents with CM receiving minimal pharmacologic treatment, which was defined as acute and preventive pharmacologic treatments supported by the best empirical and clinical data available, including guideline recommendations and supporting literature. Inclusion for each step was limited to those who had fulfilled the previous step, so consideration for current treatment was restricted to those who had traversed the consulting barrier and had received the correct diagnosis of CM.
Consulting Healthcare Professional
The CaMEO definition of “consulting HCP” was modeled after the definition used previously for the AMPP Study on barriers to care in EM.15 In the current analysis, consulting HCPs for the diagnosis and treatment of migraine were defined as primary‐care HCPs (ie, general practitioners, family physicians, internal medicine specialists, nurse practitioners, physicians' assistants, obstetricians/gynecologists) or specialists (ie, neurologists, pain specialist physicians, headache specialist physicians). “Consulters” were those respondents reporting headache management with one of the HCPs listed above and “nonconsulters” were respondents not currently consulting any of the categories of HCP defined above.
Chronic Migraine Diagnosis
In the current analysis of the CM population, the respondent was asked to self‐report whether they had received a medical diagnosis of CM or TM from the HCP they were consulting for management and treatment of their migraine headaches; if they reported receiving a diagnosis of CM or TM, they were categorized as correctly diagnosed.
Minimal Pharmacologic Treatment
Respondents were asked about their current use of prescription acute and preventive medications for headache. Acute treatment for headache/migraine was based on the previously published AMPP Study criteria utilized for the EM barriers to care assessment to assess potentially differing trends between EM and CM.15 Acute treatment for headache/migraine was considered present if the respondent reported using a prescribed nonsteroidal anti‐inflammatory drug (NSAID), triptan, or isometheptene (Midrin). Preventive medication was deemed in use if the respondent reported using a prescribed antidepressant medication, antiseizure medication, or blood pressure or heart medication to prevent or reduce the occurrence of their headaches/migraine attacks; additional treatments considered as prescribed preventives included treatments specific to migraine prevention, such as onabotulinumtoxinA and interventional medicine treatments such as nerve blocks, or trigger point injections. In summary, minimal pharmacologic treatment for CM was defined as current use of both prescribed acute and preventive treatments.
Sociodemographic Variables, Headache Characteristics, and Psychiatric Comorbidities
The baseline CaMEO modules assessed symptoms, headache‐related disability, and psychiatric comorbidities. Data from these assessments were used in the current analysis to determine predictors of consulting, diagnosis, and treatment among those with CM, MIDAS grade ≥2, and reported health insurance status.
Sociodemographic data were obtained via self‐report, and included sex, age, current employment status, body mass index (BMI; calculated using the standard algorithm taken from self‐reported height and weight measures), annual household income, and health insurance status (ie, having health insurance or not).
Headache Symptoms
The Migraine Symptom Severity Score (MSSS) is a composite score that computes headache severity through the summed responses to questions ascertaining the prevalence of the 7 ICHD‐3b criteria symptoms associated with a respondent's “most severe headaches.” These symptoms are: (1) unilateral pain; (2) pounding, pulsating or throbbing pain; (3) moderate‐to‐severe pain intensity; (4) pain made worse by routine activities; (5) nausea; (6) photophobia; and (7) phonophobia. Response values span from “1” (ie, never) to “4” (ie, half the time or more) producing a total score ranging from 7 to 28.15 The MSSS has been used frequently in AMPP Study analyses, although it is not a validated assessment.15
Headache‐Related Disability
Headache‐related disability was measured using the MIDAS, a 5‐item, self‐administered questionnaire that evaluates days of missed activity or markedly reduced activity caused by headache in the preceding 3 months in 3 areas: school work/paid employment, household work/chores, and nonwork (ie, family, social, leisure) activities.26, 27 Responses were summed to a total score that fell into 4 categories: Grade 1: little to no disability (score 0–5); Grade 2: mild disability (score 6–10); Grade 3: moderate disability (score 11–20); and Grade 4: severe disability (score 21–270). MIDAS grades were used to characterize the respondent population in the current analysis and MIDAS scores were included in the logistic modeling to assess predictors of care.
Depression and Anxiety
Levels of depression were determined using the PRIME‐MD® Patient Health Questionnaire (PHQ‐9), a validated measure of major depressive disorder based on Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) criteria, which scores 9 questions from “0” (not at all) to “3” (nearly every day) for depressive symptoms over the previous 2 weeks.28, 29 PHQ‐9 scores ≥10 indicate moderate to severe depression (severe score, 20–27). Respondents with a cumulative score ≥10 were classified with major depression for the current analysis.
Anxiety symptoms were evaluated using the validated Generalized Anxiety Disorder scale (GAD‐7), which scores 7 questions from “0” (ie, not at all) to “3” (ie, nearly every day) for the previous 2 weeks, for a total severity score ranging from 0 to 21 (moderate: 10–14; severe: 15–21).30, 31, 32 Respondents with a cumulative score ≥10 were classified as having generalized anxiety disorder. Both PHQ‐9 and GAD‐7 scores were included in the logistic models.
Consulting a Specialist With Expertise in Headache
Currently consulting a specialist was a covariate used to adjust for type of HCP that currently manages respondent's headaches. It was defined using a single survey item and was considered present if the respondent indicated that a specialist (ie, neurologist, headache specialist physician, or pain specialist physician) was currently managing or treating their headaches.
Statistical Methods
Both univariate and multivariable models were constructed for current consulting status, diagnostic status among current consulters, and diagnosed current consulters who received appropriate treatment. In the univariate analysis, binary outcomes (eg, sex, employment status, health insurance status, meeting criteria for depression or generalized anxiety disorder, consultation with an HCP) were modeled using binary logistic regression and reported as odds ratios (OR; 95% confidence interval [CI]). The mean difference in age and MSSS were compared using a 2‐sample t‐test with equal variances and reported as mean difference and 95% CI. The distribution of MIDAS most resembled a negative binomial distribution and therefore was modeled using a negative binomial regression and reported as rate ratios (RR; 95% CI). Income was divided into sequential ordered categories and modeled using ordinal regression and reported cumulative ORs. In addition, multivariable binary logistic models were developed for assessing predictors of consulting an HCP for headache, predictors of receiving a CM/TM diagnosis, and predictors of receiving minimal acute and preventive pharmacologic treatment. P values ≤.05 were considered significant for all statistical tests and models. All analyses were conducted with SPSS Statistics, version 22.0 (IBM, Armonk, NY, USA).
RESULTS
Analysis Sample
The baseline stage of the CaMEO Study was initiated during the third quarter of 2012 and completed between September 17 and October 30, 2012. Among 80,783 respondents who provided study data, 16,789 met criteria for migraine. Of the respondents who completed the Screening, Core, and Barriers to Care modules, 1476 met the criteria for CM (8.8%) and 15,313 met the criteria for EM (91.2%). Of the 1476 who met CM criteria, 1273 had MIDAS grade ≥2, and 1254 of those also responded to questions about their health insurance status (possessing or not and type) (Fig. 1). The 1254 chronic migraineurs with MIDAS grade ≥2 and reporting insurance data (85% of those with CM) comprised the eligible sample for the analyses presented herein.
Barriers to Achieving Appropriate Treatment
Figure 2 summarizes the respondents that traversed each step in the path leading to minimal acute and preventive pharmacologic treatment for CM. Of the 1254 respondents with CM eligible for analysis, 512 (40.8%) reported currently consulting with an HCP for headache. Among current consulters, 126 (24.6%) received a diagnosis of CM/TM and 56 (44.4%) of those with a correct diagnosis received both a prescribed acute and preventive treatment. In aggregate, among people with CM and MIDAS disability grade ≥2, only 56 (4.5%) successfully traversed all 3 barriers (ie, consulted an HCP for headache, received a CM diagnosis, and reported receiving prescribed acute and preventive treatments).
Figure 2.
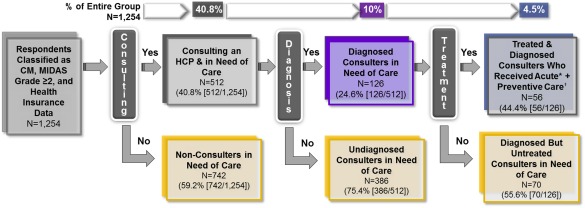
Defining respondents who traverse all 3 barriers to care. Minimal pharmacologic treatment for persons with chronic migraine may be achieved if they traverse a 3‐step series of barriers to care defined as: (1) seeking care from a healthcare professional (current consulters); (2) receiving a diagnosis of chronic migraine or transformed migraine from a healthcare professional; and (3) using minimal pharmacologic treatment, which in this analysis included both acute and preventive therapies. *Acute treatment=respondent reported currently using a prescribed NSAID, triptan, isometheptene (Midrin) to treat their headaches. †Preventive treatment=respondent reported currently using medications to prevent or reduce headache frequency including antidepressants, antiseizure medications, blood pressure/heart medications, other preventative medications including onabotulinumtoxinA, abobotulinumtoxinA, other botulinum toxins, trigger point injections. CM=chronic migraine; HCP=healthcare professional; MIDAS=Migraine Disability Assessment Scale.
Univariate Examination of Differences for Consulting, Diagnosis, and Treatment
Current Consulters vs Nonconsulters
Table 1 compares and contrasts those who did and did not consult an HCP for headache management. Those who consulted were older by almost 4 years on average (42.8 years vs 38.9 years; mean difference 3.9, 95% CI 2.37–5.37), were more likely to have health insurance (93.6% vs 77.6%; OR 4.18, 95% CI 2.82–6.20), had a mean MIDAS score that was 21% higher (77.7 vs 64.3; RR 1.21, 95% CI 1.08–1.35), and had a mean MSSS that was 1 point higher (24.7 vs 23.7; mean difference 1.03, 95% CI 0.71–1.35). No significant differences were found for sex, employment status, BMI, household income, or rates of depression/anxiety.
Table 1.
Univariate Examination of Differences for Consulting, Diagnosis, and Treatment Among Chronic Migraineurs
| Consulting Status Among CM‐Eligible Sample (N = 1254) | Diagnostic Status Among Current Consulters (N = 512) | Minimal Acute and Preventive Pharmacologic Treatment Among Diagnosed Current Consulters (N = 126) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Not Currently Consulting (n = 742) | Currently Consulting (n = 512) | Difference (95% CI) | No CM or TM Diagnosis (n = 386) | CM or TM Diagnosis (n = 126) | Difference (95% CI) | No Treatment (n = 70) | Treatment (n = 56) | Difference (95% CI) | |
| Women, N (%)a,b | 622 (83.8) | 419 (81.8) | 0.87 (0.65–1.17) | 309 (80.1) | 110 (87.3) | 1.71 (0.96–3.06) | 60 (85.7) | 50 (89.3) | 1.39 (0.47–4.09) |
| Age, mean (SD)c | 38.9 (13.7) | 42.8 (12.8) | 3.87 (2.37–5.37) | 42.7 (13.1) | 43.0 (11.6) | 0.28 (−2.29 to 2.85) | 43.0 (12.4) | 43.0 (10.8) | −0.04 (−4.18 to 4.11) |
| Body mass index, mean (SD) | 29.5 (8.2) | 30.3 (8.5) | 0.79 (−0.14 to 1.73) | 30.3 (8.6) | 30.1 (7.9) | −0.2 (−0.19 to 1.51) | 29.5 (8.3) | 30.9 (7.5) | 1.43 (−1.39 to 4.25) |
| Employed full‐time/part‐time, N (%)a,b | 438 (59.0) | 274 (53.5) | 0.80 (0.64–1.00) | 219 (56.7) | 55 (43.7) | 0.59 (0.39–0.89) | 28 (40.0) | 27 (48.2) | 1.40 (0.69–2.84) |
| Income, $ N (%) | |||||||||
| <$30,000 | 237 (32.2) | 133 (26.3) | Reference | 98 (25.7) | 35 (28.2) | Reference | 20 (29.0) | 15 (27.3) | Reference |
| $30,000–$49,999 | 154 (20.9) | 105 (20.8) | 80 (20.9) | 25 (20.2) | 15 (21.7) | 10 (18.2) | |||
| $50,000–$74,999 | 154 (20.9) | 119 (23.5) | 93 (24.4) | 26 (21.0) | 15 (21.7) | 11 (20.0) | |||
| ≥75,000e | 191 (26.0) | 149 (29.5) | 0.87 (0.71–1.06) | 111 (29.1) | 38 (30.7) | 1.14 (0.79–1.64) | 19 (27.5) | 19 (34.6) | 1.11 (0.59–2.09) |
| Insured, N (%)a,b | 576 (77.6) | 479 (93.6) | 4.18 (2.82–6.20) | 363 (94.0) | 116 (92.1) | 0.73 (0.34–1.59) | 64 (91.4) | 52 (92.9) | 1.22 (0.33–4.55) |
| MIDAS, mean (SD)d | 64.3 (65.4) | 77.7 (77.5) | 1.21 (1.08–1.35) | 73.7 (75.2) | 90.2 (83.4) | 1.22 (1.00–1.50) | 97.6 (94.9) | 81.0 (65.8) | 0.83 (0.58–1.18) |
| MSSS, mean (SD)c | 23.7 (2.9) | 24.7 (2.8) | 1.03 (0.71–1.35) | 24.3 (2.9) | 25.9 (2.1) | 1.53 (0.98–2.08) | 25.8 (2.2) | 26.0 (1.9) | 0.23 (−0.52 to 0.97) |
| Major depression, N (%)a,b | 436 (58.8) | 314 (61.3) | 1.11 (0.88–1.40) | 237 (61.4) | 77 (61.1) | 0.99 (0.65–1.49) | 42 (60.0) | 35 (62.5) | 1.11 (0.54–2.29) |
| Generalized anxiety disorder, N (%)a,b | 383 (51.6) | 251 (49.0) | 0.90 (0.72–1.13) | 188 (48.7) | 63 (50.0) | 1.05 (0.70–1.57) | 36 (51.4) | 27 (48.2) | 0.88 (0.44–1.78) |
| Consulted a specialist, N (%)b | inestimable | 122 (31.6) | 68 (54.0) | 2.54 (1.68–3.83) | 33 (47.1) | 35 (62.5) | 1.87 (0.91–3.82) | ||
Significant P values (P < .05) are bolded.
CM = chronic migraine; MIDAS = Migraine Disability Assessment; MSSS = Migraine Symptom Severity Score; TM = transformed migraine.
Reference values are men, unemployed, uninsured, no major depression, no generalized anxiety disorder.
Calculated using binary logistic regression, presented as an odds ratio.
Calculated using t‐test, presented as mean difference.
Calculated as negative binomial regression, presented as rate ratio.
Calculated as ordinal logistic regression, presented as cumulative odds ratio.
Diagnosis of CM and Minimal Pharmacologic Treatment
Among current consulters, 24.6% reported a clinical diagnosis of CM/TM. Those with a self‐reported physician diagnosis of CM/TM were less likely to be fully employed (43.7% vs 56.7%; OR 0.59, 95% CI 0.39–0.89), had a mean MIDAS score 22% higher (90.2 vs 73.7; RR 1.22, 95% CI 1.00–1.50) and mean MSSS about 1.5 points higher (25.9 vs 24.3; mean difference 1.53, 95% CI 0.98–2.08), and were about 1.5 times more likely to report currently consulting a specialist for their headaches (54.0% vs 31.6%; OR 2.54, 95% CI 1.68–3.83). No differences were found between those diagnosed vs not diagnosed with CM for sex, employment status, BMI, household income, or rates of depression/anxiety. Among those consulting an HCP and diagnosed with CM, 44.4% received acute and preventive treatment. There were no significant differences between those who were given minimal pharmacologic treatment vs not given minimal pharmacologic treatment.
Multivariable Logistic Models for Current Consulting, Diagnosis, and Receiving Minimal Pharmacologic Treatment
Using multivariable logistic regression, we modeled characteristics associated with (1) consulting behavior, (2) diagnosis among consulters, and (3) treatment with prescribed acute and preventive treatment use among diagnosed consulters (Table 2).
Table 2.
Multivariable Logistic Models for Current Consulting, Diagnosis, and Treatment Among Chronic Migraineurs
| Odds Ratio (95% CI) | Current Consulting Status (N = 1254) | CM/TM Diagnosis Among Current Consulters (N = 512) | Minimal Acute and Preventive Pharmacologic Treatment Among Diagnosed Current Consulters (N = 126) |
|---|---|---|---|
| Sexa | 0.83 (0.60–1.14) | 1.93 (1.03–3.61) | 1.21 (0.38–3.89) |
| Age (1‐year increments) | 1.02 (1.01–1.03) | 1.00 (0.98–1.02) | 1.00 (0.97–1.04) |
| Employed part‐time or full‐timea | 0.92 (0.72–1.19) | 0.68 (0.43–1.07) | 1.44 (0.66–3.14) |
| BMI (per unit increase) | 1.01 (1.00–1.03) | 1.00 (0.97–1.03) | 1.03 (0.98–1.08) |
| Income ≥$75,000a | 1.00 (0.76–1.33) | 1.33 (0.81–2.18) | 1.58 (0.68–3.67) |
| Insurancea | 4.61 (3.05–6.96) | 0.76 (0.33–1.75) | 1.37 (0.34–5.57) |
| MIDAS (10‐point increments) | 1.02 (1.00–1.04) | 1.01 (0.98–1.03) | 0.98 (0.93–1.03) |
| MSSS (1‐point increments) | 1.16 (1.11–1.22) | 1.25 (1.14–1.37) | 1.10 (0.91–1.34) |
| PHQ‐9 (Depression)a | 1.22 (0.89–1.67) | 0.90 (0.51–1.6) | 1.49 (0.53–4.14) |
| GAD‐7 (Anxiety)a | 0.79 (0.59–1.07) | 1.08 (0.63–1.85) | 0.70 (0.26–1.88) |
| Current Specialistb | inestimable | 2.38 (1.54–3.69) | 1.86 (0.88–3.97) |
Significant P values (P < .05) are bolded.
BMI = body mass index; CI = confidence interval; CM = chronic migraine; GAD = generalized anxiety disorder; MIDAS = Migraine Disability Assessment; MSSS = Migraine Symptom Severity Score; PHQ = patient health questionnaire; TM = transformed migraine.
Reference values are men, unemployed, income <$75,000, uninsured, no major depression, and no generalized anxiety disorder.
Specialist = neurologist, headache specialist, pain specialist.
Predictors of Consulting an HCP for Headache
For each 1‐year increase in age, medical consulting for headache increased 2% (OR 1.02; 95% CI 1.01–1.03). For each unit increase in BMI, consulting increased 1% (OR 1.01; 95% CI 1.00–1.03). Those possessing insurance were about 4.6 times as likely to consult (OR 4.61; 95% CI 3.05–6.96). A 10‐point increase in mean MIDAS disability scores was associated with a 2% increase in consultation (OR 1.02; 95% CI 1.00–1.04). A 1‐point mean increase in MSSS was associated with 16% increase in consulting (OR 1.16; 95% CI 1.11–1.22).
Predictors of Appropriate Diagnosis
Among those with CM consulting HCPs for headache, in comparison with men, women were almost twice as likely to receive a CM diagnosis (OR 1.93; 95% CI 1.03–3.61). A 1‐point mean increase in MSSS was associated with a 25% increase in odds of getting CM diagnosis (OR 1.25; 95% CI 1.14–1.37). Those currently consulting a specialist were nearly 1.5 times more likely to receive CM diagnoses (OR 2.38; 95% CI 1.54–3.69) than those consulting other HCPs.
Predictors of Receiving Prescribed Acute and Preventive Treatment
Among those with CM consulting HCPs for headache and who received a diagnosis, none of the variables used as predictors in this study was significantly associated with increased odds of receiving minimal acute and preventive pharmacologic treatment.
DISCUSSION
In this report, we identified people with CM and unmet medical needs based on disability and assessed 3 levels of healthcare seeking and delivery, focusing on consultation, diagnosis, and treatment. Just 41% of eligible respondents with CM were currently consulting an HCP regarding their headaches. Among consulters, only 25% reported receiving an accurate diagnosis of CM or TM. Among the diagnosed, 44% reported receiving both acute and preventive pharmacologic treatments. Thus, the percentage of individuals with CM traversing all 3 barriers is 4.5%, the product of success at each step (0.41 × 0.25 × 0.44 × 100). This approach has not been applied to medical care for CM.
In a previous study using similar methodology we found that 26.3% of people with EM and unmet medical needs successfully traversed all 3 barriers to migraine care.15 In contrasting the studies, we found that consultation rates were lower for people with CM (41%) than EM (46%). Diagnostic rates among consulters were also lower for those with CM (25%) than EM (87%). Finally, treatment rates among the diagnosed were also lower for those with CM (44%) than EM (67%).
In the present report, we examined predictors of accomplishing each step in the initial process leading to better treatment. The odds of consulting among those with CM increased with age and BMI as well as with greater levels of headache‐related disability and symptom severity. Availability of health insurance was also a very powerful predictor of consulting. In the study of barriers to EM care, predictors of consulting were similar and included indicators of migraine severity (ie, MIDAS score, MSSS) and having health insurance.15 These findings indicate that seeking healthcare for migraine is determined in part by treatment needs, as indexed by measures of headache severity, and in part by access to care, as indicated by health insurance status. According to the US Ambulatory Medical Care Survey, 72.2% of outpatient visits for migraine take place in primary care settings.33 Efforts to improve consultation for migraine are therefore best directed to primary care settings. People with disabling headache should be encouraged to discuss their headaches with their doctors, and primary care providers should be encouraged to evaluate their patients for migraine and CM.
For diagnosis of CM among those consulting, the odds of correct diagnosis were greater for women, those with severe symptoms, and those consulting a specialist. Rates of diagnosis among consulters were far lower for CM (25%) than for EM (87%).15 This difference suggests an unmet need for better CM diagnosis, particularly in primary care settings. Barriers associated with diagnosis may be addressed by encouraging HCPs to use screening tools for migraine and CM, such as the Identify (ID)‐Migraine34 and ID‐Chronic Migraine (ID‐CM).34
Rates of minimal pharmacologic treatment were greatly reduced in people diagnosed with CM (44%) compared with EM (67%).15 For CM, we required both acute and preventive medications, whereas only acute medication for migraine was required for EM.15 These requirements were based on the observation that most people with EM in the community do not meet criteria for preventive treatment,36 while virtually everyone with CM requires prevention. Although, we were unable to identify statistically significant predictors of effective treatment, this remains a crucial barrier. Healthcare professionals should aim to better understand and implement evidence‐based guidelines for optimal acute and preventive care of CM.
Of note, the magnitude of effect cannot be interpreted without examining the structure of the variable used to compute test statistic; thus, although reported ORs may have been small, they represent an incremental change in the scale of interest (eg, a 1‐point change in MIDAS [range 0–270]).
People with CM, by virtue of headache‐day frequency, report high levels of interference in activities of normal life, including the ability to work, perform routine chores, and build and maintain functional family, social, and community relationships.17 Preventive treatment regimens are often critical to ensuring optimal patient outcomes. The goals of migraine preventive therapies include: (1) reduction in attack frequency, severity, and duration; (2) improved responsiveness to acute treatments; (3) decreasing disability and improving overall function; (4) preventing analgesic overuse and “transformation” to chronic daily headache; and (5) reduction of overall cost of treatment.4, 36
This study has both strengths and limitations. We assigned diagnoses of CM and EM based on validated self‐reported measures, without access to medical assessments. Similarly, patterns of consultation, diagnosis, and treatment were based on self‐report without verification by medical claims and pharmacy data. However, this approach is common in large‐scale epidemiologic studies.37, 38 In addition, it is not possible to use medical claims and pharmacy data to assess people who remain undiagnosed and untreated. If diagnosis is unreported, diagnosis may not have been effectively communicated. Given these inherent limitations, we used validated and widely used diagnostic tools.24, 25 Some of our instruments, particularly those that examined patterns of consultation, diagnosis, and treatment, were not validated, although they had a high level of face validity. The items were also consistent with previous analyses examining barriers to EM care15 and the direction and magnitude of effect in this CM study were consistent with what would be expected for a more severe headache population.
To conduct this study, we made judgments about types of HCP providing ongoing medical care for CM. Of the 1254 respondents with MIDAS grade ≥2 and reporting health insurance status, 28 reported consulting with a type of HCP not included as an eligible provider for this study (eg, allergist; ear, nose, and throat specialist; emergency department; urgent care department; psychiatrist). For example, we judged that emergency department consultations represented one‐time evaluations and not ongoing treatment. Reasonable clinicians might disagree with these judgments, although the effect was likely small. We acknowledge that among these specialties, there may be highly qualified headache specialists. Four of these 28 individuals who consulted ineligible providers received a CM diagnosis and only one received minimal pharmacologic treatment. In this analysis of people with CM, we required preventive treatment as part of minimal pharmacologic treatment, while only acute treatments were required in the EM analysis. We also did not consider over‐the‐counter medications as part of our definition for minimal pharmacologic treatment because these medications may not be sufficient for patients with persistent disability, although they may be an appropriate choice for some patients.
In addition, we treated consultation, diagnosis, and treatment as cascade events. We used this conditional analytic strategy for 2 reasons: (1) we wanted to separately assess the predictors of each step among those who had traversed the previous step, and (2) effective treatment of CM requires an accurate diagnosis as a prerequisite to patient education and behavioral interventions. Nevertheless, people who never received or reported a diagnosis of CM might still receive minimal pharmacologic treatment, and were not captured in this analysis.
Some limitations were imposed by sample size. Power was limited to identify predictors of minimal pharmacologic treatment. We could not separately examine the influence of HCP type on diagnosis and treatment. In addition, our analyses were not powered to provide statistical comparisons and multiple comparisons were analyzed, which under certain circumstances can increase the likelihood of statistical significance being attributed to chance outcomes.39
A strength of this study is its large, systematically recruited sample. In addition, these results may be generalizable to the US migraine population, as a comparison of CaMEO and AMPP Study data yielded similar demographic characteristics, headache‐day frequency, and headache‐related disability among the respondents, despite methodologic differences.18, 40 This study also assigned migraine diagnosis based on a validated diagnostic module utilizing modified ICHD‐3b criteria, and used validated instruments to assess headache‐related disability, depression, and anxiety. Finally this analysis was able to adjust for multiple confounders.18, 40
An important next step toward developing targeted migraine education for both patients and HCPs (thus reducing the consultation barriers) is to gain additional understanding of the knowledge and motivations of patients for seeking care for their headache disorder and to understand patient satisfaction with care and expectations for treatment among those with EM and CM. The AMPP Study looked beyond the current barriers and also identified dissatisfaction with current acute treatment options and care to better understand unmet need within the EM population and found that among those with ≥1 unmet need, 37.4% were dissatisfied with their acute treatment regimen.41 Similar analyses are needed to understand treatment satisfaction among people with CM to improve understanding of motivations for consultation and expectations for management among people with migraine. These issues can be explored in future analyses of the CaMEO data.
STATEMENT OF AUTHORSHIP
Category 1
-
(a) Conception and Design
Aubrey Manack Adams, Michael Reed, Kristina Fanning, Dawn Buse, Richard Lipton, David Dodick
-
(b) Acquisition of Data
Aubrey Manack Adams, Michael Reed, Kristina Fanning
-
(c) Analysis and Interpretation of Data
Aubrey Manack Adams, Michael Reed, Kristina Fanning, Dawn Buse, Richard Lipton, David Dodick, Elizabeth Loder
Category 2
-
(a) Drafting the Manuscript
Aubrey Manack Adams, Michael Reed, Kristina Fanning, Dawn Buse, Richard Lipton, David Dodick, Elizabeth Loder
-
(b) Revising It for Intellectual Content
Aubrey Manack Adams, Michael Reed, Kristina Fanning, Dawn Buse, Richard Lipton, David Dodick, Elizabeth Loder
Category 3
-
(a) Final Approval of the Completed Manuscript
Aubrey Manack Adams, Michael Reed, Kristina Fanning, Dawn Buse, Richard Lipton, David Dodick, Elizabeth Loder
Acknowledgments
This study and analysis were sponsored by Allergan plc (Dublin, Ireland). The study sponsor was involved in the study design, data collection, data analysis, data interpretation and the writing of the article. AMA, MR, KF, DB, and RL were involved in development of the web‐based questionnaire. AMA, MR, KF, and RL were involved in development of the statistical analyses plan. DD, AMA, MR, KF, DB, and RL were involved in directing the data analyses through their activity on the CaMEO Steering Committee. All authors contributed intellectual comments to the development of this article. All authors had full access to all of the data. The corresponding author had final responsibility for submission of this article. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship. Writing and editorial assistance was provided to the authors by Amanda M. Kelly, MPhil, MSHN, and Meghan Johnson, PhD, of Complete Healthcare Communications, LLC (Chadds Ford, PA, USA), Dana Franznick, PharmD, and Kristine W. Schuler, MS, and funded by Allergan plc (Dublin, Ireland).
Conflicts of Interest:David W. Dodick, MD, in the past 12 months, has served on advisory boards and has consulted for Allergan, Amgen, Alder, CoLucid, Merck, ENeura, Eli Lilly & Company, Autonomic Technologies, Teva, Tonix, Novartis, Supernus, ScionNeurostim, and Boston Scientific. Within the past 12 months, Dr. Dodick has received royalties, funding for travel, speaking, or editorial activities from the following: Healthlogix, Haymarket Media Group, Ltd., SAGE Publishing, Synergy, Allergan, Lippincott Williams & Wilkins, Oxford University Press, and Cambridge University Press; he serves as Editor‐in‐Chief of Cephalalgia and on the editorial boards of The Neurologist, Lancet Neurology, and Postgraduate Medicine. He receives publishing royalties for Wolff's Headache, 8th edition (Oxford University Press, 2009), and Handbook of Headache (Cambridge University Press, 2010).
Elizabeth W. Loder, MD, MPH, receives salary paid to her institution from The British Medical Journal for services as a medical editor.
Richard B. Lipton, MD, has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol‐Myers Squibb, CoLucid, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff's Headache, 8th Edition (Oxford University Press, 2009).
Dawn C. Buse, PhD, in the past 12 months, has received grant support and honoraria from Allergan, Avanir, and the National Headache Foundation. She is an employee of Montefiore Medical Center, which has received research support funded by Alder, Allergan, Argus, Avanir, CoLucid, Dr. Reddy's Laboratories, Electrocore, Labrys, Merck, and Teva, both directly and via grants to the National Headache Foundation. She is on the editorial board of the Journal of Headache and Pain, Pain Pathways Magazine, and Pain Medicine News.
Aubrey Manack Adams, PhD, is an Allergan plc employee, and receives stock and stock options.
Michael L. Reed, PhD, and Kristina M. Fanning, PhD, are employees of Vedanta Research, which has received support funded by Allergan plc, CoLucid, Dr. Reddy's Laboratories, Endo Pharmaceuticals, GlaxoSmithKline, Merck & Co., Inc., NuPathe, Novartis, and Ortho‐McNeil, via grants to the National Headache Foundation.
REFERENCES
- 1. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 2. Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 2010;9:285‐298. [DOI] [PubMed] [Google Scholar]
- 3. Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: Focus on epilepsy and migraine. Lancet Neurol. 2006;5:148‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52:1456‐1470. [DOI] [PubMed] [Google Scholar]
- 5. Wang SJ, Wang PJ, Fuh JL, Peng KP, Ng K. Comparisons of disability, quality of life, and resource use between chronic and episodic migraineurs: A clinic‐based study in Taiwan. Cephalalgia. 2013;33:171‐181. [DOI] [PubMed] [Google Scholar]
- 6. Buse D, Manack A, Serrano D, et al. Headache impact of chronic and episodic migraine: Results from the American Migraine Prevalence and Prevention study. Headache. 2012;52:3‐17. [DOI] [PubMed] [Google Scholar]
- 7. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301‐315. [DOI] [PubMed] [Google Scholar]
- 8. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: Results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058‐1077. [DOI] [PubMed] [Google Scholar]
- 9. Bloudek LM, Stokes M, Buse DC, et al. Cost of healthcare for patients with migraine in five European countries: Results from the International Burden of Migraine Study (IBMS). J Headache Pain. 2012;13:361‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meletiche DM, Lofland JH, Young WB. Quality‐of‐life differences between patients with episodic and transformed migraine. Headache. 2001;41:573–578. [DOI] [PubMed] [Google Scholar]
- 11. Payne KA, Varon SF, Kawata AK, et al. The International Burden of Migraine Study (IBMS): Study design, methodology, and baseline cohort characteristics. Cephalalgia. 2011;31:1116‐1130. [DOI] [PubMed] [Google Scholar]
- 12. Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. J Neurol. 2013;260:1960‐1969. [DOI] [PubMed] [Google Scholar]
- 13. Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new‐onset chronic migraine. Neurology. 2015;84:688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine. Identifying and removing barriers to care. Neurology. 1994;44:S63‐68. [PubMed] [Google Scholar]
- 15. Lipton RB, Serrano D, Holland S, Fanning KM, Reed ML, Buse DC. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache. 2013;53:81‐92. [DOI] [PubMed] [Google Scholar]
- 16. Post MW, Boosman H, van Zandvoort MM, Passier PE, Rinkel GJ, Visser‐Meily JM. Development and validation of a short version of the Stroke Specific Quality of Life Scale. J Neurol Neurosurg Psychiatry. 2011;82:283‐286. [DOI] [PubMed] [Google Scholar]
- 17. Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: Burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559‐566. [DOI] [PubMed] [Google Scholar]
- 18. Manack Adams A, Serrano D, Buse DC, et al. The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) study methods and baseline results. Cephalalgia. 2015;35:563‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manack AN, Reed ML, Marske V, et al. CaMEO (Chronic Migraine Epidemiology & Outcomes) Study: Design, Methodology and Baseline Cohort Characteristics. Paper presented at the International Headache Congress (IHC). Boston, MA, USA. Poster P60, June 27–30, 2013.
- 20. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache. 2001;41:646‐657. [DOI] [PubMed] [Google Scholar]
- 21. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64‐69. [PubMed] [Google Scholar]
- 22. Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near‐daily headaches: Field trial of revised IHS criteria. Neurology. 1996;47:871‐875. [DOI] [PubMed] [Google Scholar]
- 23. Silberstein SD, Lipton RB, Solomon S, Mathew N. Classification of daily and near‐daily headaches in the headache clinic. Proposed revisions to the International Headache Society criteria In: Olesen J, ed. Frontiers in Headache Research. New York: Raven Press, Ltd; 1994:117‐126. [Google Scholar]
- 24. Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: Results from the American Migraine Study II. Headache. 2001;41:638‐645. [DOI] [PubMed] [Google Scholar]
- 25. Liebenstein M, Bigal M, Sheftell F, Tepper S, Rapoport A, Lipton R. Validation of the Chronic Daily Headache Questionnaire (CDH‐Q), Abstract F25. Paper presented at the 49th Annual Scientific Meeting of the American Headache Society. Chicago, IL, June 7–11, 2007.
- 26. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9‐160. [DOI] [PubMed] [Google Scholar]
- 27. Stewart WF, Lipton RB, Simon D. Work‐related disability: Results from the American migraine study. Cephalalgia. 1996;16:231‐238; discussion 215. [DOI] [PubMed] [Google Scholar]
- 28. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th ed. American Psychiatric Association: Washington, DC; 1994.
- 30. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch Intern Med. 2006;166:1092‐1097. [DOI] [PubMed] [Google Scholar]
- 31. Lowe B, Decker O, Muller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD‐7) in the general population. Med Care. 2008;46:266‐274. [DOI] [PubMed] [Google Scholar]
- 32. Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317‐325. [DOI] [PubMed] [Google Scholar]
- 33. Gibbs TS, Fleischer AB Jr, Feldman SR, Sam MC, O'Donovan CA. Health care utilization in patients with migraine: Demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330‐335. [DOI] [PubMed] [Google Scholar]
- 34. Lipton RB, Dodick D, Sadovsky R, et al. A self‐administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61:375‐382. [DOI] [PubMed] [Google Scholar]
- 35. Lipton RB, Serrano D, Buse DC, et al. Improving the detection of chronic migraine: Development and validation of the Identify Chronic Migraine (ID‐CM). Cephalalgia. 2015 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. [DOI] [PubMed] [Google Scholar]
- 37. Silberstein SD. Practice parameter: Evidence‐based guidelines for migraine headache (an evidence‐based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology . 2000;55:754‐762. [DOI] [PubMed] [Google Scholar]
- 38. McAdams MA, Maynard JW, Baer AN, et al. Reliability and sensitivity of the self‐report of physician‐diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38:135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Short ME, Goetzel RZ, Pei X, et al. How accurate are self‐reports? Analysis of self‐reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51:786‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gelman A, Hill J, Yajima M. Why we (usually) don't have to worry about multiple comparisons. J Res Educ Eff. 2012;5:189‐211. [Google Scholar]
- 41. Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A Comparison of the CaMEO (Chronic Migraine Epidemiology & Outcomes) Study and AMPP (American Migraine Prevalence and Prevention) Study: Demographics and Headache‐Related Disability. Paper presented at the 57th Annual Scientific Meeting of the American Headache Society (AHS). Washington, DC, 2015.
- 42. Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: Results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:1300‐1311. [DOI] [PubMed] [Google Scholar]


