Summary
Blood‐borne viruses, such as hepatitis B virus, hepatitis C virus, human immunodeficiency virus, and the facultative blood‐borne hepatitis E virus, are considered a major public health problem given that they are accountable for millions of deaths each year. Treatment options, including effective vaccine design, development of antiviral strategies and the implementation of antiretroviral therapy have improved substantially over the last couple of years and contribute to successful treatment and prevention of these infectious diseases. In this review, we summarise the current knowledge and concepts in prevention of transmission of these blood‐borne viruses.
Abbreviations used
- ART
antiretroviral therapy
- DAA
direct acting antivirals
- GT
genotype
- MTC
mother‐to‐child transmission
- NNRTI
non‐nucleosidic RT inhibitors
- NRTI
nucleosidic RT inhibitor
- NSP
needle/syringe programme
- OST
opioid substitution therapy
- PEG
pegylated
- PEP
Post‐exposure prophylaxis
- PrEP
Pre‐exposure prophylaxis
- PWID
people who inject drugs
- SVR
sustained virological response
Old Foes and New Faces: Epidemiology of HIV, HBV, HCV, HEV
Exchange of blood and other body fluids between individuals bears the risk of acquiring blood‐borne virus infections with often severe consequences. Blood‐borne virus infections are caused by HIV, HBV or HCV and are known for a high prevalence worldwide and significant associated morbidity and mortality 1. Besides these ‘major three agents’, there is increasing concern regarding the prevalence of HEV, even if classically not defined as a true ‘blood‐borne virus’ due to its transmission mainly via the faecal/oral route. Nevertheless, especially in the context of the safety of blood products, non‐diagnosed HEV infection can pose a significant risk for immunosuppressed patients, patients with chronic liver disease and pregnant women 2.
With more than 34 million deaths so far, HIV infection continues to be a major global health problem. It was estimated that in 2014, 1.2 million people died from HIV‐related causes globally with approximately 36.9 million people being chronically infected 3 (Figure 1 and Table 1). As the virus targets immune cells and specifically CD4+ T cells, infected individuals become immunodeficient, which results in an increased susceptibility to infections and if untreated, HIV is almost universally fatal. In the most advanced stage, the infection progresses to AIDS, which leads to the development of certain cancers, high susceptibility to various opportunistic infections and other clinical manifestation 3.
Figure 1.
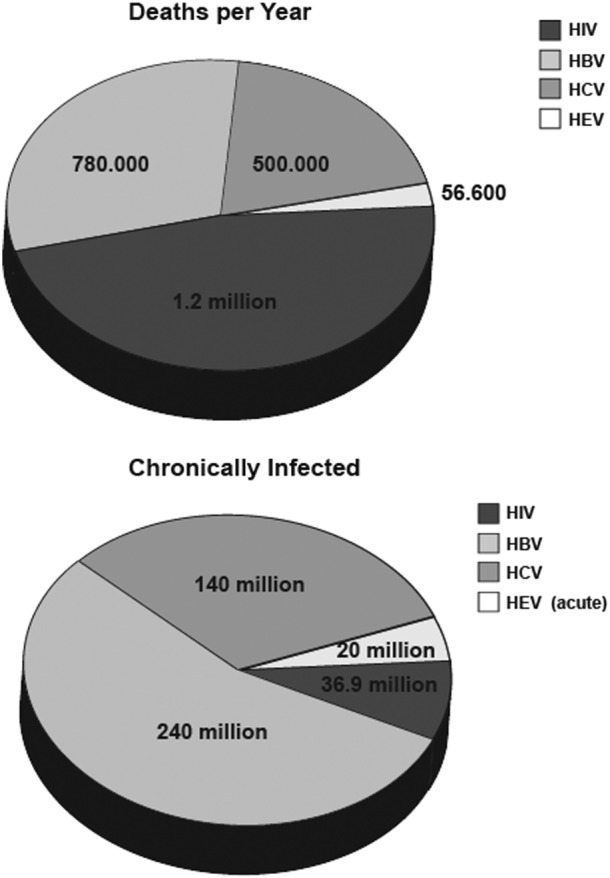
Blood‐borne viruses and related deaths. Numbers are based on data released by the World Health Organisation updated July 2015 3, 4, 5, 6
Table 1.
Characteristics of blood‐borne viruses
| Viral characteristic | HBV | HCV | HEV | HIV |
|---|---|---|---|---|
| Familiy | Hepadnaviridae | Flaviviridae | Hepeviridae | Retroviridae |
| Genome | DNA, partially ds | RNA, ss (+) | RNA, ss (+) | RNA, ss (+) |
| Genotypes | 8 | 7 | 4 | 2 |
| GT A‐H | GT 1‐7 | GT 1‐4*1 | HIV‐1, HIV2 | |
| Replication site | Liver | Liver | Liver | CD4+ T cells |
| Transmission route | Blood and body fluids | Blood and body fluids | Water borne faecal–oral, food‐borne | Blood and body fluids |
| Public health *2 | ||||
| Chronically infected | 240 million | 140 million | few cases | 36.9 million |
| New infections p.a. | 4 million | 2 million | 20 million | 2 million |
| Related deaths p.a. | 780 000 | 500 000 | 56 600 | 1.2 million |
| Clinical impact | ||||
| Outcome of infection | Chronicity: 10% adults, 20–30% children*3 95% neonates | Chronicity: 75–85% | Mostly self‐limiting*4 | Chronicity: 100% |
| Clinical manifestation | Viral hepatitis | Viral hepatitis | Viral hepatitis | AIDS |
| Treatment | Antiviral therapy | Direct acting antivirals | Ribavirin | Antiretroviral therapy |
| Vaccine | Available | Not available | Available | Not available |
HEV1 and HEV2 restricted to humans.
Numbers are based on data published from the World Health Organisation (WHO) and Averhoff et al., CID.
Age 1–5 years.
Chronicity can develop in solid‐organ transplant recipients.
The development of viral hepatitis is the shared hallmark of infections with HBV, HCV and HEV, even though the viruses differ substantially from each other. Viral hepatitis is often silent and symptomless, and it can take years until the development of significant liver disease, which then results in noticeable symptoms 7. Infection with either HBV, HCV or HEV can lead to viral hepatitis, which results in liver fibrosis, cirrhosis and hepatocellular carcinoma rendering viral hepatitis in many countries as the leading cause of liver transplantations 8. It is estimated that HBV causes approximately 780 000 deaths each year with globally 240 million people being chronically infected 4 (Figure 1 and Table 1). The risk for proceeding to a chronic infection varies according to the age of infection, with children being at the greatest risk. Approximately 90% of infants and 25–50% of children aged 1–5 years will remain chronically infected with HBV, whereas the majority of adults (95%) are able to eliminate viral infection 9.
Hepatitis C virus infections account for approximately 500 000 deaths worldwide each year. Once infected, approximately 75–85% of the individuals proceed to chronic infections leading to an estimated worldwide infection rate of 130–150 million people who are chronically infected 5 (Figure 1 and Table 1).
In comparison, HEV infections seem negligible with an annual 56 600 deaths and 20 million acute infections globally 6 (Figure 1 and Table 1). Furthermore, in contrast to HIV, HBV and HCV, infection is mainly asymptomatic and self‐limiting, and progress to chronicity is rare. However, especially in immunosuppressed patients, pregnant women and patients with liver failure, HEV‐related mortality is higher, and/or disease progression is more severe 10.
Knowing the Risks: Transmission Routes
Blood‐borne viruses are transmitted as the name implies mainly by blood or by other body fluids containing infectious virus particles. Penetration through intact skin does not occur, and airborne transmission can also be excluded for HBV, HIV, HCV and HEV. The rate of viral transmission can vary depending on the virus type, viral load in the donor, volume of blood inoculated, route of infection and the immune status of the exposed individual. The main routes of transmission for blood‐borne viruses include contaminated blood products, sharing injection equipment, sexual intercourse, mother‐to‐child (MTC) transmission and occupational exposure. HEV is an exception as it is mainly transmitted via the faecal–oral route with the most common transmission being faecal contamination of drinking water or eating uncooked meat 11. However, blood–blood transmission is possible. In the last decades, transfusion transmitted infections by virus‐contaminated blood products have decreased tremendously through the awareness of these pathogens and the introduction of screening assays for HBV, HIV and HCV in most countries. However, in the case of HEV, blood products are not routinely tested for the presence of viral RNA, and so represent a potential risk of HEV transmission 12.
Injection drug use is a clear risk factor for the acquisition and transmission of HIV, HCV and HBV. Virus cross‐transmissions may occur at various stages during the drug preparation process like sharing syringes and drug preparation equipment such as cookers, filters, water and water containers 13. It is estimated worldwide that about 3 million people who inject drugs (PWID) are HIV‐positive 14, 10 million HCV‐positive and about 1.2 million living with HBV 15.
The risk for sexual transmission of HCV is low, while an increased risk is linked to co‐infection with HIV, increasing number of sexual partners and men who have sex with men. In contrast, unprotected intercourse is one of the most common routes of infection worldwide for HBV and HIV. In case of HIV, the majority of transmissions occur through heterosexual contacts; however, depending on the country, the pattern of sexual HIV transmission varies. Risk factors for HIV and HBV sexual transmission are repeated intercourse with an infected person, the type of sexual contact and the amount of virus present in the blood or secretions of the infected partner.
Also, MTC transmission of HIV and HBV is a major transmission route and can occur during childbirth or breastfeeding. The risk of HIV transmission to the infant is about 25% at the delivery and up to an estimated 40% due to prolonged (18–24 months) breastfeeding in untreated HIV infection and in the absence of interventions 16. For HCV, MTC infection was reported in only about 5–10% of deliveries 17. The role of breastfeeding for perinatal HCV transmission is thought to be negligible, even though some studies have reported HCV RNA in breast milk and colostrum samples from infected women 18. In case of HBV, viral DNA can be detected in the breastmilk 19, but several studies suggest that breastfeeding does not add risk to MTC 20. Furthermore, all infants born to HBV‐infected mothers should receive hepatitis B immune globulin and the first dose of hepatitis B vaccine within 12 h after birth.
Other body fluids like tears, saliva and sweat could additionally harbour the risk of transmitting blood‐borne viruses. HIV as well as HCV RNA has been detected in different body fluids, including saliva; nevertheless, direct transmission via this route has never been shown 21, 22. This is in contrast to HBV, where viral DNA has been detected in tears, saliva and sweat of infected patients, and virus isolated from tear specimens of a patient have been shown to be infective in vivo, indicating a potential risk that body fluids can serve as a vehicle for HBV transmission 23. Occupational exposure to blood‐borne viruses also poses a serious risk for instance to health care workers, and although safety precautions have been introduced, occupational modes of transmission will continue to occur. These include percutaneous or mucosal exposure to blood or body fluids of infected individuals as well as needlestick or other sharp injuries with transmission risks of approximately 30% for HBV, 2% for HCV and 0.3% for HIV 24, 25, 26.
Reducing the Spread: Prevention of Infection
Primary prevention
Primary prevention seeks to prevent the onset of a viral infection by reducing risk factors in non‐infected people. It involves interventions that are applied before there is any evidence of infection or disease. Examples include an increased awareness, safety of blood products, hygiene and disinfection, reduction of viral exposure, as well as prophylaxis and induction of immunity in uninfected people by vaccination (Figure 2) 8.
Figure 2.
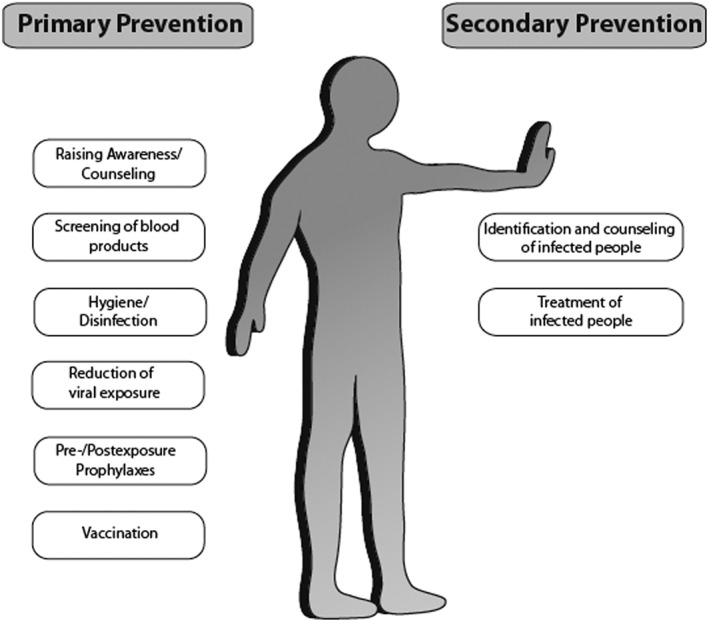
Primary and secondary prevention strategies for blood‐borne viral infections. Prevention strategies that help reduce transmission of blood‐borne viral infections
Raising awareness
Successful prevention strategies of viral infections require a high level of awareness of the infected individuals as well as a detailed knowledge among healthcare professionals and society 7. Public campaigns including world hepatitis day, which is commemorated every year on the 28th of July and world AIDS day, commemorated on the 1st of December, are helping to raise awareness in the community. Many healthcare professionals lack basic knowledge about risk factors or screening recommendations associated with viral hepatitis or AIDS 27, 28. Extensive and reiterated education of medical personnel is a prerequisite, and raising awareness of opportunities for prevention, care and treatment is a major goal to help reduce occupational transmission. Updated guidelines are available provided by different organisations including the World Health Organisation or the Centers for Disease Control and Prevention (CDC)/European CDC as well as national guidelines.
Screening of blood products
In the last decades, there have been significant reductions in the risk of transfusion‐transmitted blood‐borne infections because of the implementation of routine screening procedures of blood products. Nucleic acid amplification testing has been successfully established for the detection of HIV, HBV and HCV resulting in a reduction of transmission 29. However, there is increasing concern regarding the transmission risk of HEV by transfusion and ongoing debates whether routine HEV screening for blood donors should be implemented 12. Recent studies from different European countries reported a low prevalence of HEV RNA in blood donors 10, but due to pooling procedures, the risk can increase substantially to a rate of up to 10% HEV RNA positivity in pooled plasma 30. To date, screening of blood products for HEV has not been standardised, and also due to poor sensitivity and specificity of anti‐HEV assays, it is estimated that the risk for transfusion‐mediated transmission of HEV is far higher than for other blood‐borne viruses including HIV 12.
Hygiene/disinfection
Simple measures regarding hygiene and disinfection of possible contamination sources can help reduce viral transmission. To prevent occupational transmission, prevention strategies involve implementation of standard barrier precautions including gloves, gowns and protective eye wear as well as minimal manual manipulation of sharp instruments (e.g. by not recapping needles) as well as disposal of sharp material into suitable containers 1, 31. Furthermore, appropriate disinfection practices should be employed to facilitate a contamination‐free environment. This involves sterilisation of medical and dental equipment as well as surface disinfection of contaminated areas. Next to nosocomial environments, the usage of sterile equipment in tattoo and piercing studios is of utmost importance, and a non‐sterile environment, for example, upon tattooing and piercing in prisons, homes and other potentially non‐sterile setting, can facilitate viral transmission 32.
Reduction of viral exposure
Sexual transmission poses a major risk factor, and prevention strategies have been implemented to reduce viral spread. Behavioural interventions including condom use have been shown to successfully reduce the incidence of HIV infections 33 and are also effective measures to prevent transmission of HBV and HCV. Furthermore, medical male circumcision has been shown to effectively reduce transmission of HIV by the sexual route 34, and new intervention strategies including vaginal and rectal microbiocides are being developed to further reduce transmission 35.
Injection drug use is one of the most efficient transmission routes for blood‐borne infections in the developed world, and sharing of contaminated needles and preparation equipment have been associated with viral transmission 36. The introduction of harm reduction interventions like needle/syringe programmes (NSP) and opiate substitution therapy contributes to prevention of viral transmission. NSP have been initiated in several countries worldwide, and sterile needles have been made available for drug users at low or no costs. Opioid substitution therapy also provides an ideal context for viral screening to efficiently contribute to reducing transmission risks 37, and together with NSP and antiretroviral therapy (ART), it efficiently helps to reduce viral transmission 38.
Post‐exposure prophylaxis
In case of a high chance of getting infected, for example, by accidental blood contact with infected body fluids or to prevent viral transmission from infected mothers to newborns, measurements for HBV and HIV are available to assess the risk for viral transmission 39, 40. Post‐exposure prophylaxis (PEP) refers to immediate treatment with antiviral drugs following exposure to a pathogen in order to prevent infection. In the case of HIV, PEP should be initiated as early as possible and no later than 48–72 h. It is recommended after any high risk—usually sexual, occupational or associated with intravenous drug use—exposure 41, 42. Given the possibility of side effects such as drug induced liver injury, PEP should not be initiated if the risk of infection is deemed very low given the nature of the exposure 43. Similarly, in the event of high‐risk exposure of an unvaccinated individual to HBV and in newborns to HBsAg positive mothers, PEP should be initiated using combined active/passive vaccination 44. Newborns to HBsAg positive mothers should receive PEP irrespective of the mother's HBeAg status: the usual dose being 30–100 IU anti‐HBs per kilogramme bodyweight. No PEP protocols or recommendations exist for HCV or HEV.
Pre‐exposure prophylaxis
Pre‐exposure prophylaxis (PrEP) refers to the concept of administering antiviral drugs to individuals prior to anticipated exposures. PrEP has been shown to reduce the risk of acquisition of HIV infection in individuals at high risk and is recommended by the CDC 45. No PrEP protocols are established or recommended for HBV, HCV or HEV infection.
Vaccination
Effective immune protection in the community contributes greatly to the prevention and eradication of viral infections. A safe and effective vaccine against HBV has been available since 1982, and nowadays, vaccination against HBV has been successfully implemented with 47 European countries having adopted universal HBV vaccination programmes 46, 47. HBV vaccination leads to appearance of anti‐HBs: generally levels >100 U/l are considered to represent a good response, while levels >10 U/l are still protective. In individuals at risk from HBV infection, antibody levels should be rechecked after 10 years. Introduction of the vaccine has contributed to a 96% decline in the incidence of acute hepatitis B in children and adolescents 48.
For HEV, there is currently no global vaccine available even though in 2011, the first vaccine to prevent HEV infection (HEV239 vaccine) was registered in China 2. It has to be seen whether this will soon be available also in other countries. Up to now, there is no effective prophylactic vaccine available for HCV or HIV infections. Several vaccine candidates for HCV are in phase I/II clinical trials, but their clinical usefulness remains to be demonstrated 49, 50. Similarly, HIV vaccine research has been ongoing for several years, but an effective and safe preparation has thus far remained elusive 51, 52.
Secondary prevention
Secondary prevention strategies aim to identify infected people and to provide appropriate medical management to reduce the risk of chronic diseases (Figure 2) 8. These strategies include identification as well as treatment of infected people.
Identification and counselling of infected people
A major impediment to the potential of antiviral therapy to prevent transmission is the high number of undiagnosed infections 53, 54. It has been estimated that 45–85% of HCV‐infected individuals in the USA are unaware of their infection 55. Thus, it is important to suspect and test for blood‐borne infections in individuals at risk or showing suggestive symptoms. Screening services are crucial for a comprehensive strategy to reduce and eliminate blood‐borne viral infections 56. These services aim to identify individuals who are unaware of their infection status and provide them with counselling and education thereby allowing the infected persons to make behavioural changes and to take steps that prevent transmission to uninfected individuals. Early diagnosis of an infectious disease offers the best chance for a successful medical treatment and the prevention of viral spread 57. Next to the implementation of screening strategies, notification and counselling of blood donors who have been tested positive after blood donation provide ample opportunity to identify unaware infected people and to facilitate medical support.
Treatment of infected people
Powerful antiviral drugs with a defined molecular target exist for all pathogens discussed here with the exception of HEV. Antiviral therapy prevents transmission events either by reducing the number of infected individuals in the population (HCV) or by reducing viral load and thus the risk of person‐to‐person spread (HBV, HIV).
All oral antivirals approved against HBV infection inhibit the HBV RT 58. Taken as a single pill per day, all of them block production of new viral particles, which in turn reduces viral load and prevents the progression of liver disease. However, HBV antivirals do not clear covalently closed circular DNA from the nuclei of infected cells and thus cannot cure infection 59. Hence, antiviral therapy for HBV must be taken long term in most cases.
Numerous direct acting antivirals (DAAs) for HCV infection have reached the market starting in 2011 60. All HCV antivirals inhibit one of three molecular targets: the NS3/4A protease, the NS5A RNA binding protein and the NS5B RNA‐dependent RNA polymerase 60. The polymerase inhibitor class can be further divided into nucleoside analogues targeting the NS5B active site and non‐nucleoside analogues 61. Current treatment protocols combine two or more of these agents sometimes together with the non‐specific antiviral ribavirin (see succeeding discussion) for an 8‐ to 24‐week course with the intent to permanently cure infection 62, 63. The exact choice of drugs and treatment duration depends on viral genotype, severity of liver disease and sometimes other factors such as co‐morbidities, co‐medications, sex or pre‐treatment viral load. In almost all cases, viral load drops rapidly to undetectable levels when treatment is initiated. Undetectable HCV RNA 12 weeks after end of therapy is referred to as sustained virological response (SVR) and seen as equivalent to cure. This is achieved in over 90% of individuals treated 64. Post‐SVR individuals are clearly non‐infectious. It is likely that individuals with undetectable HCV RNA are already non‐infectious prior to reaching SVR, but this has not been formally shown.
Beyond antiviral drugs, both HBV and HCV also respond to treatment with IFN‐α 65. It is usually applied as weekly subcutaneous injections of a pegylated form (PEG) and exerts a non‐specific antiviral effect through stimulation of cellular antiviral defences. In chronic HBV infection, PEG‐IFN‐α is administered for 48 weeks instead of or in addition to antivirals 66. In a minority of cases, it achieves a lasting attenuation of viral replication or even long‐term immune control so that further antiviral treatment is not required. In the case of HCV, PEG was used in combination with ribavirin and later also different DAA, but it has now been all but replaced by oral DAA combination therapy that achieves higher SVR rates with fewer side effects 64, 66. No specific antivirals exist for HEV infection. However, ribavirin monotherapy has been shown to often cure chronic infection in immunosuppressed individuals and is sometimes also used in severe forms of acute HEV infection 67. No data exist on the effect of ribavirin treatment on HEV transmission.
Since the introduction of zidovudine in 1987, ART has evolved to highly potent combination ART that now allows infected individuals with access to good healthcare to live a lifespan very comparable with that of uninfected individuals 68, 69. However, as in HBV, treatment is suppressive and not curative and thus must be taken life‐long. Four classes of HIV antivirals are currently employed as part of first‐line combinations: nucleosidic inhibitors of the HIV RT (NRTI) form the backbone of many regimens 70. Several HIV NRTI are also active against HBV; most notably, tenofovir is widely used in both HBV and HIV infection. Other classes of first line agents are non‐nucleosidic RT inhibitors (NNRTI), protease inhibitors and integrase inhibitors 71. Agents with different mechanism of action such as fusion inhibitors and blockers of the CCR5 co‐receptor are available as second line agents 72. Additionally, other antiretroviral drugs are currently in the drug pipeline including monoclonal antibodies, maturation inhibitors and attachment inhibitors 73. Furthermore, the new long‐acting injectable antiretrovirals as well as the new boosting agent cobicistat have the potential to improve therapy 74, 75. Treatment usually combines two NRTI with a third first‐line agent from a different class 41, 42. Several fixed dose combinations allow administration of state‐of‐the‐art combination therapy as a single pill taken once daily. Choice of initial combination is based on co‐morbidities, co‐medications, viral resistance profile, anticipated side effects and convenience of administration. Given the many options and assuming that medications are taken regularly, it is now possible to suppress viral replication for life in almost all patients. Thus, the recommendations have been evolving towards early initiation of ART rather than waiting until the CD4 cell count drops below some threshold or even symptoms of immunodeficiency appear as clinical trials, for example, START have shown clear benefits of starting treatment earlier 76, 77. Following initiation of ART in most cases, viral load steadily declines eventually falling below the limit corresponding to markedly reduced risk of transmission of infection to others, which has been supported by the HPTN 052 study that showed both personal and public health benefits of HIV treatment as prevention 78.
Conclusion
There are several key challenges ahead to efficiently prevent transmission of blood‐borne pathogens. It is necessary to raise awareness and discuss risks associated with blood‐borne viruses. Global screening and detection systems need to be implemented and general treatment guidelines mediated, which are also available in low‐income countries. Vaccines for both HCV and HIV still remain elusive. In the case of HBV and HIV, avenues towards curative rather than suppressive treatments need to be explored and in the case of HEV, specific antiviral drugs are lacking.
Conflict of Interest
TVH Speaker's Fees from BMS, Johnson & Johnson and Roche.
Acknowledgements
E.S. was supported by the DFG (STE 1954/1‐1) and an intramural young investigator award of the Helmholtz Centre for Infection Research.
Pfaender, S. , von Hahn, T. , Steinmann, J. , Ciesek, S. , and Steinmann, E. (2016) Prevention strategies for blood‐borne viruses—in the Era of vaccines, direct acting antivirals and antiretroviral therapy. Rev. Med. Virol., 26: 330–339. doi: 10.1002/rmv.1890.
References
- 1. Deuffic‐Burban S, Delarocque‐Astagneau E, Abiteboul D, Bouvet E, Yazdanpanah Y. Blood‐borne viruses in health care workers: prevention and management. Journal of Clinical Virology 2011; 52: 4–10. DOI:10.1016/j.jcv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 2. Kamar N, Bendall R, Legrand‐Abravanel F, et al. Hepatitis E. Lancet (London, England) 2012; 379: 2477–2488. [DOI] [PubMed] [Google Scholar]
- 3. HIV/AIDS Fact Sheet N°360 [Media centre]. http://www.who.int/mediacentre/factsheets/fs360/en/ [accessed 12 November 2015].
- 4. Hepatitis B Fact Sheet N°204 [Media centre]. http://www.who.int/mediacentre/factsheets/fs204/en/ [accessed 12 November 2015].
- 5. Hepatitis C Fact Sheet N°164 [Media centre]. http://www.who.int/mediacentre/factsheets/fs164/en/ [accessed 12 November 2015].
- 6. Hepatitis E Fact Sheet N°280 [Media centre]. http://www.who.int/mediacentre/factsheets/fs280/en/ [accessed 12 November 2015].
- 7. Benjamin RM. Raising awareness of viral hepatitis: National Hepatitis Testing Day, May 19. Public Health Reports 2012; 127: 244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prevention and control of viral hepatitis infection: framework for global action. http://www.who.int/hiv/pub/hepatitis/Framework/en/ [accessed 20 January 2016].
- 9. Viral Hepatitis—Hepatitis B Information. http://www.cdc.gov/hepatitis/hbv/index.htm [accessed 20 January 2016].
- 10. Behrendt P, Steinmann E, Manns MP, Wedemeyer H. The impact of hepatitis E in the liver transplant setting. Journal of Hepatology 2014; 61: 1418–1429. DOI:10.1016/j.jhep.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 11. Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clinical Microbiology Reviews 2014; 27: 116–138. DOI:10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pawlotsky JM. Hepatitis E screening for blood donations: an urgent need? Lancet 2014; 384: 1729–1730. DOI:10.1016/S0140-6736(14)61187-9. [DOI] [PubMed] [Google Scholar]
- 13. Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal of Public Health 2001; 91: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta‐analysis. Bulletin of the World Health Organization 2013; 91: 102–123. DOI:10.2471/Blt.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378: 571–583. DOI:10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother‐to‐child transmission of HIV‐1: timing and implications for prevention. Lancet Infectious Diseases 2006; 6: 726–732. DOI:10.1016/S1473-3099(06)70629-6. [DOI] [PubMed] [Google Scholar]
- 17. Mohan N, Gonzalez‐Peralta RP, Fujisawa T, et al. Chronic hepatitis C virus infection in children. Journal of Pediatric Gastroenterology and Nutrition 2010; 50: 123–131. DOI:10.1097/MPG.0b013e3181c61995. [DOI] [PubMed] [Google Scholar]
- 18. Kumar RM, Shahul S. Role of breast‐feeding in transmission of hepatitis C virus to infants of HCV‐infected mothers. Journal of Hepatology 1998; 29: 191–197. [DOI] [PubMed] [Google Scholar]
- 19. de Oliveira PR, Yamamoto AY, de Souza CBS, et al. Hepatitis B viral markers in banked human milk before and after holder pasteurization. Journal of Clinical Virology 2009; 45: 281–284. DOI:10.1016/j.jcv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 20. Shi ZJ, Yang YB, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus a meta‐analysis and systematic review. Archives of Pediatrics & Adolescent Medicine 2011; 165: 837–846. DOI:10.1001/archpediatrics.2011.72. [DOI] [PubMed] [Google Scholar]
- 21. Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS 2001; 15: 837–845. [DOI] [PubMed] [Google Scholar]
- 22. Ackerman Z, Paltiel O, Glikberg F, Ackerman E. Hepatitis C virus in various human body fluids: a systematic review. Hepatology Research 1998; 11: 26–40. DOI:10.1016/S1386-6346(98)00004-7. [Google Scholar]
- 23. Komatsu H, Inui A, Sogo T, Tateno A, Shimokawa R, Fujisawa T. Tears from children with chronic hepatitis B virus (HBV) infection are infectious vehicles of HBV transmission: experimental transmission of HBV by tears, using mice with chimeric human livers. Journal of Infectious Diseases 2012; 206: 478–485. DOI:10.1093/infdis/jis293. [DOI] [PubMed] [Google Scholar]
- 24. Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood‐borne infections in health care workers. Clinical Microbiology Reviews 2000; 13: 385–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerberding JL. Management of occupational exposures to blood‐borne viruses. New England Journal of Medicine 1995; 332: 444–451. DOI:10.1056/NEJM199502163320707. [DOI] [PubMed] [Google Scholar]
- 26. Service USPH. Updated U.S. . Public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR ‐ Recommendations and Reports 2001; 50: 1–52. [PubMed] [Google Scholar]
- 27. Burke RC, Sepkowitz KA, Bernstein KT, et al. Why don't physicians test for HIV? A review of the US literature. AIDS 2007; 21: 1617–1624. DOI:10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 28. Smith BD, Jorgensen C, Zibbell JE, Beckett GA. Centers for disease control and prevention initiatives to prevent hepatitis C virus infection: a selective update. Clinical Infectious Diseases 2012; 55(Suppl 1): S49–53. DOI:10.1093/cid/cis363. [DOI] [PubMed] [Google Scholar]
- 29. Coste J, Reesink HW, Engelfriet CP, et al. Implementation of donor screening for infectious agents transmitted by blood by nucleic acid technology: update to 2003. Vox Sanguinis 2005; 88: 289–303. DOI:10.1111/j.1423-0410.2005.00636_1.x. [DOI] [PubMed] [Google Scholar]
- 30. Baylis SA, Gartner T, Nick S, Ovemyr J, Blumel J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sanguinis 2012; 103: 89–90. DOI:10.1111/j.1423-0410.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 31. Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: a global perspective. Vaccine 1999; 17: 1730–1733. [DOI] [PubMed] [Google Scholar]
- 32. Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clinical Infectious Diseases 2012; 54: 1167–1178. DOI:10.1093/cid/cir991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scott‐Sheldon LAJ, Huedo‐Medina TB, Warren MR, Johnson BT, Carey MP. Efficacy of behavioral interventions to increase condom use and reduce sexually transmitted infections: a meta‐analysis, 1991 to 2010. Jaids‐Journal of Acquired Immune Deficiency Syndromes 2011; 58: 489–498. DOI:10.1097/QAI.0b013e31823554d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database of Systematic Reviews 2009. ARTN CD003362.. DOI:10.1002/14651858.CD003362.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obiero J, Mwethera PG, Hussey GD, Wiysonge CS. Vaginal microbicides for reducing the risk of sexual acquisition of HIV infection in women: systematic review and meta‐analysis. BMC Infectious Diseases 2012; 12: 289 DOI:10.1186/1471-2334-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagan H, Jarlais DCD. HIV and HCV infection among injecting drug users. Mount Sinai Journal of Medicine 2000; 67: 423–428. [PubMed] [Google Scholar]
- 37. Larney S, Grebely J, Falster M, et al. Opioid substitution therapy is associated with increased detection of hepatitis C virus infection: a 15‐year observational cohort study. Drug and Alcohol Dependence 2015; 148: 213–216. DOI:10.1016/j.drugalcdep.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 38. Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction (Abingdon, England) 2010; 105: 844–859. [DOI] [PubMed] [Google Scholar]
- 39. Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2011; CD003510 DOI:10.1002/14651858.CD003510.pub3. [DOI] [PubMed] [Google Scholar]
- 40. Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta‐analysis. Hepatology 2016; 63: 319–333. DOI:10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 41. EACS Guidelines 8.0. http://www.eacsociety.org/guidelines/eacs‐guidelines/eacs‐guidelines.html [accessed 20 January 2016].
- 42. Clinical Guidelines Portal. https://aidsinfo.nih.gov/guidelines [20 January 2016].
- 43. Jain S, Mayer KH. Practical guidance for nonoccupational postexposure prophylaxis to prevent HIV infection: an editorial review. AIDS 2014; 28: 1545–1554. DOI:10.1097/QAD.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occupational Medicine (London) 2011; 61: 531–540. DOI:10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- 45. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2014 Clinical Practice Guideline. http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf [accessed 20 January 2016].
- 46. Romano L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Digestive and Liver Disease 2011; 43(Suppl 1): S2–7. DOI:10.1016/S1590-8658(10)60685-8. [DOI] [PubMed] [Google Scholar]
- 47. Trepo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet (London, England) 2014; 384: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 48. Viral hepatitis. http://www.cdc.gov/hepatitis/ [accessed 3 November 2015].
- 49. Baumert TF, Fauvelle C, Chen DY, Lauer GM. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. Journal of Hepatology 2014; 61: S34–44. DOI:10.1016/j.jhep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 50. Man John Law L, Landi A, Magee WC, Lorne Tyrrell D, Houghton M. Progress towards a hepatitis C virus vaccine. Emerging Microbes & Infections 2013; 2: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mann JK, Ndung'u T. HIV‐1 vaccine immunogen design strategies. Virology Journal 2015; 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen YZ, Dolin R. Novel HIV vaccine strategies: overview and perspective. Therapeutic Advances in Vaccines 2013; 1: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS 2012; 26: 893–896. DOI:10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 54. Brouard C, Le Strat Y, Larsen C, Jauffret‐Roustide M, Lot F, Pillonel J. The undiagnosed chronically‐infected HCV population in France. Implications for expanded testing recommendations in 2014. PLoS One 2015; 10: e0126920 DOI:10.1371/journal.pone.0126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith BD, Morgan RL, Beckett GA, Falck‐Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the centers for disease control and prevention. Annals of Internal Medicine 2012; 157 817‐+:. DOI:10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alfaleh FZ, Nugrahini N, Maticic M, et al. Strategies to manage hepatitis C virus infection disease burden—volume 3. Journal of Viral Hepatitis 2015; 22(Suppl 4): 42–65. DOI:10.1111/jvh.12474. [DOI] [PubMed] [Google Scholar]
- 57. Ryom L, Boesecke C, Gisler V, et al. Essentials from the 2015 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV‐positive persons. HIV Medicine 2015. DOI:10.1111/hiv.12322. [DOI] [PubMed] [Google Scholar]
- 58. Clark DN, Hu J. Hepatitis B virus reverse transcriptase—target of current antiviral therapy and future drug development. Antiviral Research 2015; 123: 132–137. DOI:10.1016/j.antiviral.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang E, Kosinska A, Lu M, Yan H, Roggendorf M. Current status of immunomodulatory therapy in chronic hepatitis B, fifty years after discovery of the virus: search for the “magic bullet” to kill cccDNA. Antiviral Research 2015; 123: 193–203. DOI:10.1016/j.antiviral.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 60. Manns MP, von Hahn T. Novel therapies for hepatitis C—one pill fits all? Nature Reviews. Drug Discovery 2013; 12: 595–610. DOI:10.1038/nrd4050. [DOI] [PubMed] [Google Scholar]
- 61. Eltahla AA, Luciani F, White PA, Lloyd AR, Bull RA. Inhibitors of the hepatitis C virus polymerase; mode of action and resistance. Viruses 2015; 7: 5206–5224. DOI:10.3390/v7102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. European Association for the Study of the L . EASL recommendations on treatment of hepatitis C 2014. Journal of Hepatology 2014; 61: 373–395. DOI:10.1016/j.jhep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 63. Sarrazin C, Berg T, Buggisch P, et al. S3 guideline hepatitis C addendum. Zeitschrift für Gastroenterologie 2015; 53: 320–334. DOI:10.1055/s-0034-1399322. [DOI] [PubMed] [Google Scholar]
- 64. Solbach P, Wedemeyer H. The new era of interferon‐free treatment of chronic hepatitis C. Viszeralmedizin 2015; 31: 290–296. DOI:10.1159/000433594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin FC, Young HA. Interferons: success in anti‐viral immunotherapy. Cytokine and Growth Factor Reviews 2014; 25: 369–376. DOI:10.1016/j.cytogfr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology 2015; 61: 712–721. DOI:10.1002/hep.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. Journal of Viral Hepatitis 2015; 22: 965–973. DOI:10.1111/jvh.12403. [DOI] [PubMed] [Google Scholar]
- 68. Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine 1998; 338: 853–860. DOI:10.1056/Nejm199803263381301. [DOI] [PubMed] [Google Scholar]
- 69. Vittinghoff E, Scheer S, O'Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. Journal of Infectious Diseases 1999; 179: 717–720. DOI:10.1086/314623. [DOI] [PubMed] [Google Scholar]
- 70. Buckhold FR. Primary care of the human immunodeficiency virus patient. Medical Clinics of North America 2015; 991105‐ + .: . DOI:10.1016/j.mcna.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 71. Robbins RN, Spector AY, Mellins CA, Remien RH. Optimizing ART adherence: update for HIV treatment and prevention. Current Hiv/Aids Reports 2014; 11: 423–433. DOI:10.1007/s11904-014-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Geretti AM, Tsakiroglou M. HIV: new drugs, new guidelines. Current Opinion in Infectious Diseases 2014; 27: 545–553. DOI:10.1097/Qco.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 73. Cihlar T, Fordyce M. Current status and prospects of HIV treatment. Current Opinion in Virology 2016; 18: 50–56. DOI:10.1016/j.coviro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 74. Landovitz RJ, Kofron R, McCauley M. The promise and pitfalls of long‐acting injectable agents for HIV prevention. Current Opinion in HIV and AIDS 2016; 11: 122–128. DOI:10.1097/COH.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sherman EM, Worley MV, Unger NR, Gauthier TP, Schafer JJ. Cobicistat: review of a pharmacokinetic enhancer for HIV infection. Clinical Therapeutics 2015; 37: 1876–1893. DOI:10.1016/j.clinthera.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 76. Franco RA, Saag MS. When to start antiretroviral therapy: as soon as possible. BMC Medicine 2013; 11Artn 147: . DOI:10.1186/1741-7015-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. WHO Guidelines Approved by the Guidelines Review Committee. In Guideline on When to Start Antiretroviral Therapy and on Pre‐Exposure Prophylaxis for HIV. World Health Organization Copyright (c) World Health Organization 2015.: Geneva, 2015. [PubMed]
- 78. Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV‐1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infectious Diseases 2014; 14: 281–290. DOI:10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


