Summary
Obesity has a negative impact on health‐related quality of life (HRQoL). The SCALE Obesity and Prediabetes study investigated the effect of liraglutide 3.0 mg, as adjunct to diet and exercise, on HRQoL in patients with obesity [body mass index (BMI) ≥ 30 kg m−2] or overweight (BMI ≥ 27 kg m−2) with comorbidity. Participants were advised on a 500 kcal d−1 deficit diet and a 150‐min week−1 exercise programme and were randomised 2:1 to once‐daily subcutaneous liraglutide 3.0 mg or placebo. HRQoL was assessed using the Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite) and Short‐Form 36 (SF‐36) v2 health questionnaires. Individuals on liraglutide 3.0 mg (n = 2046) had significantly greater improvements in IWQOL‐Lite total score (10.6 ± 13.3) vs. placebo (n = 1020) (7.7 ± 12.8) and SF‐36 physical (PCS) and mental (MCS) component summary scores (PCS, 3.6 ± 6.8; MCS, 0.2 ± 8.1) vs. placebo (PCS, 2.2 ± 7.7; MCS, −0.9 ± 9.1). The estimated treatment differences were IWQOL‐Lite total score 3.1 (95% CI: 2.2; 4.0), P < 0.0001; SF‐36 PCS 1.7 (95% CI: 1.2; 2.2), P < 0.0001 and MCS 0.9 (95% CI: 0.3; 1.5), P = 0.003. All subscales of the IWQOL‐Lite and SF‐36 were significantly improved with liraglutide 3.0 mg vs. placebo. More patients on liraglutide 3.0 mg experienced meaningful improvement on the IWQOL‐Lite total (P < 0.0001) and the SF‐36 PCS (P < 0.0001) scores.
Keywords: IWQOL‐Lite, liraglutide 3.0 mg, quality of life, SF‐36v2
What is already known about this subject?
Obesity and overweight have a negative effect on health‐related quality of life (HRQoL).
Previous studies into the effect of weight loss on HRQoL in people with obesity or overweight have produced inconsistent results.
Liraglutide 3.0 mg is a glucagon‐like peptide‐1 receptor agonist approved in the EU, US, Mexico, Brazil and Canada for chronic weight management in people with obesity or overweight with comorbidities.
What this study adds
Liraglutide 3.0 mg, as adjunct to diet and exercise, is associated with statistically significant and meaningful improvements in HRQoL in people who have obesity or are overweight with comorbidities, compared with placebo.
Improvements in HRQoL with liraglutide 3.0 mg were observed using both disease‐specific and generic instruments, across all physical and mental HRQoL subscales; however, the greatest improvements were seen in the physical aspects of HRQoL and self‐esteem.
Introduction
Obesity is a chronic disease associated with an increased risk of type 2 diabetes, cardiovascular disease, select cancers and a reduced life expectancy. A number of other comorbidities have also been linked with obesity, including urinary incontinence, joint and mobility problems and obstructive sleep apnoea 1, 2, 3, 4. Studies have shown that the health risks of obesity, alongside social stigmatization and discrimination, have a negative impact on health‐related quality of life (HRQoL), the degree of which is dependent upon the level of obesity – higher body mass index (BMI) being correlated with poorer HRQoL 5, 6, 7, 8. HRQoL is often defined as the subjective assessment of the impact of disease and treatment across the physical, psychological and social domains of functioning and well‐being 9. In addition to the acute health effects of obesity, there are also economic consequences for individuals and society through lower rates of productivity and increased absenteeism 10, 11, 12, 13. There is a clinical imperative for improving HRQoL in people with obesity as it may represent tangible physical benefits to their daily lives, for example, walking to the shops, playing football with their children or grandchildren in the park or tying their shoelaces. Regarding the mental aspects of HRQoL, improvements related to psychosocial functioning could mean feeling more energetic, freedom from anxiety and depression or improved well‐being 14.
Weight loss can improve overall health and HRQoL in people with obesity, although it should be noted that improvements in HRQoL have not been universally observed in clinical studies 8, 15, 16, 17, 18. Even when statistically significant differences in HRQoL are observed in clinical trials, they are not always of a magnitude that is meaningful to the patient 19. Recognizing the importance of HRQoL, there has been growing support for the inclusion of HRQoL scales in clinical trials to inform the decisions of drug‐licensing agencies and healthcare payers 20, 21.
Weight loss strategies involving diet and exercise are the first‐line treatment for obesity; however, the success rates of such interventions are relatively low, particularly over the long term, with up to two‐thirds of dieters regaining more weight than they lost on their diets. In many cases, pharmacological intervention, alongside diet and exercise, is ultimately required 22, 23, 24, 25, 26, 27.
Liraglutide, a glucagon‐like peptide‐1 (GLP‐1) analogue, is currently licensed for the treatment of type 2 diabetes at doses of up to 1.8 mg, and a 3.0 mg d−1 dose has been approved in the EU, US, Mexico, Brazil and Canada for weight management in people with obesity or overweight with comorbidity. Weight loss with liraglutide is dose‐dependent up to 3.0 mg once daily and is mediated by reduced appetite and energy intake rather than by increased energy expenditure 28, 29. Satiety and Clinical Adiposity – Liraglutide Evidence (SCALE) Obesity and Prediabetes, a phase 3a clinical trial, found that liraglutide 3.0 mg induced weight loss of 8.0% (8.4 kg) of baseline body weight over 56 weeks compared to 2.6% (2.8 kg) with placebo, both in combination with lifestyle intervention, and improved a range of weight‐related comorbidities 30. The aim of this analysis was to investigate the effects of liraglutide 3.0 mg, as an adjunct to diet and exercise, on HRQoL in people with obesity or overweight with comorbidity in the SCALE Obesity and Prediabetes trial.
Materials and methods
The study was a 56‐week, randomized, double‐blind, placebo‐controlled, parallel group, multi‐centre, multinational trial. It enrolled adults (18 years of age or older) with obesity (BMI ≥ 30 kg m−2) or overweight (BMI ≥ 27 kg m−2), with treated or untreated hypertension or dyslipidaemia. People with type 2 diabetes were excluded, whilst patients with prediabetes were permitted to enrol. Additional details of the inclusion and exclusion criteria are reported elsewhere 30. Participants were advised on a 500 kcal d−1 deficit diet and a 150‐min week−1 exercise programme and were randomized 2:1 to once‐daily subcutaneous liraglutide 3.0 mg (n = 2487) or placebo (n = 1244), which was initiated at 0.6 mg d−1 and escalated in weekly increments of 0.6 mg until the target dose of 3.0 mg was reached.
Two separate questionnaires were used to evaluate HRQoL: Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite) and the Short Form‐36 version 2 (SF‐36v2) health survey. Participants were assessed at baseline, mid trial (28 weeks) and end of trial (56 weeks) in those countries with linguistically validated translations (14 of 27 countries; ~82% of participants). The IWQOL‐Lite is a validated, 31‐item, self‐reported measure of obesity‐specific quality of life 31, 32. The IWQOL‐Lite provides a total score and scores for five subscales: physical function, self‐esteem, sexual life, public distress and work. The IWQOL‐Lite is sensitive to the degree of obesity and responsive to weight loss and weight gain 33, 34, 35. Scores range from 0 to 100 (with lower scores indicating greater impairment). The SF‐36v2 is a generic measure of health status, comprising 36 questions distributed across eight subscales. Two summary measures – the physical component summary (PCS) and the mental component summary (MCS) – are calculated from the eight scales 36. High scores represent good health status, with a score of 50 being the mean for the US general population. The SF‐36 has previously been validated in large‐scale, population‐based surveys and in health surveys involving people with obesity 37, 38, 39.
Statistical methods
Baseline characteristics were evaluated using descriptive statistics for both the entire trial population and the study population assessed for HRQoL. This was an exploratory analysis (without adjustment for multiple testing). Absolute change in HRQoL scores from Week 0 to Week 56 was compared between liraglutide 3.0 mg and placebo using an analysis of covariance (ancova) model, with treatment, country, gender, BMI stratification factor (27.0–29.9 kg m−2, 30.0–34.9 kg m−2, 35.0–39.0 kg m−2, >40 kg m−2), prediabetes status at screening, interaction between BMI strata and prediabetes status at screening as fixed factors and baseline HRQoL scores (at Week 0) as covariates. The analysis was based on the full analysis set (FAS; all randomized participants exposed to at least one dose of the trial product and with at least one post‐baseline measurement) from countries where HRQoL was assessed. From this model, the differences between the treatment groups were estimated together with the corresponding two‐sided 95% confidence interval (CI) and p‐value. Endpoints evaluated were absolute change from baseline (Week 0) to Week 56 in the IWQOL‐Lite total score and the SF‐36 PCS and MCS scores and corresponding subscales. Missing values for HRQoL questionnaires at 56 weeks were imputed using last observation carried forward (LOCF). As a sensitivity analysis, repeated measures analysis of covariance (ancova) was also carried out as an alternative to LOCF.
Meaningful change was defined differently for the IWQOL‐Lite and the SF‐36 according to published algorithms. For the IWQOL‐Lite total score, the cut‐off for ‘improvement' and ‘deterioration' varied depending on baseline severity 40. Cut‐off values are provided in Table S1, Supporting Information. For the SF‐36 PCS, ‘improvement' was defined as a change from baseline ≥3.8; ‘no change' between −3.8 and 3.8; and ‘deterioration' was a change from baseline ≤ −3.8. The corresponding values for the MCS were ‘improvement' as a change from baseline ≥4.6; ‘no change' −4.6 and 4.6; and ‘deterioration' ≤ −4.6 41. Proportional odds models are the most popular model for ordinal logistic regression. The resulting odds ratio (OR) for a predictor can be interpreted as a summary of the odds ratios obtained from separate binary logistic regressions using all possible cut‐offs of the ordinal outcome 42. The odds of achieving a meaningful improvement in the HRQoL score were estimated using an ordinal logistic regression/proportional odds model with a cumulative logit link. The model included treatment, gender, prediabetes status at screening, BMI stratification factor and an interaction between prediabetes status at screening and BMI stratification factor as fixed factors and the baseline HRQoL score (Week 0) as a covariate.
Changes in HRQoL scores were also evaluated by categories of weight change (weight gain, weight loss 0–4.9%, weight loss 5–9.9%, weight loss 10–14.9% and weight loss ≥15%) to evaluate how the magnitude of weight loss related to HRQoL.
Results
Participant characteristics
During the study period, a total of 2487 and 1244 participants were treated with liraglutide 3.0 mg and placebo, respectively. Of these, 2046 (82%) participants in the liraglutide 3.0 mg treatment arm and 1020 (82%) participants in the placebo treatment arm were assessed for HRQoL. Patient demographic and baseline characteristics were similar for both treatment arms as well as for the entire trial population and the study population in which HRQoL was assessed (Table 1). The percentage of patients who had valid IWQOL‐Lite and SF‐36 assessments at Week 56 was similar to the percentage of patients who completed the trial in countries where the health questionnaires were administered (data not shown).
Table 1.
Subject demographics and baseline characteristics for the entire trial population and participants in whom HRQoL was assessed
| Total trial population | HRQoL‐assessed population | |||
|---|---|---|---|---|
| Liraglutide 3.0 mg (n = 2487) | Placebo (n = 1244) | Liraglutide 3.0 mg (n = 2046) | Placebo (n = 1020) | |
| Age, years ± SD | 45.2 ± 12.1 | 45.0 ± 12.0 | 45.5 ± 12.1 | 45.2 ± 12.0 |
| Age group | ||||
| 18–39 years | 856 (34.4%) | 415 (33.4%) | 686 (33.5%) | 331 (32.5%) |
| 40–64 years | 1495 (60.1%) | 760 (61.1%) | 1242 (60.7%) | 631 (61.9%) |
| 65–74 years | 130 (5.2%) | 68 (5.5%) | 113 (5.5%) | 57 (5.6%) |
| ≥75 years | 6 (0.2%) | 1 (<0.1%) | 5 (0.2%) | 1 (<0.1%) |
| Gender | ||||
| Female | 1957 (78.7%) | 971 (78.1%) | 1639 (80.1%) | 803 (78.7%) |
| Male | 530 (21.3%) | 273 (21.9%) | 407 (19.9%) | 217 (21.3%) |
| BMI, kg m−2 ± SD | 38.3 ± 6.4 | 38.3 ± 6.3 | 38.5 ± 6.5 | 38.6 ± 6.5 |
| BMI group | ||||
| 27.0–29.9 kg m−2 | 66 (2.7%) | 44 (3.5%) | 55 (2.7%) | 39 (3.8%) |
| 30.0–34.9 kg m−2 | 806 (32.4%) | 388 (31.2%) | 644 (31.5%) | 301 (29.5%) |
| 35.0–39.9 kg m−2 | 787 (31.6%) | 398 (32.0%) | 649 (31.7%) | 318 (31.2%) |
| >40 kg m−2 | 828 (33.3%) | 414 (33.3%) | 698 (34.1%) | 362 (35.5) |
| Waist circumference, cm ± SD | 115.0 ± 14.4 | 114.5 ± 14.3 | 114.9 ± 14.7 | 114.8 ± 14.6 |
| Body weight, kg ± SD | 106.2 ± 21.2 | 106.2 ± 21.7 | 106.4 ± 21.5 | 107.0 ± 22.1 |
| Prediabetes status | ||||
| With prediabetes | 1528 (61.4%) | 757 (60.9%) | 1218 (59.5%) | 594 (58.2%) |
| Without prediabetes | 959 (38.6%) | 487 (39.1%) | 828 (40.5%) | 426 (41.8) |
| Glycated haemoglobin, % ± SD | 5.6 ± 0.4 | 5.6 ± 0.4 | 5.6 ± 0.4 | 5.6 ± 0.4 |
| Fasting glucose, mg dL−1 ± SD | 95.9 ± 10.6 | 95.5 ± 9.8 | 95.6 ± 10.6 | 95.1 ± 9.8 |
| Fasting serum insulin, uIU mL−1 ± CV | 16.3 ± 79.8 | 16.1 ± 89.3 | 16.4 ± 81.5 | 16.4 ± 87.6 |
| History of cardiovascular disease*, yes (%) | 216 (8.7) | 105 (8.5) | 192 (9.4) | 93 (9.1) |
| Blood pressure, mmHg ± SD | ||||
| Systolic | 123.0 ± 12.9 | 123.2 ± 12.8 | 122.7 ± 13.0 | 122.7 ± 12.7 |
| Diastolic | 78.7 ± 8.6 | 78.9 ± 8.5 | 78.6 ± 8.6 | 78.7 ± 8.4 |
| Cholesterol, mg dL−1 ± CV | ||||
| Total | 193.7 ± 19.1 | 194.3 ± 18.8 | 193.9 ± 19.2 | 193.9 ± 18.6 |
| LDL | 111.6 ± 27.9 | 112.2 ± 27.6 | 111.6 ± 28.3 | 111.6 ± 27.5 |
| HDL | 51.4 ± 26.2 | 51.0 ± 26.4 | 51.8 ± 26.1 | 51.3 ± 26.1 |
| Free fatty acids, mmol L−1 ± CV | 0.45 ± 40.5 | 0.46 ± 39.7 | 0.5 ± 40.5 | 0.5 ± 38.8 |
| Triglycerides, mg dL−1 ± CV | 126.2 ± 56.9 | 128.9 ± 61.0 | 125.7 ± 56.2 | 128.6 ± 50.4 |
Based on standardized MedDRA queries, ischaemic heart disease, cardiac failure, central nervous system haemorrhages, cerebrovascular conditions, embolic and thrombotic events.
BMI, body mass index; CV, coefficient of variation; HDL, high‐density lipoprotein; HRQoL, health‐related quality of life; LDL, low‐density lipoprotein; MedDRA, Medical Dictionary for Regulatory Activities; SD, standard deviation (sample).
IWQOL‐Lite results
Baseline IWQOL‐Lite scores were similar for the liraglutide 3.0 mg and placebo groups, with mean total scores indicating ‘moderate' impairment according to the IWQOL‐Lite Manual 43 (Table 2). The proportion of participants with scores reaching the maximum value (100) varied depending on the subscale, ranging from 1.1% (total score, baseline, liraglutide 3.0 mg) up to 64.7% (work score, Week 56, liraglutide 3.0 mg) (Table 2). Analysis of treatment interaction with glycaemic status (prediabetes vs. normoglycaemia) yielded no significant differences across IWQOL‐Lite total or subscales (data not shown).
Table 2.
Change in total and subscale HRQoL scores between baseline and Week 56, by treatment arm, for IWQOL‐Lite and SF‐36
| Summary/subscale score | Liraglutide 3.0 mg | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline score (LS mean ± SD) | Change from baseline score (LS mean ± SE) | Proportion of participants having maximum score at baseline/Week 56 (%) | n | Baseline score (LS mean) | Change from baseline score (LS mean ± SE) | Proportion of participants having maximum score at baseline/Week 56 (%) | |
| IWQOL‐Lite total | 1891 | 73.13 ± 18.01 | 10.66 ± 0.25 | 1.1/8.1 | 890 | 72.49 ± 17.86 | 7.54 ± 0.37 | 1.0/3.9 |
| Physical function | 1891 | 69.39 ± 21.65 | 13.36 ± 0.31 | 3.2/15.2 | 891 | 69.04 ± 21.98 | 8.55 ± 0.46 | 2.6/10.4 |
| Self‐esteem | 1893 | 61.12 ± 25.90 | 13.88 ± 0.41 | 6.1/20.3 | 891 | 58.99 ± 26.00 | 10.64 ± 0.59 | 5.3/15.0 |
| Sexual life | 1853 | 77.48 ± 26.45 | 8.51 ± 0.40 | 38.1/54.5 | 877 | 77.63 ± 26.20 | 6.03 ± 0.58 | 38.0/51.6 |
| Public distress | 1893 | 83.96 ± 20.59 | 6.03 ± 0.28 | 38.3/55.2 | 891 | 83.58 ± 20.45 | 4.44 ± 0.41 | 37.3/51.3 |
| Work | 1890 | 86.78 ± 18.43 | 5.56 ± 0.27 | 46.7/64.7 | 889 | 86.73 ± 18.57 | 4.51 ± 0.40 | 45.8/62.6 |
| SF‐36 PCS | 1690 | 48.25 ± 8.35 | 3.66 ± 0.15 | 0.0/0.0 | 799 | 47.67 ± 8.70 | 1.93 ± 0.21 | 0.0/0.0 |
| SF‐36 MCS | 1690 | 53.84 ± 8.08 | 0.14 ± 0.17 | 0.0/0.0 | 799 | 53.94 ± 7.93 | −0.76 ± 0.25 | 0.0/0.0 |
| Physical functioning | 1689 | 47.89 ± 8.47 | 3.64 ± 0.15 | 15.0/32.8 | 799 | 47.53 ± 8.76 | 2.08 ± 0.22 | 12.7/25.2 |
| Role physical | 1690 | 50.00 ± 8.25 | 2.76 ± 0.16 | 39.1/57.0 | 799 | 49.59 ± 8.64 | 1.29 ± 0.23 | 38.1/49.8 |
| Bodily Pain | 1690 | 50.80 ± 9.72 | 1.86 ± 0.21 | 27.6/35.7 | 799 | 50.33 ± 9.81 | −0.02 ± 0.30 | 25.0/27.7 |
| General health | 1689 | 49.50 ± 8.76 | 3.30 ± 0.16 | 2.9/6.1 | 797 | 49.03 ± 8.70 | 1.43 ± 0.23 | 1.8/4.2 |
| Vitality | 1689 | 52.57 ± 8.88 | 2.43 ± 0.19 | 1.9/5.0 | 799 | 52.12 ± 8.69 | 1.10 ± 0.27 | 1.9/2.9 |
| Social functioning | 1689 | 51.89 ± 7.80 | 1.11 ± 0.16 | 61.5/68.9 | 799 | 51.91 ± 7.83 | 0.09 ± 0.24 | 61.1/64.5 |
| Role emotional | 1690 | 51.53 ± 7.82 | 0.17 ± 0.16 | 64.6/69.2 | 799 | 51.99 ± 7.16 | −0.35 ± 0.24 | 66.5/66.5 |
| Mental health | 1689 | 53.40 ± 8.29 | 0.76 ± 0.18 | 8.1/11.4 | 799 | 53.00 ± 8.19 | −0.45 ± 0.27 | 7.0/8.6 |
IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; MCS, mental component summary; PCS, physical component summary; SD, standard deviation; SE, standard error; SF‐36, Short‐Form 36; LS, least square.
At Week 56, all subscales of the IWQOL‐Lite showed a significantly greater increase from baseline in the liraglutide 3.0 mg group compared with placebo. Changes in IWQOL‐Lite total score were also significantly different for liraglutide 3.0 mg vs. placebo, favouring liraglutide 3.0 mg (Table 2), estimated treatment difference 3.13 (95% CI: 2.24, 4.01), P < 0.0001 (Fig. 1). The greatest difference between liraglutide 3.0 mg and placebo was observed in the physical function subscale [estimated treatment difference 4.80 (95% CI: 3.72, 5.89), P < 0.0001] (Fig. 1). In addition, the odds of achieving a meaningful improvement for the IWQOL‐Lite total score were higher with liraglutide 3.0 mg than with placebo [OR 1.59 (95% CI: 1.35; 1.88), P < 0.0001] (Fig. 2).
Figure 1.
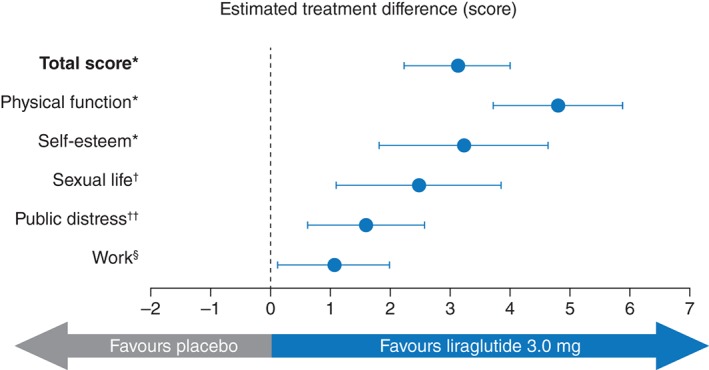
IWQOL‐Lite estimated treatment difference for total and subscale scores at Week 56. Data are estimated treatment difference and 95% confidence intervals. *P < 0.0001, † P = 0.0004, †† P = 0.0013, § P = 0.028. IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite.
Figure 2.

Meaningful change in HRQoL at Week 56 for IWQOL‐Lite and SF‐36. Meaningful change was defined differently for the IWQOL‐Lite and the SF‐36 according to published algorithms. For the IWQOL‐Lite total score, the cut‐off for 'improvement' and 'deterioration' varied depending on baseline severity40. Cut‐off values are provided in Table S1. For the SF‐36 PCS, 'deterioration' was defined as a change from baseline ≤ −3.8; 'no change' between −3.8 and 3.8; and 'improvement' was a change from baseline ≥3.8. The corresponding values for the MCS were 'deterioration' as change from baseline ≤ −4.6; 'no change’, ‐4.6 and 4.6 and 'improvement' ≥4.6. CI, confidence interval; HRQoL, health‐related quality of life; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; MCS, mental component summary; OR, odds ratio; PCS, physical component summary; SF‐36, Short‐Form 36.
Estimated treatment differences using repeated measures ancova as an alternative to LOCF confirmed that scores for liraglutide 3.0 mg were significantly improved for all subscales and total score compared to placebo (data not shown). Estimated treatment difference for IWQOL‐Lite total score was 3.48 [(95% CI: 2.52; 4.45), P < 0.0001] at end of trial.
SF‐36 results
Baseline SF‐36 scores were similar between the liraglutide 3.0 mg and placebo groups, with mean PCS scores below the normative mean of 50 and mean MCS scores above the normative mean (Table 2). There were no participants with the maximum score in either of the summary scores. The proportion of participants with maximum score on a subscale ranged from 1.9% (general health scale, baseline, placebo) up to 69.2% (role emotional scale, Week 56, liraglutide 3.0 mg) (Table 2). Analysis of treatment interaction with glycaemic status (prediabetes vs. normoglycaemia) yielded no significant differences across SF‐36 summary scores or subscales (data not shown).
At Week 56, all subscales of the SF‐36 showed a significantly greater increase from baseline in the liraglutide 3.0 mg group compared with placebo (Table 2). Changes in PCS and MCS scores were also significantly different for liraglutide 3.0 mg vs. placebo, favouring liraglutide 3.0 mg (Table 2). Estimated treatment differences for PCS and MCS scores were 1.73 [(95% CI: 1.22, 2.24), P < 0.0001] and 0.90 [(95% CI: 0.30, 1.50), P = 0.0034], respectively (Fig. 3). The greatest difference between liraglutide 3.0 mg and placebo was observed in the bodily pain subscale [estimated treatment difference 1.88 (95% CI: 1.17, 2.59), P < 0.0001]. In addition, the odds of achieving a meaningful improvement for the PCS score were significantly greater with liraglutide 3.0 mg than with placebo [OR 1.60 (95% CI: 1.35, 1.90), P < 0.0001); however, the effect for the MCS score was not statistically significant [OR 1.14 (95% CI: 0.96, 1.35), P = 0.1427] (Fig. 2).
Figure 3.
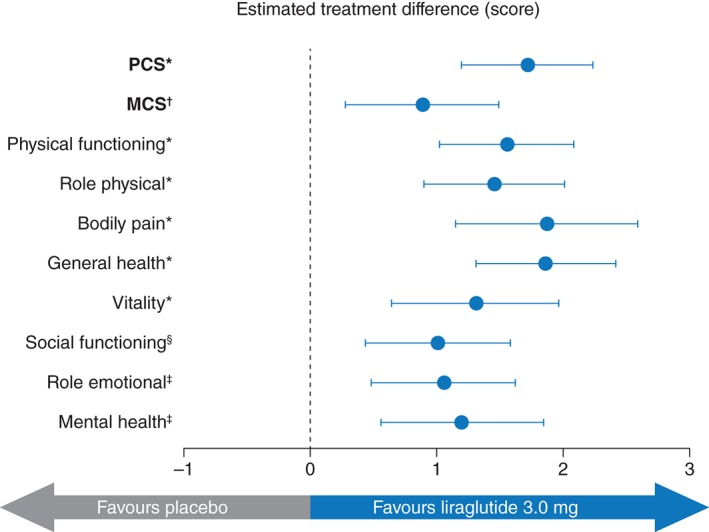
SF‐36 estimated treatment difference for PCS and MCS and subscale scores at Week 56. Data are estimated treatment difference and 95% confidence intervals. *P < 0.0001, † P = 0.0034, ‡ P = 0.0002, § P = 0.0004. MCS, mental component summary; PCS, physical component summary; SF‐36, Short‐Form 36.
Repeated measures ancova confirmed that changes in score were significantly improved with liraglutide 3.0 mg for all subscales, as well as PCS and MCS, compared to placebo (data not shown). Estimated treatment difference for SF‐36 PCS and MCS scores were, respectively, 1.78 [(95% CI: 1.24; 2.33), P < 0.0001] and 0.97 [(95% CI: 0.33; 1.60), P = 0.0028].
Categorical weight loss and health‐related quality of life
The proportion of patients in each weight loss category by treatment arm for IWQOL‐Lite total, SF‐36 PCS and SF‐36 MCS scores is shown in Fig. 4. For both liraglutide 3.0 mg and placebo, there was a pattern for greater improvement in IWQOL‐Lite total score and SF‐36 PCS score in groups with higher weight loss (Fig. 4). However, there did not appear to be any pattern to changes in MCS by weight loss category for either liraglutide 3.0 mg or placebo.
Figure 4.
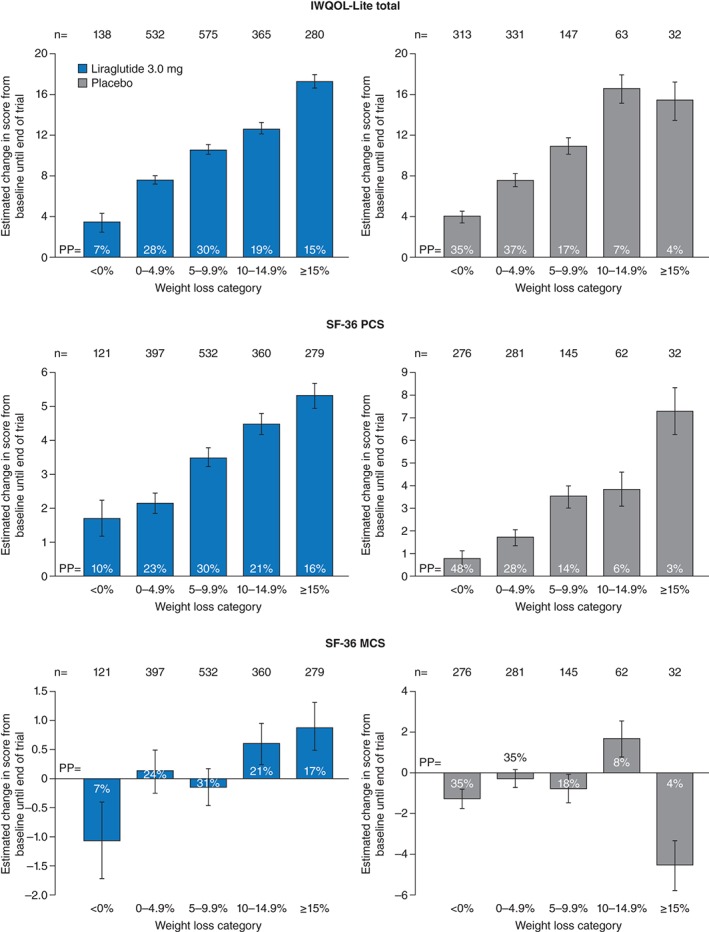
Estimated change in HRQoL score (IWQOL‐Lite total and SF‐36 PCS and MCS) by weight loss category for liraglutide 3.0 mg and placebo. Error bars are standard error. IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; MCS, mental component summary; PCS, physical component summary; PP, proportion of participants; SF‐36, Short‐Form 36.
Discussion
This analysis demonstrates that treatment with liraglutide 3.0 mg, as adjunct to diet and exercise, is associated with statistically significant improvements in HRQoL in people who have obesity or are overweight with comorbidity, compared with placebo. Improvements in HRQoL with liraglutide 3.0 mg were observed using both disease‐specific and generic instruments across physical and mental HRQoL subscales; however, the greatest improvements were seen in the physical aspects of HRQoL and self‐esteem. More people in the liraglutide group had meaningful improvements in their IWQOL‐Lite total and SF‐36 PCS scores. The categorical weight‐loss results of this study suggest that increasing weight loss was associated with greater improvements in HRQoL regardless of treatment arm; however, the liraglutide 3.0 mg group experienced greater improvements in HRQoL than the placebo group, presumably because they lost more weight.
Previous studies using generic and obesity‐specific health questionnaires have shown mixed results concerning the effect of weight loss on HRQoL 8, 15, 16, 17, 18, 44. Where improvements in HRQoL have been associated with weight loss, physical health has been the main driver, especially as measured by the SF‐36 8. The results of our study confirm the importance of physical health in HRQoL for people with obesity or overweight undergoing weight‐loss interventions. However, our findings may not extend to individuals with class III obesity (BMI ≥ 40 kg m−2). A meta‐analysis of a broad range of study populations reports that while both physical and mental HRQoL are impaired in individuals with obesity, the patterns of impairment differ by BMI: physical HRQoL is impaired among individuals with overweight (BMI = 25–29.9 kg m−2) and/or obesity (BMI = 30–39.9 kg m−2), while mental HRQoL is only affected in individuals who have class III obesity 7.
The results of our analysis are strengthened by the double‐blinded, randomized, placebo‐controlled trial design, which reduces the risk of bias. A large and diverse population of participants was recruited to the study, giving the results a high degree of external validity. Furthermore, both the disease‐specific and generic health questionnaires used in this analysis produced similar results for liraglutide 3.0 mg vs. placebo. This reinforces the results by allowing comparisons of HRQoL through different methods.
The observations in our analysis are subject to limitations. As HRQoL was not the primary endpoint of the trial, these results are exploratory rather than confirmatory. The proportions of participants with the maximum score (ceiling effect) were high in the IWQOL‐Lite sexual life, public distress and work and, in SF‐36, role physical, social functioning and role emotional subscales, which may have caused us to underestimate the potential effects of liraglutide within these domains. Although not all participants in the trial had HRQoL assessed, the majority (82%) did, and the HRQoL study population was representative of the entire study population, as evidenced by similar baseline characteristics and demographics.
The results of our analysis are highly clinically relevant because they demonstrate that liraglutide 3.0 mg is associated with improvements in HRQoL, particularly in physical functioning and self‐esteem, alongside weight loss, when compared with placebo; however, further investigation is needed to evaluate whether these improvements are maintained over the long term.
In summary, obesity has a serious detrimental effect on HRQoL, with negative consequences for both the individual and wider society. The results of this analysis demonstrate that treatment with liraglutide 3.0 mg, in addition to diet and exercise, is associated with better HRQoL in people with obesity or overweight with comorbidity compared with placebo. These findings will help to inform patients, clinicians and healthcare payers on the benefits of treatment with liraglutide 3.0 mg in people with obesity or overweight with comorbidity.
Conflict of interest statement
RLK has received travel support from Orexigen Therapeutics and Novo Nordisk; served as a consultant to Orexigen Therapeutics, Novo Nordisk and Eisai; served on an advisory panel for Novo Nordisk and Janssen; and receives royalties from Duke University for the IWQOL‐Lite. JBB is an employee of Optum, who distributes the SF‐36v2, and has served as consultant to Novo Nordisk. JHB is a full‐time employee of Novo Nordisk Inc. and a stockholder in Novo Nordisk A/S. MLW is a full‐time employee and stockholder of Novo Nordisk A/S. KF has served on the scientific advisory board for Novo Nordisk and received honoraria as a speaker.
Supporting information
Table S1. Cut‐off values for improvement and deterioration in IWQOL‐Lite total score by baseline severity.
Acknowledgements
KF was involved in the study design and implementation. RLK and JBB contributed to the statistical analyses. All authors were involved in the data interpretation, writing the manuscript and had final approval of the submitted and published versions. We thank all investigators, trial staff and participants. We also thank Paul Tisdale Ph.D. and Izabel James MD, of Watermeadow Medical, UK, funded by Novo Nordisk, for providing medical writing and editorial support. The study was sponsored by Novo Nordisk. Novo Nordisk contributed to study design and conduct, data collection, analysis and interpretation (NCT01272219).
References
- 1. Guh DP, Zhang W, Bansback N et al. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health 2009; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens 2007; 20: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prospective Studies Collaboration , Whitlock G, Lewington S, Sherliker P et al. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eheman C, Henley SJ, Ballard‐Barbash R et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012; 118: 2338–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myers A, Rosen J. Obesity stigmatization and coping: relation to mental health symptoms, body image and self‐esteem. Int J Obes Relat Metab Disord 1999; 23: 221–230. [DOI] [PubMed] [Google Scholar]
- 6. Søltoft F, Hammer M, Kragh N. The association of body mass index and health‐related quality of life in the general population: data from the 2003 Health Survey of England. Qual Life Res 2009; 18: 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ul‐Haq Z, Mackay DF, Fenwick E, Pell JP. Meta‐analysis of the association between body mass index and health‐related quality of life among adults, assessed by the SF‐36. Obesity (Silver Spring) 2013; 21: E322–E327. [DOI] [PubMed] [Google Scholar]
- 8. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health‐related quality of life: systematic review and meta‐analysis of randomized trials. Obes Rev 2014; 15: 169–182. [DOI] [PubMed] [Google Scholar]
- 9. Ware JE. Methodological considerations in the selection of health status assessment procedures In: Wenger J, Mattson M, Furberg C, Elinson J. (eds). Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. Le Jacq Pub: Greenwich, CT, 1984, pp. 87–111. [Google Scholar]
- 10. Kesztyüs D, Wirt T, Kobel S et al. “Komm mit in das gesunde Boot – Grundschule” – Research Group. Is central obesity associated with poorer health and health‐related quality of life in primary school children? Cross‐sectional results from the Baden‐Württemberg Study. BMC Public Health 2013; 13: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodbard HW, Fox KM, Grandy S, Shield Study Group . Impact of obesity on work productivity and role disability in individuals with and at risk for diabetes mellitus. Am J Health Promot 2009; 23: 353–360. [DOI] [PubMed] [Google Scholar]
- 12. Langley P, Pérez Hernández C, Margarit Ferri C, Ruiz Hidalgo D, Lubián LM. Pain, health related quality of life and healthcare resource utilization in Spain. J Med Econ 2011; 14: 628–638. [DOI] [PubMed] [Google Scholar]
- 13. Kleinman N, Abouzaid S, Andersen L, Wang Z, Powers A. Cohort analysis assessing medical and nonmedical cost associated with obesity in the workplace. J Occup Environ Med 2014; 56: 161–170. [DOI] [PubMed] [Google Scholar]
- 14. Karlsson J, Taft C, Sjöström L, Torgerson JS, Sullivan M. Psychosocial functioning in the obese before and after weight reduction: construct validity and responsiveness of the obesity‐related problems scale. Int J Obes Relat Metab Disord 2003; 27: 617–630. [DOI] [PubMed] [Google Scholar]
- 15. Kaukua JK, Pekkarinen TA, Rissanen AM. Health‐related quality of life in a randomised placebo‐controlled trial of sibutramine in obese patients with type II diabetes. Int J Obes Relat Metab Disord 2004; 28: 600–605. [DOI] [PubMed] [Google Scholar]
- 16. Merideth CH. A single‐center, double‐blind, placebo‐controlled evaluation of lamotrigine in the treatment of obesity in adults. J Clin Psychiatry 2006; 67: 258–262. [DOI] [PubMed] [Google Scholar]
- 17. Astrup A, Madsbad S, Breum L et al. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double‐blind, placebo‐controlled trial. Lancet 2008; 372: 1906–1913. [DOI] [PubMed] [Google Scholar]
- 18. Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health‐related quality of life. J Clin Epidemiol 2005; 58: 568–578. [DOI] [PubMed] [Google Scholar]
- 19. Warkentin LM, Majumdar SR, Johnson JA et al. Weight loss required by the severely obese to achieve clinically important differences in health‐related quality of life: two‐year prospective cohort study. BMC Med 2014; 12: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The George Washington University . Obesity drug outcome measures: a consensus report of considerations regarding pharmacologic intervention, 2012. [WWW document]. URL https://publichealth.gwu.edu/pdf/obesitydrugmeasures.pdf (accessed December 2015).
- 21. Kahan S, Ferguson C, David S, Divine L. Obesity drug outcome measures: results of a multi‐stakeholder critical dialogue. Curr Obes Rep 2013; 2: 128–133. [Google Scholar]
- 22. Jensen MD, Ryan DH, Apovian CM et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 Suppl. 2): S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garber AJ, Abrahamson MJ, Barzilay JI et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement – executive summary. Endocr Pract 2013; 19: 536–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mann T, Tomiyama AJ, Westling E et al. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol 2007; 62: 220–233. [DOI] [PubMed] [Google Scholar]
- 25. Wadden TA, Berkowitz RI, Womble LG et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005; 353: 2111–2120. [DOI] [PubMed] [Google Scholar]
- 26. Bray GA, Blackburn GL, Ferguson JM et al. Sibutramine produces dose‐related weight loss. Obes Res 1999; 7: 189–198. [DOI] [PubMed] [Google Scholar]
- 27. James WP, Astrup A, Finer N et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet 2000; 356: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 28. Astrup A, Carraro R, Finer N et al. NN8022‐1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once‐daily human GLP‐1 analog, liraglutide. Int J Obes 2012; 36: 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes 2014; 38: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pi‐Sunyer X, Astrup A, Fujioka K et al. SCALE Obesity and Prediabetes NN8022‐1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 31. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001; 9: 102–111. [DOI] [PubMed] [Google Scholar]
- 32. Kolotkin RL, Crosby RD. Psychometric evaluation of the Impact Of Weight On Quality Of Life‐Lite Questionnaire (IWQOL‐Lite) in a community sample. Qual Life Res 2002; 11: 157–171. [DOI] [PubMed] [Google Scholar]
- 33. Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health‐related quality of life and weight loss. Obes Res 2001; 9: 564–571. [DOI] [PubMed] [Google Scholar]
- 34. Engel SG, Crosby RD, Kolotkin RL et al. The impact of weight loss and regain on obesity‐specific quality of life: mirror image or differential effect. Obes Res 2003; 11: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 35. White MA, O'Neil PM, Kolotkin RL, Byrne TK. Gender, race, and obesity‐related quality of life at extreme levels of obesity. Obes Res 2004; 12: 949–955. [DOI] [PubMed] [Google Scholar]
- 36. Ware JE, Kosinski M, Bayliss MS et al. Comparison of methods for the scoring and statistical analysis of SF‐36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995; 33: AS264–AS279. [PubMed] [Google Scholar]
- 37. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I: conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 38. Doll HA, Petersen SE, Stewart‐Brown SL. Obesity and physical and emotional well‐being: associations between body mass index, chronic illness, and the physical and mental components of the SF‐36 questionnaire. Obes Res 2000; 8: 160–170. [DOI] [PubMed] [Google Scholar]
- 39. Larsson U, Karlsson J, Sullivan M. Impact of overweight and obesity on health‐related quality of life – a Swedish population study. Int J Obes Relat Metab Disord 2002; 26: 417–424. [DOI] [PubMed] [Google Scholar]
- 40. Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health‐related quality of life. J Clin Epidemiol 2004; 57: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 41. Maruish ME. (ed). User's Manual for the SF‐36v2 Health Survey, 3rd edn. QualityMetric Inc.: Lincoln, RI, 2011. [Google Scholar]
- 42. Scott SC, Goldberg MS, Mayo NE. Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol 1997; 50: 45–55. [DOI] [PubMed] [Google Scholar]
- 43. Kolotkin RL, Crosby RD. Manual for the Impact of Weight on Quality of Life Measure (IWQOL and IWQOL‐Lite). Obesity and Quality of Life Consulting: Durham, NC, 2008. [Google Scholar]
- 44. Kolotkin RL, Chen S, Klassen P, Gilder K, Greenway FL. Patient‐reported quality of life in a randomized placebo‐controlled trial of naltrexone/bupropion for obesity. Clin Obes 2015; 5: 237–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cut‐off values for improvement and deterioration in IWQOL‐Lite total score by baseline severity.


