Abstract
The regulatory mechanisms underlying food intake in chickens have been a focus of research in recent decades to improve production efficiency when raising chickens. Lines of evidence have revealed that a number of brain‐gut peptides function as a neurotransmitter or peripheral satiety hormone in the regulation of food intake both in mammals and chickens. Glucagon, a 29 amino acid peptide hormone, has long been known to play important roles in maintaining glucose homeostasis in mammals and birds. However, the glucagon gene encodes various peptides that are produced by tissue‐specific proglucagon processing: glucagon is produced in the pancreas, whereas oxyntomodulin (OXM), glucagon‐like peptide (GLP)‐1 and GLP‐2 are produced in the intestine and brain. Better understanding of the roles of these peptides in the regulation of energy homeostasis has led to various physiological roles being proposed in mammals. For example, GLP‐1 functions as an anorexigenic neurotransmitter in the brain and as a postprandial satiety hormone in the peripheral circulation. There is evidence that OXM and GLP‐2 also induce anorexia in mammals. Therefore, it is possible that the brain‐gut peptides OXM, GLP‐1 and GLP‐2 play physiological roles in the regulation of food intake in chickens. More recently, a novel GLP and its specific receptor were identified in the chicken brain. This review summarizes current knowledge about the role of glucagon‐related peptides in the regulation of food intake in chickens.
Keywords: appetite, brain, chicken, glucagon, hypothalamus
Introduction
Broiler chickens, which are bred for rapid growth and high meat yield, do not adequately control voluntary food intake to meet their energy requirements (Richards & Proszkowiec‐Weglarz 2007). Consequently, their overconsumption of food can lead to excessive accumulation of visceral fat, which is regarded as an animal by‐product or as waste. Furthermore, data on energy from fat and protein in chicken (1870‐2004) reveal that while chicken was at one time a lean, low‐fat food, this is no longer true (Wang et al. 2010). Thus, the regulatory mechanisms underlying food intake in chickens have been a focus of research in recent decades to improve production efficiency when raising chickens (Kuenzel 1994; Furuse 2002; Richards & Proszkowiec‐Weglarz 2007; Bungo et al. 2011).
In mammals, a single glucagon gene encodes three distinct structurally related peptides: oxyntomodulin (OXM), glucagon‐like peptide (GLP)‐1 and GLP‐2 are produced in the intestine and brain, whereas glucagon is produced from the same precursor in the pancreas (Janssen et al. 2013). In contrast, nonmammalian vertebrates utilize more complex mechanisms for the production of proglucagon‐derived peptides. For example, chickens have a single proglucagon gene that expresses multiple messenger RNA (mRNA) transcripts (Fig. 1) (Richards & McMurtry 2009). Fish and amphibians have either multiple proglucagon genes or exons that are likely the result of duplication events (Ng et al. 2010). Recently, a novel GLP (GCGL) and its receptor (GCGLR) have been identified in chickens as well as in other nonmammalian vertebrates (Wang et al. 2012). To date, GCGL and its receptor genes have not been identified in any mammalian species, implying that they might have been lost during evolution. Figure 2 shows the alignment of glucagon‐related peptides from different species. The amino acid sequence of glucagon was highly conserved between mammals and chickens, whereas that of GLP‐2 was not. These findings raise the hypothesis that glucagon‐related peptides play different physiological roles among vertebrates. This review provides current knowledge about the possible roles of glucagon‐related peptides in the appetite regulatory systems in chickens.
Figure 1.
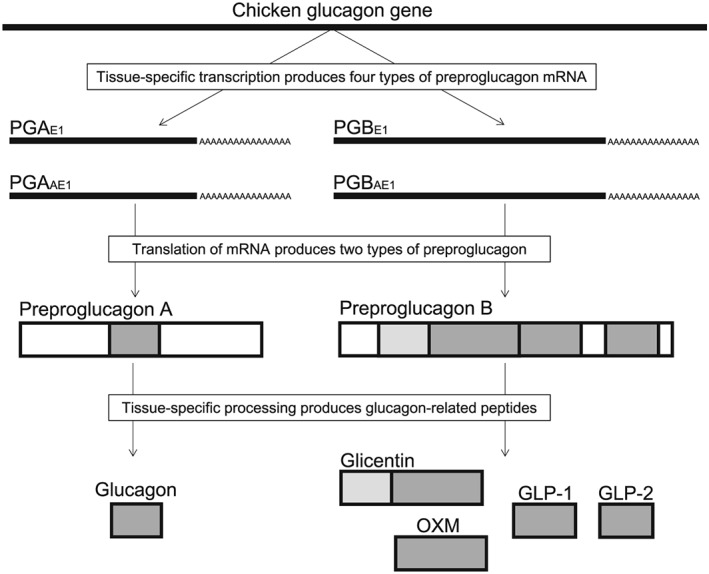
Chicken glucagon, oxyntomodulin (OXM), glucagon‐like peptide (GLP)‐1 and GLP‐2 are produced in a tissue‐specific manner. Two messenger RNA (RNA) classes, PGA (pancreas) and PGB (brain and gut), are produced by alternate promoter and alternate first exon usage. Translation of these mRNAs produces preproglucagon A or preproglucagon B. In the pancreas, proglucagon A is cleaved by PC1/3 to generate glucagon. In the brain and gut, the proglucagon B is cleaved by PC2 to generate glicentin, OXM, GLP‐1 and GLP‐2.
Figure 2.

Alignment of glucagon‐related peptides from different species. Dashes indicate amino acids identical to chicken peptides. Amino acid sequences of glucagon‐related peptides were retrieved from articles (Ng et al. 2010; Wang et al. 2012; Honda et al. 2015b).
Glucagon
The anorexigenic effect of glucagon was first reported in mammals: intracerebroventricular (ICV) administration of glucagon in rats significantly decreased food intake (Inokuchi et al. 1984). The anorexigenic effect of ICV administration of glucagon was also found in chicks (Honda et al. 2007a) and sheep (Kurose et al. 2009). Although the mechanisms underlying the anorexigenic action of glucagon in mammals and birds are not fully understood, the possible mechanisms are similar in some respects.
ICV administration of glucagon induced hyperglycemia in chicks (Honda et al. 2007a, 2012, 2015a). Intravascular administration of glucose significantly decreased food intake in chicks (Honda et al. 2007a). It is therefore likely that hyperglycemia‐mediated pathways are also involved in the anorexigenic action of glucagon in chicks. Central glucagon‐induced hyperglycemia was observed in rats (Marubashi et al. 1985), mice (Amir 1986), dogs (Agarwala et al. 1986) and sheep (Kurose et al. 2009). Phentolamine, an α‐adrenergic receptor antagonist, significantly attenuated glucagon‐induced hyperglycemia in rats (Marubashi et al. 1985) and chicks (Honda et al. 2012). These findings suggest that glucagon induces hyperglycemia at least partly via the α‐adrenergic neural pathway both in mammals and chicks.
ICV administration of glucagon dramatically increased plasma corticosterone concentration in chicks, suggesting the activation of the hypothalamic pituitary adrenal (HPA) axis (Honda et al. 2012). Corticotropin‐releasing factor (CRF), the upstream regulator of HPA axis, was also suggested to be a mediator for a number of anorexigenic peptides in chickens (Meade & Denbow 2003; Tachibana et al. 2004, 2006; Honda et al. 2007b; Kamisoyama et al. 2007). ICV administration of glucagon significantly increased the hypothalamic CRF mRNA level in chicks (Honda et al. 2007a). These findings suggest that CRF acts as a downstream molecule for a glucagon‐induced appetite‐suppressive pathway in chicks.
Electrophoretic application of glucagon to hypothalamic neurons in rats suppressed the neuronal activity of glucose‐sensitive neurons in the lateral hypothalamic area (LHA) but not in the ventromedial hypothalamic nucleus (VMH) and dorsomedial hypothalamic nucleus (DMH) (Inokuchi et al. 1986). Glucagon administration into the paraventricular nucleus in rats did not influence food intake (Atrens & Menendez 1993). These findings suggest that LMH is the target site of glucagon to exert its anorexogenic effect in mammals. In birds, the greatest expression of glucagon receptor was consistently detected in the hypothalamus of chickens (Wang et al. 2008). Montaron et al. (1994) used radioautography to detect and localize glucagon receptors in the duck brain. Further study will be needed to identify the target site of glucagon in the chicken hypothalamus.
There is evidence that glucagon can be transported from blood into the cerebrospinal fluid in humans (Graner & Abraira 1985). It is therefore possible that peripheral glucagon can access the target site of the brain in chicks. However, plasma glucagon level is elevated under fasting condition in chickens (Dupont et al. 2008; Richards & McMurtry 2008). Therefore, it is not reasonable to assume that glucagon suppresses appetite under conditions of hunger. One possible explanation is proposed by Filippi et al. (2013): elevations in circulating glucagon levels that elevate brain glucagon levels may occur during conditions of stress, during which suppression of the desire to eat may be beneficial to divert the body's attention to a ‘fight‐or‐flight’ response. Further study will be needed to clarify this possibility in chickens.
Oxyntomodulin
OXM is released from the intestines into the blood in response to food ingestion and in proportion to energy intake (Ghatei et al. 1983; Le Quellec et al. 1992). Central or peripheral administration of OXM suppressed food intake in mammals (Dakin et al. 2001; Cohen et al. 2003), suggesting that OXM plays a physiological role as a satiety signal and anorexigenic neuropeptide in mammals. In chickens, the anorexigenic effect of OXM was reported by two different groups: ICV administration of bovine OXM significantly suppressed food intake (Cline et al. 2008; Moghaddam et al. 2010). However, as shown in Figure 2, the amino acid sequence of bovine OXM differs from that of chicken OXM: chicken OXM contains the 29 amino acid sequence of chicken glucagon followed by a 26 amino acid C‐terminal extension, whereas bovine OXM contains the 29 amino acid sequence of bovine glucagon followed by a shorter sequence of eight residues at its C‐terminal. Mammalian peptide, which has a similar but not identical amino acid sequence to chicken peptide, showed different effects on food intake when centrally administered (Shiraishi et al. 2009; Saneyasu et al. 2011a). In fact, ICV administration of chicken OXM significantly suppressed food intake in broiler and layer chicks (Honda et al. 2014a). It is therefore possible that OXM functions as an anorexigenic peptide in chicks.
Chicken OXM significantly increased plasma glucose and corticosterone levels (Honda et al. 2014a). Similar changes in plasma glucose and corticosterone are induced by glucagon (Honda et al. 2007a, 2012) but not by GLP‐1 (Honda et al. 2014a) or GLP‐2 (Honda et al. 2015b) when centrally administered. OXM receptor gene or its mRNA was not identified in the chicken genome or chicken expressed sequence tag (EST) databases. Therefore, it seems likely that OXM suppresses food intake via glucagon receptor in chicks.
OXM contains the 29 amino acid glucagon sequence in the N‐terminal region. Prévost et al. (2012) reported that F22, V23, M27 and D15 of glucagon are the most important residues for receptor binding. These four amino acid residues are conserved in chicken and bovine OXM. In addition, both glucagon and OXM induced anorexia and hyperglycemia in chicks (Honda et al. 2012, 2014a). These findings suggest that the N‐terminal glucagon region plays an important role in the anorexigenic action of OXM. However, in mammals, growing evidence suggests that OXM suppresses food intake via GLP‐1 receptor. For example, the anorexigenic effect of OXM was blocked by co‐administration of the GLP‐1 receptor antagonist exendin in rats (Dakin et al. 2001). The anorexigenic effect of OXM is preserved in glucagon receptor null mice but abolished in GLP‐1 receptor null mice (Baggio et al. 2004). It is therefore likely that OXM‐induced anorexigenic pathways are different between mammals and chicks.
Dakin et al. (2004) reported that OXM increases c‐FOS in the arcuate nucleus of rats. In chickens, c‐FOS immunoreactivity was significantly decreased in the Leghorn male hepatoma and significantly increased in the nucleus infundibuli hypothalami (equivalent of the mammalian arcuate nucleus) by ICV administration of OXM (Cline et al. 2008). Therefore, the OXM induced anorexigenic pathway in the brain might be partly conserved between mammals and chickens.
Glucagon‐like peptide‐1
In 1996, Turton et al. (1996) first reported that ICV administration of GLP‐1 suppresses food intake in mammals. The GLP‐1‐producing neuron in the nucleus tractus solitarii (NTS) of the brainstem is an important site within the regulatory pathways of feeding in mammals (Van Bloemendaal et al. 2014). Thus, GLP‐1 is considered as an anorexigenic neurotransmitter in mammals. In chickens, ICV administration of chicken GLP‐1 significantly suppressed food intake (Furuse et al. 1997a). GLP‐1‐immunoreactive perikarya were found in the NTS (Tachibana et al. 2005). Proglucagon mRNA level in the brainstem or medulla oblongata was decreased after 24 h fasting in chicks (Tachibana et al. 2005; Honda et al. 2015c). Chicken GLP1R was highly expressed in the telencephalon, midbrain, hindbrain and hypothalamus (Huang et al. 2012). All these findings suggest that GLP‐1 functions as an anorexigenic peptide in the central nervous system in chicks.
GLP‐1 is released from the intestine in response to food ingestion in mammals (Tolhurst et al. 2009). Meta‐analysis revealed that intravascular administration of GLP‐1 dose‐dependently reduces energy intake in humans (Verdich et al. 2001). Thus, GLP‐1 is also considered as a peripheral satiety hormone in mammals (Van Bloemendaal et al. 2014). In birds, intraperitoneal administration of 3 nmol/kg body weight GLP‐1 did not affect food intake in layer chicks under 15 h fasting (Tachibana et al. 2003). On the other hand, intraperitoneal administration of 0.5 nmol/body rat GLP‐1 significantly suppressed food intake in Japanese quails (Shousha et al. 2007). In addition, intravascular administration of 1.5 nmol/kg body weight GLP‐1 in broiler chicks significantly suppressed food intake under ad libitum feeding conditions (Honda K, unpublished data). Recent findings in immunohistochemical and morphometric studies of chickens suggested that GLP‐1 plays physiological roles as one of the common hormones secreted by L cells in the chicken small intestine (Nishimura et al. 2013; 2014a, 2014b). For example, frequencies of occurrence of GLP‐1‐immunoreactive cells were influenced by food deprivation in chickens (Monir et al. 2014a) and dietary protein levels (Monir et al. 2014b). It is therefore likely that GLP‐1 functions as an anorexigenic peptide not only in the central nervous system but also in the peripheral circulation in chickens.
Turton et al. (1996) reported that deletion of N‐terminal histidine of GLP‐1 eliminated the anorexigenic effect in rats, suggesting that the N‐terminal histidine has an important role in the anorexigenic action of GLP‐1. In chickens, the anorexigenic effects of modified mammalian GLP‐1 in which N‐terminal histidine was substituted for tyrosine was 11‐ to 13‐fold less than that of non‐modified GLP‐1 (Bungo et al. 1999a). Furuse et al. (1998a) reported that the threshold of full‐length mammalian GLP‐1‐induced anorexia was lower than that of C‐terminal‐truncated GLP‐1 in chicks. These findings suggest that both the N‐ and C‐terminal regions of GLP‐1 may have important roles for the regulation of food intake in chickens.
ICV administration of the GLP‐1 receptor antagonist exendin, enhanced food intake in rats (Turton et al. 1996) and layer chicks (Furuse et al. 1998b; Tachibana et al. 2001) but not in broiler chicks (Tachibana et al. 2001). Broiler chicks eat more food than layer chicks (Saneyasu et al. 2011b). It is therefore possible that the expression of endogenous GLP‐1 in broiler chicks is lower than that in layer chicks, which in turn results in hyperphagia in boiler chicks. However, exendin also attenuates GLP‐2‐induced anorexia in chicks (Honda K, unpublished data), suggesting that exendin acts as a dual antagonist of GLP‐1 receptor and GLP‐2 receptor in chickens. Further study will be needed to investigate the ligand specificity of chicken glucagon‐related receptors in more detail using commonly used antagonists and agonists for mammalian glucagon‐related receptors.
CRF is suggested to be a downstream mediator of the GLP‐1‐induced anorexigenic pathway in rats (Larsen et al. 1997; Sarkar et al. 2003; Gotoh et al. 2005). In layer chicks, ICV administration of GLP‐1 significantly increased plasma corticosterone concentration, suggesting that GLP‐1 activates the HPA axis (Tachibana et al. 2006). Furthermore, the anorexigenic effect of GLP‐1 was attenuated by the co‐administration of the CRF antagonist, astressin, in layer chicks (Tachibana et al. 2006). In contrast, ICV administration of GLP‐1 did not alter plasma corticosterone concentration in broiler chicks (Furuse et al. 1997b). These findings suggest that CRF is involved in the inhibitory mechanisms of GLP‐1 for feeding, at least in layer chicks as well as in mammals.
GLP‐1 (Bungo et al. 1999b) and noradrenalin (Denbow et al. 1981) induced sedation in chicks when centrally administered. In addition, ICV administration of fusaric acid, an inhibitor of noradrenaline synthesis, attenuated the suppression of food intake by GLP‐1 in a dose‐related fashion (Bungo et al. 2001). ICV administration of GLP‐1 in chicks decreased monoamine concentrations and stimulated the expression of FOS‐like immunoreactive cells in the ventromedial nuclei (Tachibana et al. 2002). These findings suggest that the noradrenergic system in the brain is involved in the GLP‐1‐induced anorexigenic pathway in chicks.
Glucagon‐like peptide‐2
GLP‐2 is produced in the brain and gut as described above. ICV administration of GLP‐2 suppressed food intake and increased c‐FOS immunoreactive nuclei in the DMN in rat brain (Tang‐Christensen et al. 2000). There is evidence that GLP‐2 receptor was expressed in the appetite regulation‐related brain regions, such as DMH, VMH and NTS in rats (Tang‐Christensen et al. 2000; Lovshin et al. 2004). Thus, GLP‐2 is considered as an anorexigenic neurotransmitter in mammals. In birds, Shousha et al. (2007) first reported that ICV administration of rat GLP‐2 did not influence food intake in Japanese quails. However, mammalian GLP‐2 shares only 51‐55% amino acid identity with chicken GLP‐2 (Fig. 1). There is evidence that GLP‐2 receptor was expressed in the brain of chickens (Richards & McMurtry 2008; Mo et al. 2014). In fact, ICV administration of chicken GLP‐2, but not human GLP‐2, potently suppressed food intake (Honda et al. 2015b). Also, ICV administration of chicken GLP‐2 significantly decreased plasma glucose concentration and did not affect plasma corticosterone concentration, suggesting that the mechanism underlying the anorexigenic action of GLP‐2 differs from that of glucagon and OXM in chicks (Honda et al. 2015b). Furthermore, ICV administration of an equimolar amount of GLP‐1 and GLP‐2, but not OXM, significantly suppressed food intake in chicks (Honda et al. 2015b). These findings suggest that GLP‐2 may function as a powerful anorexigenic peptide in the central nervous system in chicks.
Peripheral GLP‐2 is known to be an intestinotrophic peptide (Drucker et al. 1996). Studies in humans have not demonstrated a satiety effect of peripheral GLP‐2 (Schmidt et al. 2003; Sorensen et al. 2003). However, recent data have shown that intraperitoneal injection of GLP‐2 reduces food intake in lean and obese mice, suggesting a role of GLP‐2 in the short‐term regulation of food intake (Baldassano et al. 2012). Also, intravascular administration of chicken GLP‐2 significantly suppressed food intake in chicks (Honda et al. 2015c), although peripheral administration of mammalian GLP‐2 did not influence food intake in chickens and Japanese quails (Shousha et al. 2007; Hu et al. 2010). Immunohistochemical and morphometric studies of chickens suggested that GLP‐2 colocalized with GLP‐1 in the same secretory granules of L cells in the chicken small intestine (Nishimura et al. 2013; Monir et al. 2014c). These findings suggest that GLP‐2 may act as a postprandial satiety hormone in chickens.
Novel glucagon‐like peptide
Recently, GCGL and its receptor GCGLR were identified in the chicken brain (Wang et al. 2012). Genomic analysis revealed that the GCGL gene is located in a synteny conserved in tilapia, coelacanth, Xenopus, and chickens, but not in humans (Wang et al. 2012). GCGL and GCGLR were detected in chicken and Xenopus tropicalis brains by RT‐PCR (Irwin & Prentice 2011; Wang et al. 2012), implying that GCGL and its receptor may play important roles in the central nervous system of nonmammalian vertebrates.
A comparatively high mRNA expression level of GCGL was detected in the hypothalamus of chickens (Wang et al. 2012). The hypothalamus plays a critical role in appetite regulation in mammals (Schneeberger et al. 2014) and birds (Richards & Proszkowiec‐Weglarz 2007; Bungo et al. 2011). In fact, ICV administration of GCGL significantly suppressed food intake in both layer and broiler chicks (Honda et al. 2014b). The anorexigenic effect of GCGL was attenuated by the co‐administration of the CRF antagonist, α‐helical CRF, in chicks (Honda et al. 2014b). Twenty‐four hours of fasting did not affect hypothalamic mRNA levels of GCGL and GCGLR in chicks, suggesting that endogenous GCGL does not change in response to nutritional status in chicks. However, it is possible that GCGL functions as an anorexigenic peptide in the brain in chicks. Further study will be needed to determine the changes of physiological concentration of neuronal GCGL in chicks.
Ligand specificiity of receptors of glucagon‐related peptides
Lines of evidence suggest that glucagon, GLP‐1, GLP‐2 and GCGL specifically activate their receptors in chickens. For example, chicken glucagon receptor was activated by glucagon but not by GLP‐1 and GLP‐2 in vitro (Wang et al. 2012). Chicken GLP‐1 receptor expressed in Chinese hamster ovary cells was potently activated by GLP‐1 but not by glucagon and GLP‐2 (Huang et al. 2012). Recently, Mo et al. (2014) demonstrated that chicken GLP‐2 receptor expressed in Chinese hamster ovary cells was potently activated by chicken GLP‐2 but not by other structurally related peptides, including chicken GLP‐1 and glucagon. Wang et al. (2012) reported that GCGL was 300‐fold more potent in activating GCGLR than any other peptide, including glucagon, GLP‐1 and GLP‐2, suggesting that GCGLR is a receptor specific to GCGL.
To date, a distinct receptor for OXM has not yet been identified. Therefore, OXM seems to be a dual agonist of GLP‐1 receptor and the glucagon receptor in mammals (Pocai 2012). However, recent findings suggest that OXM suppresses food intake via glucagon receptor in chicks (Honda et al. 2014b), as described earlier. Therefore, it is possible that the physiological roles of OXM are different between mammals and chickens.
Routes of the anorexigenic actions of peripheral GLP‐1 and GLP‐2
In mammals, GLP‐1 can directly stimulate anorectic pathways in the hypothalamus and brainstem, and may also act through the vagus nerve (Murphy & Bloom 2006; Van Bloemendaal et al. 2014). For example, GLP‐1 receptors in the subfornical organ and the area postrema are accessible to circulating GLP‐1 (Orskov et al. 1996). A radiolabelled GLP‐1 analogue has been demonstrated to easily cross the blood‐brain barrier in mice (Kastin et al. 2002). Vagotomy has been reported to block or attenuate the anorexgenic effect of GLP‐1 in rats (Abbott et al. 2005). In chickens, GLP‐1 receptor and GLP‐2 receptor mRNAs are widely distributed in the gastrointestinal tract (Huang et al. 2012; Mo et al. 2014). Therefore, it is possible that both GLP‐1 and GLP‐2 signals are received by the brainstem as neural inputs through the vagal afferent nerves from the gastrointestinal tract in chickens. Further studies in chickens will be needed to clarify: (i) whether circulating GLPs can directly access these receptors in the brain; (ii) whether the vagal afferent nerves are related to the anorexigenic effect of GLPs; and (iii) whether circulating GLPs postprandially elevate.
Conclusion
Recent evidence suggests that glucagon‐related peptides show anorexigenic action in chicks. Lines of evidence suggest that the physiological roles of these peptides in chickens differ from those of mammals. In addition, the anorexigenic effects of GLP‐1 and GLP‐2 were expressed at a very low dose when compared to other glucagon‐related peptides in chicks (Table 1), suggesting that GLP‐1 and GLP‐2 may play an important role in the regulation of food intake in chicks. Further studies will be needed to evaluate the physiological importance of glucagon‐related peptides in the regulation of food intake in chickens.
Table 1.
The minimal effective doses of chicken glucagon‐related peptides that suppress food intake for 2 h after intracerebroventricular administration in 8‐day‐old layer chicks
Acknowledgments
I thank Dr. Hiroshi Kamisoyama and Dr. Takaoki Saneyasu for their valuable discussions. This work was partly supported by a Grant‐in‐Aid (Number 17208023) for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Honda, K. (2016) Glucagon‐related peptides and the regulation of food intake in chickens. Anim Sci J, 87: 1090–1098. doi: 10.1111/asj.12619.
References
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, et al. 2005. The inhibitory effects of peripheral administration of peptide YY (3‐36) and glucagon‐like peptide‐1 on food intake are attenuated by ablation of the vagal‐brainstem‐hypothalamic pathway. Brain Research 1044, 127–131. [DOI] [PubMed] [Google Scholar]
- Agarwala GC, Mishra R, Jaiswal G, Bapat V. 1986. Effect of centrally administered glucagon on blood lipids in anesthetized dogs. Indian Journal of Physiology and Pharmacology 30, 280–288. [PubMed] [Google Scholar]
- Amir S. 1986. Central glucagon‐induced hyperglycemia is mediated by combined activation of the adrenal medulla and sympathetic nerve endings. Physiology & Behavior 37, 563–566. [DOI] [PubMed] [Google Scholar]
- Atrens DM, Menendez JA. 1993. Glucagon and the paraventricular hypothalamus:modulation of energy balance. Brain Research 630, 245–251. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. 2004. Oxyntomodulin and glucagon‐like peptide‐1 differentially regulate murine food intake and energy expenditure. Gastroenterology 127, 546–558. [DOI] [PubMed] [Google Scholar]
- Baldassano S, Bellanca AL, Serio R, Mule F. 2012. Food intake in lean and obese mice after peripheral administration of glucagon‐like peptide 2. Journal of Endocrinology 213, 277–284. [DOI] [PubMed] [Google Scholar]
- Bungo T, Kawakami S‐I, Ohgushi A, Sashihara K, Saito N, Sugahara K, et al. 2001. Intracerebroventricular injection of fusaric acid attenuates the anorexia by glucagon‐like peptide‐1 in the neonatal chick. Pharmacology Biochemistry and Behavior 70, 251–255. [DOI] [PubMed] [Google Scholar]
- Bungo T, Kawakami S‐I, Ohgushi A, Shimojo M, Masuda Y, Saito N, et al. 1999b. Intracerebroventricular administration of glucagon‐like peptide‐1 induces sleep‐like behavior in the neonatal chick. Japanese Poultry Science 36, 377–381. [Google Scholar]
- Bungo T, Shimojo M, Masuda Y, Saito N, Sugahara K, Hasegawa S, et al. 1999a. Effects of food intake in the neonatal chick of substitution of N‐terminal amino acid of glucagon‐like peptide‐1 (7‐36). Life Sciences 64, 295–299. [DOI] [PubMed] [Google Scholar]
- Bungo T, Shiraishi J, Kawakami S. 2011. Hypothalamic melanocortin system on feeding regulation in birds: a review. The Journal of Poultry Science 48, 1–13. [Google Scholar]
- Cline MA, Bowden CN, Nandar W, Rogers JO. 2008. Central oxyntomodulin causes anorexigenic effects associated with the hypothalamus and alimentary canal in chicks (Gallus gallus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 149, 405–410. [DOI] [PubMed] [Google Scholar]
- Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, et al. 2003. Oxyntomodulin Suppresses Appetite and Reduces Food Intake in Humans. The Journal of Clinical Endocrinology & Metabolism 88, 4696–4701. [DOI] [PubMed] [Google Scholar]
- Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, et al. 2001. Oxyntomodulin inhibits food intake in the rat. Endocrinology 142, 4244–4250. [DOI] [PubMed] [Google Scholar]
- Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, et al. 2004. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 145, 2687–2695. [DOI] [PubMed] [Google Scholar]
- Denbow DM, Cherry JA, Siegel PB, van Krey HP. 1981. Eating, drinking and temperature response of chicks to brain catecholamine injection. Physiology and Behavior 27, 265–269. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Erlich P, Asa SL, Brubaker PL. 1996. Induction of intestinal epithelial proliferation by glucagon‐like peptide 2. Proceedings of the National Academy of Sciences in the United States of America 93, 7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J, Tesseraud S, Derouet M, Collin A, Rideau N, Crochet S, et al. 2008. Insulin immuno‐neutralization in chicken: effects on insulin signaling and gene expression in liver and muscle. Journal of Endocrinology 197, 531–542. [DOI] [PubMed] [Google Scholar]
- Filippi BM, Abraham MA, Yue JT, Lam TK. 2013. Insulin and glucagon signaling in the central nervous system. Reviews in Endocrine & Metabolic Disordors 14, 365–375. [DOI] [PubMed] [Google Scholar]
- Furuse M. 2002. Central regulation of food intake in the neonatal chick. Animal Science Journal 73, 83–94. [Google Scholar]
- Furuse M, Bungo T, Shimojo M, Masuda Y, Saito N, Hasegawa S, et al. 1998a. Effects of various N‐terminal fragments of glucagon‐like peptide‐1 (7‐36) on food intake in the neonatal chick. Brain Research 807, 214–217. [DOI] [PubMed] [Google Scholar]
- Furuse M, Bungo T, Shimojo M, Masuda Y, Saito N, Hasegawa S, et al. 1998b. Influence of intracerebroventricular administration of Exendin (9‐39) on food intake of the newly‐hatched chick. Japanese Poultry Science 35, 376–380. [Google Scholar]
- Furuse M, Matsumoto M, Okumura J, Sugahara K, Hasegawa S. 1997a. Intracerebroventricular injection of mammalian and chicken glucagon‐like peptide‐1 inhibits food intake of the neonatal chick. Brain Research 755, 167–169. [DOI] [PubMed] [Google Scholar]
- Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S. 1997b. The central corticotropin‐releasing factor and glucagon‐like peptide‐1 in food intake of the neonatal chick. European Journal of Pharmacology 339, 211–214. [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR. 1983. Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. The Journal of Clinical Endocrinology & Metabolism 57, 488–495. [DOI] [PubMed] [Google Scholar]
- Gotoh K, Fukagawa K, Fukagawa T, Noguchi H, Kakuma T, Sakata T, et al. 2005. Glucagon‐like peptide‐1, corticotropin‐releasing hormone, and hypothalamic neuronal histamine interact in the leptin‐signaling pathway to regulate feeding behavior. FASEB Journal 19, 1131–1133. [DOI] [PubMed] [Google Scholar]
- Graner JL, Abraira C. 1985. Glucagon in the cerebrospinal fluid. The New England Journal of Medicine 312, 994–995. [PubMed] [Google Scholar]
- Honda K, Kamisoyama H, Saito N, Kurose Y, Sugahara K, Hasegawa S. 2007a. Central administration of glucagon suppresses food intake in chicks. Neuroscience Letters 416, 198–201. [DOI] [PubMed] [Google Scholar]
- Honda K, Kamisoyama H, Saneyasu T, Sugahara K, Hasegawa S. 2007b. Central administration of insulin suppresses food intake in chicks. Neuroscience Letters 423, 153–157. [DOI] [PubMed] [Google Scholar]
- Honda K, Kamisoyama H, Uemura T, Yanagi T, Saito N, Kurose Y, et al. 2012. The mechanism underlying the central glucagon‐induced hyperglycemia and anorexia in chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 163, 260–264. [DOI] [PubMed] [Google Scholar]
- Honda K, Saneyasu T, Okuda M, Uemura T, Kamisoyama H. 2015a. Glucagon and neuromedin U suppress food intake in broiler chicks. The Journal of Poultry Science 52, 268–278. [Google Scholar]
- Honda K, Saneyasu T, Shimatani T, Aoki K, Yamaguchi T, Nakanishi K, et al. 2015b. Intracerebroventricular administration of chicken glucagon‐like peptide‐2 potently suppresses food intake in chicks. Animal Science Journal 86, 312–318. [DOI] [PubMed] [Google Scholar]
- Honda K, Saneyasu T, Yamaguchi T, Shimatani T, Aoki K, Nakanishi K, et al. 2014a. Intracerebroventricular administration of chicken oxyntomodulin suppresses food intake and increases plasma glucose concentration in chicks. Neuroscience Letters 564, 57–61. [DOI] [PubMed] [Google Scholar]
- Honda K, Saneyasu T, Yamaguchi T, Shimatani T, Aoki K, Nakanishi K, et al. 2014b. Intracerebroventricular administration of novel glucagon‐like peptide suppresses food intake in chicks. Peptides 52, 98–103. [DOI] [PubMed] [Google Scholar]
- Honda K, Shimatani T, Aoki K, Yamaguchi T, Kondo M, Saneyasu T, et al. 2015c. Glucagon‐like peptide‐2 functions as anorexigenic peptide not only in the central nervous system but also in the peripheral circulation in broiler chicks. The Journal of Poultry Science 3, 183–187. [Google Scholar]
- Hu XF, Guo YM, Huang BY, Bun S, Zhang LB, Li JH, et al. 2010. The effect of glucagon‐like peptide 2 injection on performance, small intestinal morphology, and nutrient transporter expression of stressed broiler chickens. Poultry Science 89, 1967–1974. [DOI] [PubMed] [Google Scholar]
- Huang G, Li J, Fu H, Yan Z, Bu G, He X, et al. 2012. Characterization of glucagon‐like peptide 1 receptor (GLP1R) gene in chickens: functional analysis, tissue distribution, and identification of its transcript variants. Domestic Animal Endocrinology 43, 1–15. [DOI] [PubMed] [Google Scholar]
- Inokuchi A, Oomura Y, Nishimura H. 1984. Effect of intracerebroventricularly infused glucagon on feeding behavior. Physiology & Behavior 33, 397–400. [DOI] [PubMed] [Google Scholar]
- Inokuchi A, Oomura Y, Shimizu N, Yamamoto T. 1986. Central action of glucagon in rat hypothalamus. American Journal of Physiology, Regulatory Integrative Comparative Physiology 250, R120–R126. [DOI] [PubMed] [Google Scholar]
- Irwin DM, Prentice KJ. 2011. Incretin hormones and the expanding families of glucagon‐like sequences and their receptors. Diabetes, Obesity & Metabolism 13 (Suppl 1), 69–81. [DOI] [PubMed] [Google Scholar]
- Janssen P, Rotondo A, Mulé F, Tack J. 2013. Review article: a comparison of glucagon‐like peptides 1 and 2. Alimentary Pharmacology & Therapeutics 37, 18–36. [DOI] [PubMed] [Google Scholar]
- Kamisoyama H, Honda K, Saneyasu T, Sugahara K, Hasegawa S. 2007. Central administration of neuromedin U suppresses food intake in chicks. Neuroscience Letters 420, 1–5. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. 2002. Interactions of glucagon‐like peptide‐1 (GLP‐1) with the blood‐brain barrier. Journal of Molecular Neuroscience 18, 7–14. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ. 1994. Central neuroanatomical systems involved in the regulation of food intake in birds and mammals. The Journal of Nutrition 124, 1355S–1370S. [PubMed] [Google Scholar]
- Kurose Y, Kamisoyama H, Honda K, Azuma Y, Sugahara K, Hasegawa S, et al. 2009. Effects of central administration of glucagon on feed intake and endocrine responses in sheep. Animal Science Journal 80, 686–690. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang‐Christensen M, Jessop DS. 1997. Central Administration of glucagon‐like peptide‐1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 138, 4445–4455. [DOI] [PubMed] [Google Scholar]
- Le Quellec A, Kervran A, Blache P, Ciurana AJ, Bataille D. 1992. Oxyntomodulin‐like immunoreactivity: diurnal profile of a new potential enterogastrone. The Journal of Clinical Endocrinology & Metabolism 74, 1405–1409. [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Huang Q, Seaberg R, Brubaker PL, Drucker DJ. 2004. Extrahypothalamic expression of the glucagon‐like peptide‐2 receptor is coupled to reduction of glutamate‐induced cell death in cultured hippocampal cells. Endocrinology 145, 3495–3506. [DOI] [PubMed] [Google Scholar]
- Marubashi S, Tominaga M, Katagiri T, Yamatani K, Yawata Y, Hara M, et al. 1985. Hyperglycaemic effect of glucagon administered intracerebroventricularly in the rat. Acta Endocrinologica 108, 6–10. [DOI] [PubMed] [Google Scholar]
- Meade S, Denbow DM. 2003. The interaction of bombesin and corticotropin‐releasing hormone on ingestive behavior in the domestic fowl. Physiology & Behavior 78, 611–614. [DOI] [PubMed] [Google Scholar]
- Mo C, Zhong Y, Wang Y, Yan Z, Li J. 2014. Characterization of glucagon‐like peptide 2 receptor (GLP2R) gene in chickens: functional analysis, tissue distribution, and developmental expression profile of GLP2R in embryonic intestine. Domestic Animal Endocrinology 48, 1–6. [DOI] [PubMed] [Google Scholar]
- Moghaddam AG, Yaghoobi MM, Jonaidi H, Mahani MT, Sepehri H. 2010. Oxyntomodulin reduces expression of glucagon‐like peptide 1 receptor in the brainstem of chickens. Journal of Animal Physiology and Animal Nutrition 94, 422–428. [DOI] [PubMed] [Google Scholar]
- Monir MM, Hiramatsu K, Matsumoto S, Nishimura K, Takemoto C, Shioji T, et al. 2014a. Influences of protein ingestion on glucagon‐like peptide (GLP)‐1‐immunoreactive endocrine cells in the chicken ileum. Animal Science Journal 85, 581–587. [DOI] [PubMed] [Google Scholar]
- Monir MM, Hiramatsu K, Nishimura K, Takemoto C, Watanabe T. 2014c. Distribution of glucagon‐like peptide (GLP)‐2‐immunoreactive cells in the chicken small intestine: antigen retrieval immunohistochemistry. The Journal of Veterinary Medical Science 76, 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monir MM, Hiramatsu K, Yamasaki A, Nishimura K, Watanabe T. 2014b. The influence of restricted feeding on glucagon‐like peptide‐1 (GLP‐1)‐containing cells in the chicken small intestine. Anatomia, Histologia, Embryologia 43, 153–158. [DOI] [PubMed] [Google Scholar]
- Montaron A, Moyse E, Barre H. 1994. Radioautographic demonstration and localization of glucagon receptors in duck brain. Brain Research 663, 121–130. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR. 2006. Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859. [DOI] [PubMed] [Google Scholar]
- Ng SY, Lee LT, Chow BK. 2010. Insights into the evolution of proglucagon‐derived peptides and receptors in fish and amphibians. Annals of the New York Academy of Sciences 1200, 15–32. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Hiramatsu K, Monir MM, Takemoto C, Watanabe T. 2013. Ultrastructural study on colocalization of glucagon‐like peptide (GLP)‐1 with GLP‐2 in chicken intestinal L‐cells. The Journal of Veterinary Medical Science 75, 1335–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C, Poulsen SS, Moller M, Holst JJ. 1996. Glucagon‐like petide 1 receptors in the subfornical organ and the area postrema are accessible to circulating glucagon‐like peptide 1. Diabetes 45, 832–835. [DOI] [PubMed] [Google Scholar]
- Pocai A. 2012. Unraveling oxyntomodulin, GLP1's enigmatic brother. Journal of Endocrinology 215, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost M, Vertongen P, Waelbroeck M. 2012. Identification of key residues for the binding of glucagon to the N‐terminal domain of its receptor: an alanine scan and modeling study. Hormone and Metabolic Research 44, 804–809. [DOI] [PubMed] [Google Scholar]
- Richards MP, McMurtry JP. 2008. Expression of proglucagon and proglucagonderived peptide hormone receptor genes in the chicken. General and Comparative Endocrinology 156, 323–338. [DOI] [PubMed] [Google Scholar]
- Richards MP, McMurtry JP. 2009. The avian proglucagon system. General and Comparative Endocrinology 163, 39–46. [DOI] [PubMed] [Google Scholar]
- Richards MP, Proszkowiec‐Weglarz M. 2007. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poultry Science 86, 1478–1490. [DOI] [PubMed] [Google Scholar]
- Saneyasu T, Honda K, Kamisoyama H, Ikura A, Nakayama Y, Hasegawa S. 2011b. Neuropeptide Y effect on food intake in broiler and layer chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 159, 422–426. [DOI] [PubMed] [Google Scholar]
- Saneyasu T, Honda K, Kamisoyama H, Nakayama Y, Ikegami K, Hasegawa S. 2011a. Alpha‐melanocyte stimulating hormone plays an important role in the regulation of food intake by the central melanocortin system in chicks. Peptides 32, 996–1000. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Fekete C, Légrádi G, Lechan RM. 2003. Glucagon like peptide‐1 (7‐36) amide (GLP‐1) nerve terminals densely innervate corticotropin‐releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Research 985, 163–168. [DOI] [PubMed] [Google Scholar]
- Schmidt PT, Näslund E, Grybäck P, Jacobsson H, Hartmann B, Holst JJ, et al. 2003. Peripheral administration of GLP‐2 to humans has no effect on gastric emptying or satiety. Regulatory Peptides 116, 21–25. [DOI] [PubMed] [Google Scholar]
- Schneeberger M, Gomis R, Claret M. 2014. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. Journal of Endocrinology 220, T25–T46. [DOI] [PubMed] [Google Scholar]
- Shiraishi J, Yanagita K, Nishikawa F, Tahara Y, Fujita M, McMurtry JP, et al. 2009. A Comparison of the Anorexic Effects of Chicken, Porcine, Human and Bovine Insulin on the Central Nervous System of Chicks. The Journal of Poultry Science 46, 144–148. [Google Scholar]
- Shousha S, Nakahara K, Nasu T, Sakamoto T, Murakami N. 2007. Effect of glucagon‐like peptide‐1 and ‐2 on regulation of food intake, body temperature and locomotor activity in the Japanese quail. Neuroscience Letters 415, 102–107. [DOI] [PubMed] [Google Scholar]
- Sorensen LB, Flint A, Raben A, Hartmann B, Holst JJ, Astrup A. 2003. No effect of physiological concentrations of glucagon‐like peptide‐2 on appetite and energy intake in normal weight subjects. International Journal of Obesity 27, 450–456. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Hiramatsu K, Furuse M, Hasegawa S, Yoshizawa F, Sugahara K. 2005. Distribution of proglucagon mRNA and GLP‐1 in the brainstem of chicks. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 140, 203–207. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Matsumoto M, Furuse M, Hasegawa S, Yoshizawa F, Sugahara K. 2003. Central, but not peripheral, glucagon‐like peptide‐1 inhibits crop emptying in chicks. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 134, 777–781. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Saito ES, Takahashi H, Saito S, Tomonaga S, Boswell T, et al. 2004. Anorexigenic effects of pituitary adenylate cyclase‐activating polypeptide and vasoactive intestinal peptide in the chick brain are mediated by corticotrophin‐releasing factor. Regulatory Peptides 120, 99–105. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sato M, Oikawa D, Furuse M. 2006. Involvement of CRF on the anorexic effect of GLP‐1 in layer chicks. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 143, 112–117. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sugahara K, Ohgushi A, Ando R, Sashihara K, Yoshimatsu T, et al. 2001. Intracerebroventricular injection of exendin (5‐39) increases food intake of layer‐type chicks but not broiler chicks. Brain Research 915, 234–237. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Tanaka S, Furuse M, Hasegawa S, Kato H, Sugahara K. 2002. Intracerebroventricular injection of glucagon‐like peptide‐1 decreases monoamine concentrations in the hypothalamus of chicks. British Poultry Science 43, 122–126. [DOI] [PubMed] [Google Scholar]
- Tang‐Christensen M, Larsen PJ, Thulesen J, Rømer J, Vrang N. 2000. The proglucagon‐derived peptide, glucagon‐like peptide‐2, is a neurotransmitter involved in the regulation of food intake. Nature Medicine 6, 802–807. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Reimann F, Gribble FM. 2009. Nutritional regulation of glucagon‐like peptide‐1 secretion. The Journal of Physiology 587, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. 1996. A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature 379, 69–72. [DOI] [PubMed] [Google Scholar]
- Van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. 2014. Effects of glucagon‐like peptide 1 on appetite and body weight: focus on the CNS. Journal of Endocrinology 221, T1–T16. [DOI] [PubMed] [Google Scholar]
- Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, et al. 2001. A meta‐analysis of the effect of glucagon‐like peptide‐1 (7‐36) amide on ad libitum energy intake in humans. The Journal of Clinical Endocrinology and Metabolism 86, 4382–4389. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lehane C, Ghebremeskel K, Crawford MA. 2010. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public Health Nutrition 13, 400–408. [DOI] [PubMed] [Google Scholar]
- Wang Y, Meng F, Zhong Y, Huang G, Li J. 2012. Discovery of a novel glucagon‐like peptide (GCGL) and its receptor (GCGLR) in chickens: evidence for the existence of GCGL and GCGLR genes in nonmammalian vertebrates. Endocrinology 153, 5247–5260. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Y, Li X, Li J, Leung FC. 2008. Cloning, tissue distribution, and functional characterization of chicken receptor. Poultry Science 87, 2678–2688. [DOI] [PubMed] [Google Scholar]


