Summary
Although a successful vaccine against HBV has been implemented in 184 countries, eradication of hepatitis B virus (HBV) is still not on the horizon. There are over 240 million chronic carriers of HBV globally. The risk of developing chronic hepatitis ranges from >90% in newborns of hepatitis Be antigen (HBeAg)‐positive mothers, 25%–35% in children under 5 years of age and <5% in adults. HBeAg, a non‐particulate viral protein, is a marker of HBV replication. This is the only HBV antigen to cross the placenta, leading to specific unresponsiveness of helper T cells to the capsid protein and HBeAg in newborns. HBeAg is tolerated in utero and acts as a tolerogen after birth. Perinatal transmission is frequent when mothers are HBeAg‐positive, whereas it occurs less frequently when mothers are HBeAg‐negative. Sequence heterogeneity is a feature of HBV. Based on an intergroup divergence >7.5% across the complete genome, HBV is classified phylogenetically into at least nine genotypes. With between ~4% and 8% intergroup nucleotide divergence, genotypes A–D, F, H and I are classified further into subgenotypes. HBV genotypes/subgenotypes may have distinct geographical distribution and can develop different mutations in the regions of the HBV genome that code for HBeAg. These differences can be related to the role of HBV genotypes to the natural history of infection and mode of transmission. Thus genotypes/subgenotypes of HBV can be responsible for the different natural history of infection and modes of transmission in children, found in various regions of the world, where different genotypes/subgenotypes prevail. Copyright © 2016 John Wiley & Sons, Ltd.
Abbreviations Used
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BCP
basic core promoter
- ELISA
enzyme linked immunosorbent assay
- ε
encapsidation signal
- GBD
global burden of disease
- HBcAg
hepatitis B c antigen or core protein
- HDV
hepatitis D virus
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B s antigen
- HCC
hepatocellular carcinoma
- LiPa™
line probe assay
- MTCT
mother‐to‐child transmission
- P
polymerase
- pgRNA
pregenomic ribonucleic acid
- preC/C
precore/core
- TLR‐2
toll‐like receptor 2
- WHO
World Health Organization
Global Epidemiology and Pathogenesis of Hepatitis B Virus (HBV) Infection
Two of the 7.3 billion of the world's population have been exposed the hepatitis B virus (HBV). It is estimated that 240 million individuals are currently chronically infected with this virus 1. Twenty to thirty percent of the chronic carriers of HBV will develop complications including acute liver failure, hepatic decompensation, cirrhosis and hepatocellular carcinoma (HCC). Annually approximately 786 000 people die as a result of complications caused by chronic HBV infection. These deaths are ranked 15th in the Global Burden of Disease (GBD) 2010 2 and account for 50% and 33% of deaths as a result of HCC and cirrhosis, respectively 2.
With the exception of Ecuador, Peru, Bolivia, and southern eastern Asia, Indonesia and East Timor, the prevalence of HBV infection in children (5 to 9 years) coincides with that in adults (19 to 49 years) (Figure 1) 3, 4. In these south American countries, the prevalence in children is higher than in adults, whereas the opposite is true in the Asian countries, except for Indonesia and East Timor, where it is higher 3. Between 1990 and 2005 there was an increase in chronic HBV infections among younger age groups in southern‐eastern sub‐Saharan Africa, whereas in central sub‐Saharan Africa there was a corresponding decrease 3. In central and eastern Europe, the most affected age group was younger than 9 years old, with a minimal decrease between 1990 and 2005. Between 1990 and 2005, the strongest reduction in prevalence of HBsAg in children was recorded in south east Asia 3. This decrease is attributable to the introduction of effective vaccination programs.
Figure 1.
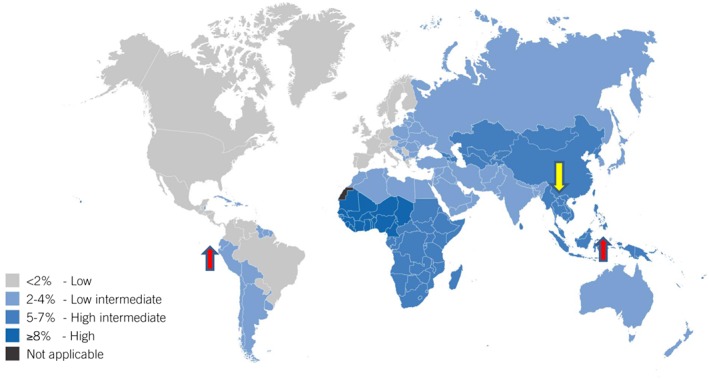
The map shows the prevalence of HBsAg‐positive adults in the world. The arrows indicate where the prevalence in children does not coincide with that in adults. The red arrows indicate higher levels of HBsAg‐positivity in children compared to adults, whereas the yellow arrows indicate lower levels in children compared to adult. Reprinted with permission from: Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection, WHO, page 12, © World Health Organization 2015 [http://www.who.int/hiv/pub/hepatitis/hepatitis‐b‐guidelines/en/]
Molecular Biology of HBV and HBeAg
HBV, the smallest DNA virus infecting man, belongs to the family Hepadnaviridae and is the prototype member of the genus Orthohepadnavirus. HBV has a partially double‐stranded, circular DNA genome of ~3 200 base pairs. This compact genome contains four partly or completely overlapping open reading frames (ORFs): precore/core (preC/C) that encodes the e antigen (HBeAg) and core protein (HBcAg); P for polymerase (reverse transcriptase), PreS1/PreS2/S for surface proteins (three forms of HBsAg, small (S), middle (M) and large (L)) and X for a transcriptional trans‐activator protein 5 (Figure 2).
Figure 2.
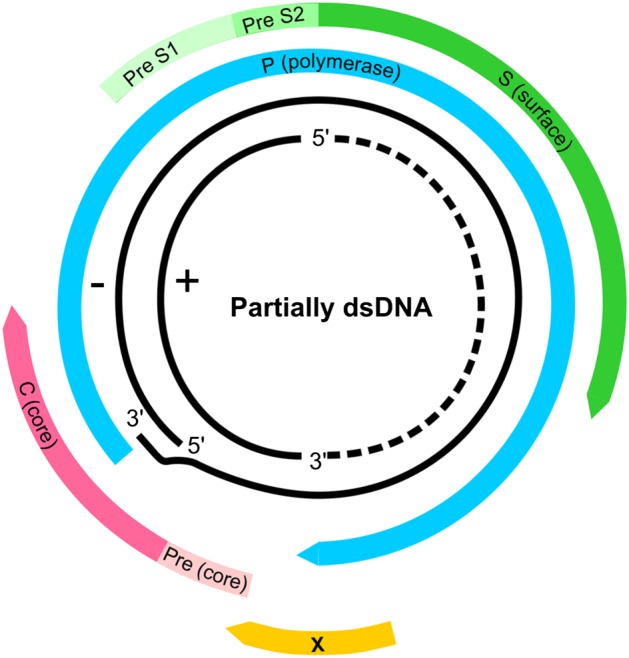
The genome of hepatitis B virus (HBV). The partially double stranded DNA (dsDNA) with the complete minus (−) strand and the incomplete (+) strand. The four open reading frames (ORFs) are shown: precore/core (preC/C) that encodes the e antigen (HBeAg) and core protein (HBcAg); P for polymerase (reverse transcriptase), PreS1/PreS2/S for surface proteins (three forms of HBsAg, small (S), middle (M) and large (L)) and X for a transcriptional trans‐activator protein
HBV replicates by reverse transcription of the pregenomic RNA (pgRNA), a 3.5 kb RNA intermediate 6, which is transcribed, by the cellular RNA polymerase II, from the covalently closed circular form of HBV DNA in the hepatocyte nucleus 7. In order to be reverse transcribed the pgRNA has to be folded into a secondary structure, known as the encapsidation signal (ε) 8 (reviewed in 9) and enclosed in the viral capsid, which is comprised of HBcAg. Anti‐HBc antibodies are a measure of exposure to HBV but not necessarily immunity. Surrounding the capsid is an envelope made up of lipid membrane from the host, in which are embedded HBsAg proteins coded by the virus (Figure 3). The presence of anti‐HBs signals immunity to HBV. In addition to the structural proteins, HBc and HBs antigens, HBV codes for two non‐particulate proteins X (a transcriptional trans‐activator protein) and HBeAg 10.
Figure 3.
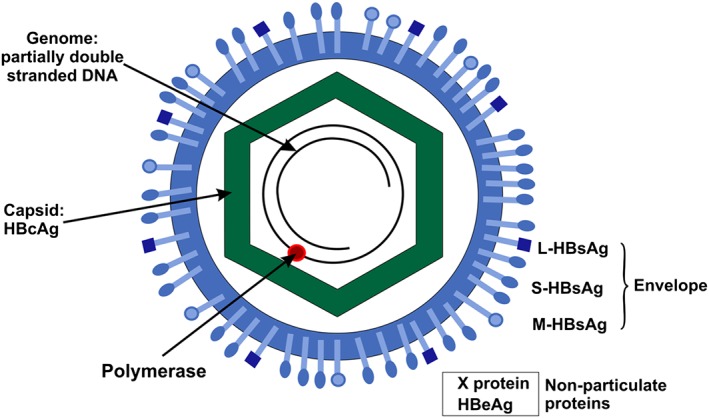
Schematic representation of hepatitis B virus (HBV), showing the structure of the virion, composed of a partially double stranded DNA genome, enclosed by a capsid, comprised of HBcAg and surrounded by a lipid envelope containing large (L)‐HBsAg, middle (M)‐HBsAg and small (S)‐HBsAg. The virus also expresses two non‐particulate proteins X protein and HBeAg
HBeAg is encoded by the preC/C ORF (1814 – 2452/2488 from the EcoRI site, 11) and the basic core promoter (BCP) of HBV controls the transcription of the preC/C region 12, 13 (reviewed in 14). The preC/C fusion protein, which is the precursor of HBeAg has a signal peptide on its amino end that targets it to the endoplasmic reticulum, where it is post‐translationally modified 15. The amino end is truncated at amino acid 19, whereas the carboxyl end is cleaved at variable sites. The mature HBeAg is secreted and is soluble in serum 16 (Figure 4).
Figure 4.
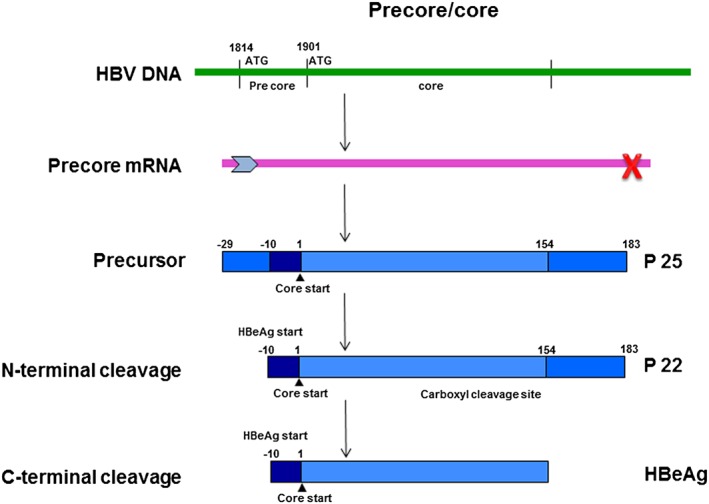
Schematic representation of the expression of HBeAg in hepatitis B virus (HBV). Nucleotide numbering is from the EcoRI cleavage site on the DNA genome and amino acid numbering is from position 1 of the core protein
Although HBeAg is not required for viral assembly or replication it is conserved in all orthohepadnaviruses 17 and is important for natural infection in vivo 18. Clinically, HBeAg is an index of viral replication, infectivity, inflammation, severity of disease and response to antiviral therapy. Seroconversion from HBeAg‐positive to HBeAg‐negative/anti‐HBeAg‐positive phase usually heralds resolution of infection 19. However, the emergence of BCP and precore mutations can lead to HBeAg‐negative infection, where viral replication and inflammation remain high. While the exact function of HBeAg has not been elucidated, it has been shown to be an immunoregulatory protein, which acts as a tolerogen 20 and an immunogen 21, triggers an interleukin‐1 response 22 and regulates toll‐like receptor 2 (TLR‐2) expression 23. Immune regulation mediated by HBeAg may lead to chronicity and persistence following perinatal infection and in adults prevent severe liver injury as result of infection 18. Chronic infection will result following perinatal transmission from HBeAg‐positive mothers. On the other hand, transmission from HBeAg‐negative mothers can result in acute hepatitis or acute liver failure 24. In addition to being an important milestone of chronic HBV infection, HBeAg status (HBeAg‐positivity versus HBeAg‐negativity) is also a determinant of the mode of transmission of the virus.
Natural History of HBV Infection in Children
Development of chronic infection, following acute hepatitis B, requires the expression of HBeAg 25 and thus the risk of developing chronic hepatitis is:
>90% in newborns of HBeAg‐positive mothers 26, 27. This is probably because of the immature immune system of the infant 28 and/or the foetus developing immune tolerance as a result of the transplacental crossing of either the virion or HBeAg, the only HBV antigen that can cross the placenta 18. Transplacental crossing of HBeAg induces a specific unresponsiveness of helper T cells to both HBcAg and HBeAg 29.
25%–35% in infants and children under the age of 5 years
6% in children 5 to 15 years
<5% in adults 30
Differentiated by the level of viral replication and the host immune response, chronic HBV infection acquired perinatally or in early infancy is broadly divided into four phases in children (Figure 5):
-
1
The high replicative, low inflammatory phase (previously immune tolerant phase) 31 is HBsAg‐positive, HBeAg‐positive, with high HBV DNA levels (>2 × 105 IU/ml) 32, with minimal liver inflammation, normal aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels and asymptomatic 33. The duration of this phase can be up to 3 decades if transmission occurred perinatally, whereas following horizontal transmission it may be very short and undetected 34.
-
2
The immune clearance or reactive phase is characterized by fluctuating ALT and HBV DNA levels and ending with spontaneous HBeAg loss 32, 35. HBeAg seroconversion is accompanied by elevated ALT and decreased HBV DNA levels 32. Spontaneous HBeAg seroconversion occurs at a lower rate in children born to HBsAg‐positive mothers compared to HBsAg‐negative mothers 36 and less frequently in children, who acquired their infection perinatally compared those infected horizontally 37. In south east Asia, the annual HBeAg seroconversion rate is 4%–5% in children older than 3 years, but only 2% in those younger than 3 years 38. In contrast, in Euro‐Mediterranean and African countries, HBeAg seroconversion is more frequent, occurring at an annual rate of 14%–16% 25, 39. Thus close to 90% of the individuals in Euro‐Mediterranean and African countries are HBeAg‐negative/anti‐HBeAg positive by the age of 20 years 39, 40, 41, compared to only 5% in south east Asia 25. On average, HBeAg seroconversion usually occurs in children younger than 15 years 42. Prognosis for these children is generally good 27.
Figure 5.
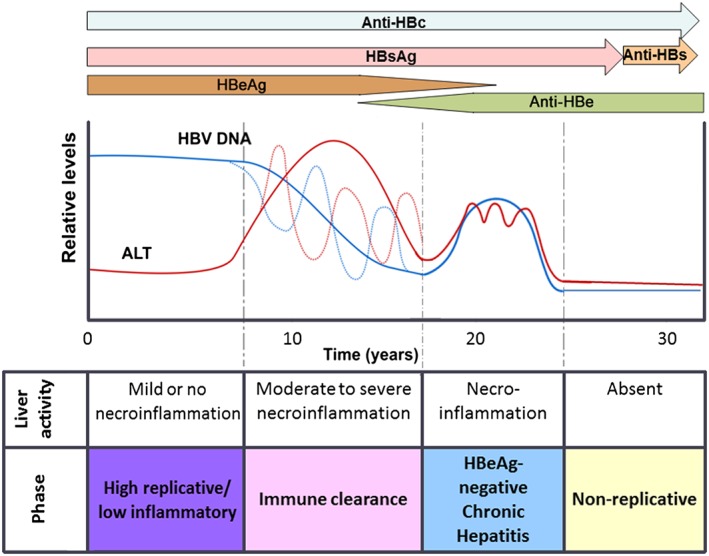
Natural history of infection of hepatitis B virus (HBV) in children over time showing the four phases, the liver histology and activity, the expression of serological markers (antigens: HBsAg and HBeAg: antibodies: anti‐HBc, anti‐HBe and anti‐HBs) and the relative levels of HBV DNA and ALT. The recently suggested nomenclature is used 31
The duration of this phase is variable. The shorter duration of the HBeAg‐positive phase in Euro‐Mediterranean and African regions compared to that in South‐East Asia leads to significantly lower rates of development of advance liver disease in these regions 25. Delayed HBeAg seroconversion may prolong the inflammatory response and lead to more severe liver disease 43. Then again, if HBeAg seroconversion occurs very early, before the age of 3 years, this can also lead to increased ALT levels, with severe liver injury and the rapid development of HCC before the age of 10 years 38, 44. The genotype or subgenotype of HBV can affect the age at which HBeAg loss occurs as well as its frequency in a population (see below).
-
The HBeAg‐negative chronic hepatitis phase 31, where necroinflammation persists with high or fluctuating ALT levels and immune clearance is ineffective. Viral loads are moderate to high and liver disease is progressive.
-
The low replicative phase (previously “inactive HBsAg‐positive carrier” phase) 31 (post‐HBeAg seroconversion) is the phase characterized by the absence of HBeAg, anti‐HBe positivity, normal ALT and low or undetectable HBV DNA levels (<2 × 103 IU/ml).
Two additional phases, the reactivation phase and the HBsAg loss or occult phase 45, have been described in the natural history of HBV infection 31 but these are infrequent in children. The reactivation phase is characterized by the recurrence of viremia, reversion to HBeAg‐positivity and hepatic flares 46. During the HBsAg loss phase, the entire, episomal, replication‐competent genomes can persist intrahepatically, in the presence or absence of serological markers (occult infection) 45. However, spontaneous HBsAg loss is rare in children, occurs at 0.6%–1% per annum, especially if children were infected perinatally and have minimal liver injury 47, 48, 49. This seroconversion rate is lower than that seen in individuals infected as adolescents or in adulthood 50. More than 90% of patients, infected in childhood, remain HBsAg carriers in adulthood 50. Acquisition of infection horizontally is associated with a higher HBsAg clearance rate, as is being born to a HBsAg‐negative mother 46. The levels of anti‐HBs after loss of HBsAg are higher in children born to HBsAg‐positive mothers compared to those born to HBsAg‐negative mothers. 46
Albeit that liver damage is minimal in the majority of children, some can manifest with mild inflammation and acute hepatitis 49, as well serious complications of HBV infection, including cirrhosis and HCC, 2 to 7 years after infection 44. Risk factors for early HCC development include cirrhosis and HBeAg seroconversion before 3 years 44. Normal ALT levels and anti‐HBeAg seroconversion are no guarantee against cirrhosis and HCC, especially in untreated individuals 44. In a European study, children developed HBeAg‐negative chronic hepatitis and/or HCC after an average 5‐year follow‐up 50. It is possible that there are different mechanisms for the development of HCC in adults and children. The former require higher viral loads and liver inflammation, whereas integration of HBV in the human genome may trigger HCC in children 51.
Although genetic and environmental factors, including socioeconomic and hygiene levels, can play a role in the natural history of HBV infection and the development of advanced liver disease, it is becoming increasingly evident that the genotypes/subgenotypes of HBV and specific mutations can play a leading role 25, 52.
Genotypes and Subgenotypes of HBV
Sequence heterogeneity is a feature of HBV, because the viral encoded polymerase lacks proofreading ability. To date, based on an intergroup divergence of greater than 7.5% across the complete genome, HBV has been classified phylogenetically into 9 genotypes, A to I 11, 53, 54, 55, with a putative 10th genotype, “J”, isolated from a single individual 56, which is a recombinant of genotype C and gibbon HBV in the S region 57. With between ~4% and 8% intergroup nucleotide difference across the complete genome and good bootstrap support, genotypes A – D, F, H and I are classified further into at least 35 subgenotypes 55. The genotypes differ in genome length, the size of ORFs and the proteins translated 11, as well as the development of various mutations 52. Based on HBsAg heterogeneity 11, nine serological subtypes, ayw1, ayw2, ayw3, ayw4, ayr, adw2, adw4, adwq, adr and adrq, have been identified. A broad, highly statistically significant correlation exists between serological subtypes and genotypes: adw is associated with genotypes A, B, F, G and H, adr with C and ayw with D and E 58 but many exceptions exist (Figure 6).
Figure 6.
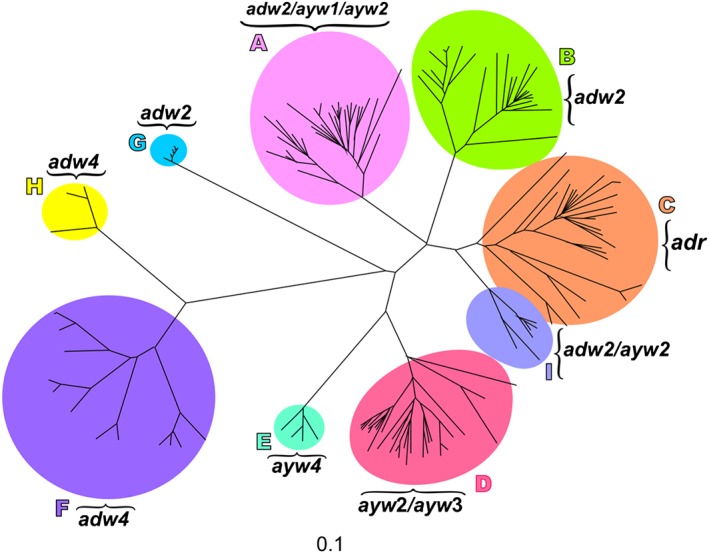
An unrooted phylogenetic tree of full genome sequences of hepatitis B virus (HBV) representative of the nine major genotypes, established using neighbor‐joining. Letters A to I represent the nine genotypes and the associated serological subtypes are indicated
The genotype of HBV can influence the outcome of HBV infection in children because it can affect the frequency of HBeAg‐positivity, the age at which HBeAg loss occurs and the mode of transmission. Therefore the natural history of HBV infection can differ in different geographical regions (52, 55, 59, 60 and references cited therein).
Geographic Distribution of Genotypes
Globally and locally the various genotypes, and in some cases the subgenotypes, have distinct geographical distributions 11, 55 (Figure 7). Genotype A is found in Africa, Europe and the Americas whereas genotypes B and C predominate in south east Asia. Although genotype D is distributed in all continents and is referred to as the cosmopolitan genotype, its subgenotypes can be geographically distributed 61. Genotype E is confined to western Africa and distributed to other regions of the world following emigration from Africa. Central and South America are the regions where genotype F and H originate. Although genotype G was originally described in Georgia, United States of America, it has been isolated in the United Kingdom, Germany and Italy. Subgenotype A1 predominates in Africa and in regions outside Africa where there has been mass migration from Africa 62. The subgenotype of A found outside Africa is subgenotype A2. Subgenotype B1 prevails in Japan and B2 in south east Asia, with B5 (formerly B6) found in Alaska. Thus in the two regions of the world where HBV occurs at high endemicity different genotypes prevail. Subgenotype A1, genotypes D and E circulate in sub‐Saharan Africa and genotypes B and C in south eastern Asia.
Figure 7.
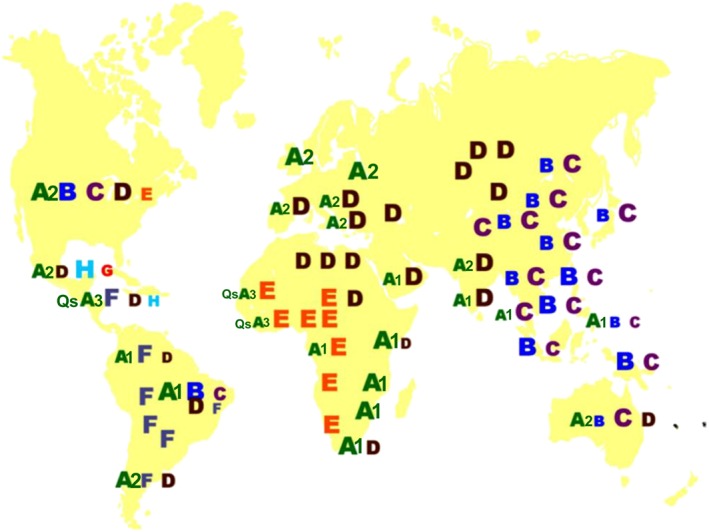
The geographic distribution of the hepatitis B virus (HBV) genotypes and subgenotypes of A (A1, A2, QsA3: Quasi‐subgenotype A3) 55. The relative size of the letter/number indicates the relative prevalence of the genotype or subgenotype.
Recombination Between Genotypes
In geographical regions where a number of genotypes co‐circulate, recombination between genotypes can occur, and this provides a mechanism of variation within individuals and in the population in general 11. Genotype A and D recombinants have been found in Africa 63, whereas in Asia genotype B/C recombinants occur 64, 65. Tibet has a 26.2% HBV carrier rate, and 96% of the isolates sequenced were C/D recombinants 66. The breakpoints occurred most frequently in the BCP/PC region, with breakpoints found in the small S and core regions 67, 68. The recombination may provide a selection advantage to the viral strains, and the recombinants may become the dominant strains of a quasispecies and persist in a population. Genotype I is composed entirely of recombinants, and 93% of genotype B strains represent recombinants 68. Thus, four of the six subgenotypes of genotype B (B2–B4) represent genotype B recombined with genotype C in the precore/core region, whereas only subgenotypes B1 found mainly in Japan 65 and B5 (previously B6) from a Canadian Inuit population 69 represent genotype B without this recombination. Subgenotypes B2–B4 show a higher risk of serious complications of HBV infection including cirrhosis and development of HCC, compared to B1 and B5 60.
Genotyping and Subgenotyping Methods
Although the HBV S gene sequence is generally adequate to assign genotypes 58, the complete sequence of the HBV genome provides additional information with respect to phylogenetic relatedness 70, 71. Moreover, recombinants may not be identified when using a single region of the HBV genome for phylogenetic analysis. Nevertheless, although complete genome sequencing, followed by phylogenetic analysis, provides the gold standard for genotyping, it does not allow for rapid and direct analysis on a large scale basis 11 and requires expertise and thus capacity development in computer processing coupled with phylogenetic analyses. In order to expedite and facilitate genotyping a number of methods have been developed 11, 72, 73. A number of commercially available assays are available, for example, genotype‐specific probes assay (Smitest HBV Genotyping Kit, Genome Science, Fukushima, Japan), reverse hybridization of PCR products to probes on nitrocellulose strips (the line probe assay, LiPa™, Innogenetic Inc, Gent, Belgium) and enzyme linked immunosorbent assay (ELISA) (HBV Genotype EIA, Institute of Immunology, Tokyo, Japan). Each one has its advantages and disadvantages 11, 72, 73, which should be taken into account, when selecting the genotyping method appropriate for a particular study or application 74.
The Effect of Genotype/Subgenotype on HBeAg Expression
HBeAg, discovered in 1972 10, has been the classical marker for HBV replication, and its presence for longer than 10 weeks can herald the transition to chronic infection 35. Transition from the immune clearance phase to the HBeAg‐negative chronic hepatitis phase is accompanied by HBeAg seroconversion, the movement of HBcAg from the nucleus to the cytoplasm 75 and an increase in the frequency of HBV strains with BCP and precore mutations 76, 77. Mutations in the BCP and preC can influence the expression of HBeAg at the transcriptional, translational and post‐translational levels. Transcription of the preC mRNA is affected by A1762T/G1764A 78. At the translational level, mutations in the Kozak sequence preceding the preC start codon (1814 from the EcoRI site) can affect the expression of HBeAg by a leaky scanning mechanism 79 whereas G1896A introduces a stop codon that leads to the truncation of the HBeAg precursor 80. The G1862T mutation in the preC affects expression at a post‐translational level 81.
The propensity to develop precore mutations can be influenced by the HBV genotype or subgenotype. As described previously, the preC/C region is transcribed into the precore mRNA that is translated into the precursor of HBeAg (Figure 4). The preC/C region also overlaps with the region that is transcribed into the pgRNA, which is the RNA intermediate of HBV replication. In order to be successfully encapsidated, the pgRNA has to be folded into a secondary structure known as ε, which has to be stable, for viral replication to proceed. The sequence of the preC/C region differs in the different genotypes, and in some cases, the subgenotypes 17. For example, 1858C in the preC/C region is positively associated with genotypes A, F and H and 1858T with genotypes B, D and E 58. Subgenotype C2 has 1858T as opposed to C1 that has 1858C and F1/F4 can be differentiated from F2/F3 by having 1858T instead of 1858C 58. 1888A is positively associated with subgenotype A1 58.
Subgenotypes A1, A2 and genotype D, which circulate in southern Africa, will be used to explain and illustrate how nucleotide differences can influence HBeAg expression. Comparison of the sequences of BCP/PreC region of the different (sub) genotypes A1, A2 and D reveals variations that can account for the differences in HBeAg expression (Figure 8A). Furthermore, the frequency of these mutations/variations also differs between these (sub) genotypes (Figure 8B) 82. The presence of 1858C in genotype A precludes the G1896A mutation found in genotype D because the presence of 1858C and 1896A would destabilize ε and compromise viral replication (Figure 8C) 83, 84. 1896A converts the codon for tryptophan to a stop codon, leading to the truncation of the HBeAg precursor and abrogation of HBeAg expression (Figure 9A) 80. This is the mutation that prevails in isolates obtained from HBeAg‐negative individuals infected with genotype D. The only mutations that can influence HBeAg expression in strains isolated from individuals infected with subgenotype A2, which has 1858C, are 1762T/1764A (Figure 9B). On the other hand, in addition to the 1762T/1764A, subgenotype A1 strains, which also have 1858C, can develop mutations in the Kozak sequence (1809–1812) and the G1862T (Figure 9C) 85, accounting for the higher frequency of HBeAg‐negativity observed in individuals infected with this subgenotype. In a case control study, comparing individuals infected with subgenotypes A1, A2 and genotype D, respectively, it was shown, regardless of the age group, the frequency of HBeAg‐positivity was lower in individuals infected with subgenotype A1 compared to the other (sub) genotypes 86. This difference reached statistical significance in individuals younger than 30 years of age 86.
Figure 8.
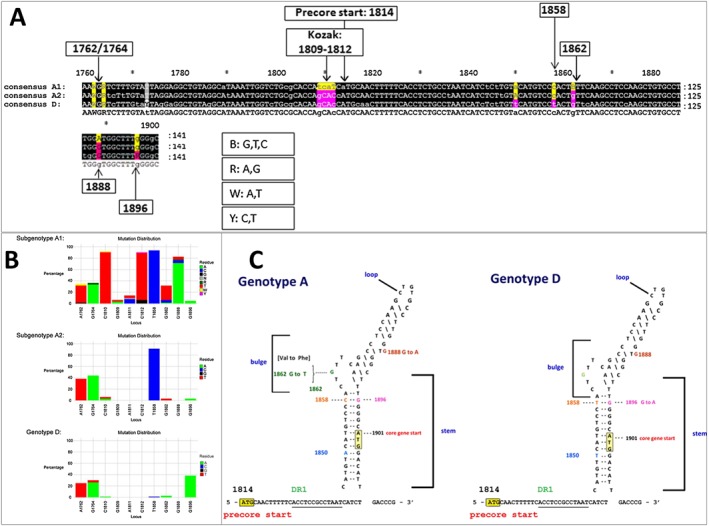
A comparison of (sub) genotypes A1, A2 and D. (A) Comparison of the consensus sequence of the basic core promoter/precore (BCP/PC) region of (sub) genotypes A1, A2 and D. Numbering from the EcoRI site. (B) Mutation distribution graphs generated using the Mutation Reporter Tool 82 showing the percentage of mutant residues relative to the reference motif found at 10 loci of interest specified (1762, 1764, 1809–1812, 1858, 1862, 1888, 1896). Three data sets were submitted to the tool to produce the three graphs showing the mutation distribution for 63 subgenotype A1 samples, 34 subgenotype A2 samples and 76 genotype D samples. The reference motif used was AGGCACTGGG. This is also shown by the letter preceding each locus on the X‐axis. To facilitate direct comparisons between the graphs, conserved loci were not suppressed, and the Y‐axis was scaled to 100% by selecting the appropriate controls on the input page. (C) Nucleotide sequence and predicted secondary structure of the hepatitis B virus (HBV) pregenome encapsidation signal (ε) 8. The DNA instead of the RNA sequence is shown for ease of interpretation. The sequence for genotypes A and D is shown. The two sequences differ in nucleotides 1850 and 1858 in this region. The initiation codons of the precore (1814) and core (1901) are labeled. Nucleotide substitutions found in the two genotypes are shown at the sides of the stem‐loop structure. Please note 1862T and 1888A only occur in subgenotype A1 and not subgenotype A2 of genotype A. Numbering from the EcoRI site
Figure 9.
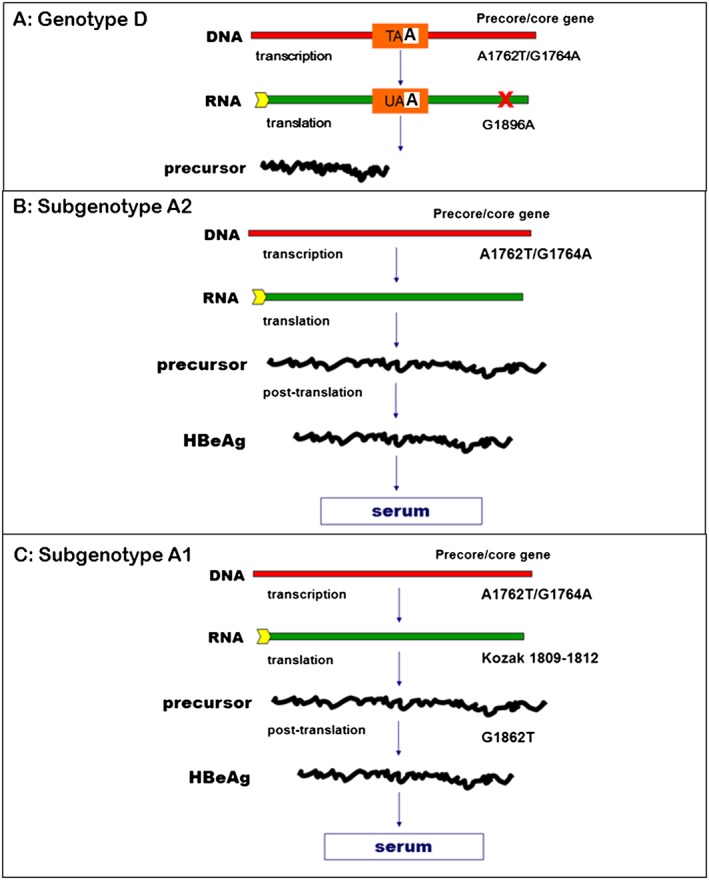
Schematic representation of the expression of HBeAg in hepatitis B virus (HBV) (sub) genotype D, A2 and A1. A1762T/G1764A, which decreases the expression of transcription of precore mRNA and therefore HBeAg expression can occur in (sub) genotypes A1, A2 and D. (A) Genotype D can develop the G1896A mutation, converting the codon TAG for tryptophan to a stop codon TAA, leading to the truncation of the precursor, abrogating HBeAg, expression. (B) Subgenotype A2 can only develop A1762T/G1764A. (C) Subgenotype A1 has unique characteristics and can develop mutations in the Kozak (1809 – 1812), which affects the translation of HBeAg and G1862T that interferes with HBeAg expression at the post‐translational level. Nucleotide numbering is from the EcoRI cleavage site on the DNA genome
As a result of the distinct geographic distributions of the (sub) genotypes globally, the percentage of HBeAg‐negative chronic hepatitis in the world also varies. Thus in the Mediterranean areas and Maghreb, where genotype D, which can develop G1896A, prevails, 80% to 90% of chronic hepatitis patients are HBeAg‐negative. This percentage is 30%–50% in southern east Asia where genotypes B and C circulate. In Chinese patients infected with genotype B, loss of HBeAg occurs earlier and more frequently than in those infected with genotype C 87. On the other hand, in regions where subgenotype A2 is found, such as northern America and Europe only 10% of the chronic HBV carriers are HBeAg‐negative. HBeAg loss occurs in native Alaskans infected with subgenotype A2, genotypes B, D and F before the age of 20, whereas in those infected with genotype C it occurs at 40 years or older 88. Ghanaian blood donors, infected with genotype E with normal ALT, had lower frequency of HBeAg‐positivity (25%) 89 compared to Taiwanese blood donors infected with genotype B (30%) or C (41%) 90. The HBeAg‐positive blood donors infected with genotype E had a median age of 22 years 89 and were 10 years younger than those infected with either genotype B or C 90. The frequency of HBeAg‐positivity in genotype E infected blood donors, with normal ALT, remained stable between the ages 16 and 52 years, whereas it decreased in the blood donors with elevated ALT 89. Likewise, chronic hepatitis B Chinese patients showed higher HBeAg‐positivity when infected with genotype B (53%) or genotype C (69%) compared to Ghanaians chronically infected with genotype E (34%) 89. This difference in frequency of HBeAg‐positivity was highly significant in individuals younger than 30 years 89. Similarly, as already stated, relatively earlier seroconversion occurs in patients infected with subgenotype A1 compared to those infected with other (sub) genotypes A2 and D 86.
In a comprehensive analysis, Ott et al. (2012) estimated the prevalence of HBeAg‐positivity in females from 21 regions of the world in 1990 and 2005 91. In 1990, the highest prevalence was seen in girls aged 0 to 9 years (55%–91%) and decreased to 12%–16% in the 60 – 69 year age group. In the childbearing age group of 20 – 29 years the levels ranged from 30% to 43%. The highest levels in all age groups were found in Oceania (91% in the 0 – 9 age group), with the lowest levels in southern and western sub‐Saharan Africa (55%–62%) 91. Although the trends were similar in 2005, there were some changes. The prevalence dropped most dramatically in the birth to 9 year olds in Oceania (~23%) with minimal or no decrease in southern (7%) and western (0.2%) sub‐Saharan Africa, respectively. The reduction evident in some geographical regions was a result of the good implementation of successful vaccination programs 91.
The expression of HBeAg is one of the major factors influencing the frequency and mode of transmission of HBV 92.
Frequency of Transmission
In a study carried out in Iran 93, where a data‐mining approach was used, the overall transmission rate was 15.7% (5.4% and 27.3% for male and female index cases, respectively). The frequency of transmission was more than double for HBeAg‐positive females (49%) compared to HBeAg‐negative females (23.4%). There was a lower intrafamilial transmission rate of HBV in patients with hepatitis D virus (HDV) co‐infection, being statistically significant between patients positive and negative for anti‐HDV antibody. This was most pronounced for HBeAg‐negative female index cases, where the frequency of transmission was only 5% in those who were anti‐HDV‐positive compared to 25% in anti‐HDV‐negative females 93. A number of factors can influence the interaction of HBV and HDV including HBV genotype. Interference of HBV replication by HDV was more apparent in patients infected with HBV genotype A compared to those infected with genotypes D or E 94. The prevalence and characteristics of HDV infection in the pediatric population have not been widely researched.
Modes of Transmission of HBV in Children
Children can be infected ante‐natally, peri‐natally or horizontally. Mother‐to‐child transmission (MTCT) can occur by all three modes:
Ante‐natal transmission or infection in utero occurs in the third trimester of pregnancy possibly when HBV, from maternal blood, traverses the placenta or as a result of placental leakages, infects the foetus 27, 95. The presence of HBV in placental villous capillary endothelial cells 96 and trophoblastic cells 97 implicates “cellular transfer” of HBV from mother to foetus. This intrauterine transmission, occurs infrequently and accounts for <5% of MTCTs from HBsAg‐positive, HBeAg‐positive mothers 29. Maternal blood levels of HBV DNA of >108 copies/ml 97 and HBeAg‐positivity 96 were shown to be a risk factors for intrauterine transmission of HBV. Moreover, a direct correlation between maternal HBV DNA levels and those in cord blood exists 97. The administration of hepatitis B immune globulin (HBIg) from 28 weeks gestation onwards reduced this risk of transmission 98. However, this mode of transmission cannot be prevented by the administration of both HBIg and vaccine after birth 99.
Perinatal transmission occurs at or near the time of delivery (28 weeks of gestation up to 7 days after birth) by percutaneous and/or permucosal exposure to maternally infected fluids in the birth canal 100, 101. HBsAg has been detected in 33% of amniotic fluid samples, 50% of cord blood samples, 98% of vaginal fluid samples, 71% of breast milk samples and 95.3% of samples of gastric contents from newborns 102. However, only a minority of amniotic fluid and cord blood samples were shown to be HBV DNA‐positive 103. Perinatal transmission is the major mode of transmission in geographical regions where there is a high frequency of HBeAg‐positivity in females at gestational age. HBeAg is associated with higher viral loads and acts as a tolerogen and immunogen 20, 21; thus, 70% to 90% of babies born to HBeAg‐positive mothers will become chronic carriers of the virus within 3 months of birth 104, 105, 106, 107, 108, 109, 110. In contrast, chronic infection occurs in only 10% of babies born to mothers who are HBeAg‐negative 111.
Post‐natal or horizontal transmission occurs parenterally via apparent or inapparent percutaneous or permucosal exposure to bodily fluids 35. In addition to blood, saliva, urine and semenal fluid have also been implicated in transmission of HBV 112. Horizontal transmission can be intra‐ and inter‐familial 113, iatrogenic, by the indiscriminate use of injections with non‐sterilized equipment and as a result of cultural practices, including scarification and tattooing 40, 114, 115. MTCT can occur during childcare activities including breastfeeding 116, although no difference was noted between transmission from HBsAg‐positive mothers to infants that were breast‐fed compared to those that were bottle‐fed 102. Children infected perinatally can be a source of infection for siblings and playmates 116, 117, 118, 119. The risk of infection among children increases with age 113. The behaviors implicated in intra‐familial transmission include sharing of bath towels, sharing of chewing gum or partially eaten sweets, sharing of toothbrushes and biting of fingernails in conjunction with scratching the backs of carriers 113. HBV exposure during childhood can lead to a large proportion of adolescents being infected by the time they reach the age of sexual maturity, when sexual transmission becomes the dominant route of transmission 120, 121. In low incidence regions of HBV, transmission mainly occurs horizontally, iatrogenically in health care personnel, drug addicts and by sexual contact in heterosexual couples or men‐who‐have‐sex‐with men 116.
The Influence of Genotypes and Subgenotypes on Mode of Transmission
HBV is classified in at least nine genotypes and at least 35 subgenotypes, which can have distinct geographical distributions. The different (sub) genotypes can develop various mutations that can affect HBeAg expression, which can determine the dominant mode of transmission and natural history of infection depending on the frequency of HBeAg‐positivity in a population. Even before (sub) genotypes were defined and their geographical distribution well known, it was recognized that the frequency and mode of transmission of HBV varied in the different ethnic groups around the world and that these were influenced by the frequency of HBeAg‐positivity 107.
A German study showed that three times as many west Asian HBsAg‐positive carriers were HBeAg‐positive compared to those of European descent 122. Similarly, Chinese mothers in Singapore and Malaysia were more likely to be HBeAg‐positive compared to either Indian or Malay mothers 123, 124. A higher percentage of HBsAg‐positive mothers were HBeAg‐positive and had higher viraemia in south east Asia (40%) compared to mothers in Africa (5%) 125. MTCT occurs in 40%–64% Chinese mother–infant pairs, but only in 30% and 10% of their African and European counterparts, respectively 25, 126. Differences can also be observed within a single locale and is dependent on ethnicity. In a study carried out in Thrace, Greece, children of Turkish descent were more likely to be infected peri‐natally (61.8%) compared to native Greek children (39%) and immigrant children from the former Union of Soviet Socialist Republic (USSR) (22%), who were infected by percutaneous exposure 127. Horizontal MTCT occurs frequently in the Chinese at 6 weeks to 3 months after birth 128, 129, whereas in other regions such as Senegal 130 and Saudi Arabia 131 transmission occurs later in infancy and childhood. Thus perinatal MTCT can account for 50% of chronic infections in endemic regions of Asia and the Pacific Islands 46 whereas it is less frequent in Africa, the other geographical region, where HBV is endemic and horizontal transmission dominates 40.
Today we know that these differences can be accounted for because of the distribution of different (sub) genotypes in the various ethnic groups and geographical regions (Figure 7). In south east Asia, the prevalent genotypes are B and C and HBeAg to anti‐HBe seroconversion, in 90% of carriers of these genotypes, occurs at the mean age of 30–35 years 132, 133, 134. Thus women of gestational age are frequently HBeAg‐positive, have high viral loads and consequently MTCT is frequent 87, 90. On the other hand, in Africa where the prevalent genotypes are subgenotype A1, genotypes D and E 40, carriers of these genotypes seroconvert earlier than the average gestational age. Thus MTCT occurs infrequently in less than 10% of the cases 135, 136, 137, 138, 139, but also associated with HBeAg‐positivity and high viral loads 136.
The Influence of Genotypes and Subgenotypes on the Natural History of HBV Infection in Children
The few studies that have looked at the natural history of HBV infection in children have demonstrated geographical variations that can also be attributed to the different distributions of (sub) genotypes and their influence of HBeAg expression.
Taiwanese children of mothers, who were older than 40 years when they lost HBeAg, had delayed HBeAg seroconversion 140. The presence of maternal HBeAg but not HBsAg at birth, delayed the children's HBeAg seroconversion significantly and this delay was enhanced when maternal HBeAg persisted 140.
HBeAg expression also determines whether acute HBV infection develops into a chronic infection; thus, a necessary prerequisite is that the strains of HBV infecting an individual express HBeAg and thus have a wild‐type BCP/PC region 25, 141, 142. Wild‐type at position 1896 of the precore region is the strongest predictor of MTCT in mothers infected with genotype E 136. However, transmission of strains carrying G1896A can occur perinatally 143. Genotype C patients, compared to genotype B patients, have a delayed HBeAg seroconversion in the immune clearance phase of chronic HBV infection, which may contribute to a more progressive liver disease and more refractory response to antiviral therapy 43.
Prevention of HBV Infection and Eradication of HBV
Since the early 1980s, there has been a successful vaccine against HBV, which can prevent HBV infection by eliciting an immune response in 95% of vaccinees 144. The success of this vaccine was first demonstrated in Taiwan, which was one of the first countries to implement universal vaccination. HBsAg carrier rates in children decreased from 10% in 1984 to <1% in 2004, with a concomitant 68% decrease of acute liver failure in infants younger than 12 months, and a 75% reduction of HCC in children aged 6 to 14 years 42, 145. However, despite the effectiveness of this vaccine and the implementation of universal vaccination, following a recommendation by the World Health Organization (WHO) in the early 1990s, in over 184 countries, eradication of HBV is still not on the horizon.
To reduce MTCT the WHO recommends administration of both the vaccine and HBIg within 24 h of birth, which reduce transmission by 90% – 98% 1. Children of HBeAg‐positive mothers are at greater risk of developing chronic hepatitis even after immunization 146, and although administration of HBIg born to HBeAg‐negative mothers did not reduce the rate of chronic hepatitis, it can prevent infantile acute liver failure 146.
Active and passive immunoprophylaxis in children cannot prevent MTCT in 10% of children 147. The reasons for this outcome include:
Occult infection
Occult HBV infection is defined as the presence of HBV DNA in the liver (with and without HBV DNA detected in the serum) in HBsAg‐negative individuals 151
-
Reactivation of HBV infection, often as a result of immunosuppression.
-
Immigration into countries with low HBsAg prevalence, where universal vaccination has not been implemented. This has led to a change in the epidemiological profile, with the prevalence of HBsAg, HBeAg and the genotype of HBV in immigrant children reflecting that of their country of origin 62, 152.
The unprecedented human migrations, which the world is currently facing as a result of the refugee crisis, will result in the change of the geographical distribution of HBV genotypes and subgenotypes and a concomitant change in the natural history of HBV infection in different regions. Considering that the (sub) genotypes can affect the frequency and rate of HBeAg seroconversion, knowledge of the (sub) genotypes circulating in a region, as well as the mutations found in strains from infected children, can inform on better prevention, management and treatment options, which can be customized to a certain degree. In order be effectively manage HBV infection and to respond to the call of the inaugural World Hepatitis Summit of September 2015 153, for the global eradication of HBV by 2030, it is necessary that pediatricians understand the natural history of the infection, particularly the course of spontaneous HBeAg seroconversion. A priority for all pediatricians and maternity service providers should be the implementation of the prevention recommendations of the WHO for all neonates to receive the first dose of hepatitis B vaccine within 24 h of birth. This is essential in all regions of the world, especially regions such as Africa, where HBV is still hyperendemic and vaccine coverage is not optimal.
Conflict of Interest
The authors have no competing interest
Acknowledgements
The Cancer Association of South Africa (CANSA), the National Research Foundation (GUN#65530), the South African Medical Research Council and the University of the Witwatersrand are acknowledged for their financial support.
Kramvis, A. (2016) The clinical implications of hepatitis B virus genotypes and HBeAg in pediatrics. Rev. Med. Virol., 26: 285–303. doi: 10.1002/rmv.1885.
Part of this work was presented at Workshop on Paediatric Virology that was included in the official program of the 20th World Congress on Advances in Oncology and the 18th International Symposium on Molecular Medicine held in Athens, Greece in October 2015, and the abstract was published in Mammas, I.N., Greenough, A., Theodoridou, M., Kramvis, A., Christaki, I., Koutsaftiki, C. … Spandidos, D.A. (2016). Current views and advances on Paediatric Virology: An update for paediatric trainees (Review). Experimental and Therapeutic Medicine, 11, 6–14. http://dx.doi.org10.3892/etm.2015.2890
References
- 1. Hepatitis B Fact sheet no 204. http://www.who.int/mediacentre/factsheets/fs204/en/ [October 2015].
- 2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. DOI:10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age‐specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30: 2212–2219. DOI:10.1016/j.vaccine.2011.12.116 S0264‐410X(11)02077‐9 [pii]. [DOI] [PubMed] [Google Scholar]
- 4. Komatsu H, Inui A. Hepatitis B virus infection in children. Expert Review of Anti‐Infective Therapy 2015; 13: 427–450. DOI:10.1586/14787210.2015.1019867. [DOI] [PubMed] [Google Scholar]
- 5. Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature 1985; 317: 489–495. [DOI] [PubMed] [Google Scholar]
- 6. Summers J, Mason WS. Replication of the genome of a hepatitis B—like virus by reverse transcription of an RNA intermediate. Cell 1982; 29: 403–415. [DOI] [PubMed] [Google Scholar]
- 7. Beck J, Nassal M. Hepatitis B virus replication. World Journal of Gastroenterology 2007; 13: 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Junker‐Niepmann M, Bartenschlager R, Schaller H. A short cis‐acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO Journal 1990; 9: 3389–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramvis A, Kew MC. Structure and function of the encapsidation signal of hepadnaviridae. Journal of Viral Hepatitis 1998; 5: 357–367. [DOI] [PubMed] [Google Scholar]
- 10. Magnius LO, Espmark A. A new antigen complex co‐occurring with Australia antigen. Acta Pathologica et Microbiologica Scandinavica. Section B: Microbiology and Immunology 1972; 80: 335–337. [DOI] [PubMed] [Google Scholar]
- 11. Kramvis A, Kew M, Francois G. Hepatitis B virus genotypes. Vaccine 2005; 23: 2409–2423. DOI:10.1016/j.vaccine.2004.10.045 S0264‐410X(04)00849‐7. [DOI] [PubMed] [Google Scholar]
- 12. Yuh CH, Chang YL, Ting LP. Transcriptional regulation of precore and pregenomic RNAs of hepatitis B virus. Journal of Virology 1992; 66: 4073–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu X, Mertz JE. Promoters for synthesis of the pre‐C and pregenomic mRNAs of human hepatitis B virus are genetically distinct and differentially regulated. Journal of Virology 1996; 70: 8719–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kramvis A, Kew MC. The core promoter of hepatitis B virus. Journal of Viral Hepatitis 1999; 6: 415–427. DOI:10.1046/j.1365-2893.1999.00189.x jvh189 [pii]. [DOI] [PubMed] [Google Scholar]
- 15. Ou JH, Laub O, Rutter WJ. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proceedings of the National Academy of Sciences of the United States of America 1986; 83: 1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jean‐Jean O, Levrero M, Will H, Perricaudet M, Rossignol JM. Expression mechanism of the hepatitis B virus (HBV) C gene and biosynthesis of HBe antigen. Virology 1989; 170: 99–106. [DOI] [PubMed] [Google Scholar]
- 17. Revill P, Yuen L, Walsh R, Perrault M, Locarnini S, Kramvis A. Bioinformatic analysis of the hepadnavirus e‐antigen and its precursor identifies remarkable sequence conservation in all orthohepadnaviruses. Journal of Medical Virology 2010; 82: 104–115. DOI:10.1002/jmv.21645. [DOI] [PubMed] [Google Scholar]
- 18. Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 2003; 38: 1075–1086. DOI:10.1053/jhep.2003.50453 S027091390300836X [pii]. [DOI] [PubMed] [Google Scholar]
- 19. Hoofnagle JH, Dusheiko GM, Seeff LB, Jones EA, Waggoner JG, Bales ZB. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Annals of Internal Medicine 1981; 94: 744–748. [DOI] [PubMed] [Google Scholar]
- 20. Chen M, Sallberg M, Hughes J, et al. Immune tolerance split between hepatitis B virus precore and core proteins. Journal of Virology 2005; 79: 3016–3027. DOI:10.1128/JVI.79.5.3016-3027.2005 79/5/3016 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen MT, Billaud JN, Sallberg M, et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proceedings of the National Academy of Sciences of the United States of America 2004; 101: 14913–14918. DOI:10.1073/pnas.0406282101 0406282101 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang CY, Kuo TH, Ting LP. Human hepatitis B viral e antigen interacts with cellular interleukin‐1 receptor accessory protein and triggers interleukin‐1 response. Journal of Biological Chemistry 2006; 281: 34525–34536. DOI:10.1074/jbc.M510981200 M510981200 [pii]. [DOI] [PubMed] [Google Scholar]
- 23. Visvanathan K, Skinner NA, Thompson AJ, et al. Regulation of Toll‐like receptor‐2 expression in chronic hepatitis B by the precore protein. Hepatology 2007; 45: 102–110. DOI:10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 24. Chang MH, Lee CY, Chen DS, Hsu HC, Lai MY. Fulminant hepatitis in children in Taiwan: the important role of hepatitis B virus. Journal of Pediatrics 1987; 111: 34–39. [DOI] [PubMed] [Google Scholar]
- 25. Hadziyannis SJ. Natural history of chronic hepatitis B in Euro‐Mediterranean and African countries. Journal of Hepatology 2011; 55: 183–191. DOI:10.1016/j.jhep.2010.12.030 S0168‐8278(11)00023‐7 [pii]. [DOI] [PubMed] [Google Scholar]
- 26. Tang JR, Hsu HY, Lin HH, Ni YH, Chang MH. Hepatitis B surface antigenemia at birth: a long‐term follow‐up study. Journal of Pediatrics 1998; 133: 374–377. DOI:10.1016/S0022-3476(98)70272-0. [DOI] [PubMed] [Google Scholar]
- 27. Ni YH. Natural history of hepatitis B virus infection: pediatric perspective. Journal of Gastroenterology 2011; 46: 1–8. DOI:10.1007/s00535-010-0304-7. [DOI] [PubMed] [Google Scholar]
- 28. Choe HJ, Choe BH. What physicians should know about the management of chronic hepatitis B in children: East side story. World Journal of Gastroenterology 2014; 20: 3582–3589. DOI:10.3748/wjg.v20.i13.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu HY, Chang MH, Hsieh KH, et al. Cellular immune response to HBcAg in mother‐to‐infant transmission of hepatitis B virus. Hepatology 1992; 15: 770–776 DOI: 10.1002/hep.1840150505. [DOI] [PubMed] [Google Scholar]
- 30. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45: 507–539. DOI:10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 31. Gish RG, Given BD, Lai CL, et al. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Research 2015; 121: 47–58. DOI:10.1016/j.antiviral.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 32. Ni YH, Chang MH, Chen PJ, et al. Viremia profiles in children with chronic hepatitis B virus infection and spontaneous e antigen seroconversion. Gastroenterology 2007; 132: 2340–2345. DOI:10.1053/j.gastro.2007.03.111 S0016‐5085(07)00637‐3 [pii]. [DOI] [PubMed] [Google Scholar]
- 33. Chang MH, Hwang LY, Hsu HC, Lee CY, Beasley RP. Prospective study of asymptomatic HBsAg carrier children infected in the perinatal period: clinical and liver histologic studies. Hepatology 1988; 8: 374–377 S0270913988000576 [pii]. [DOI] [PubMed] [Google Scholar]
- 34. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 2006; 43: S173–181. DOI:10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 35. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009; 373: 582–592. DOI:10.1016/S0140-6736(09)60207-5 S0140‐6736(09)60207‐5 [pii]. [DOI] [PubMed] [Google Scholar]
- 36. Bortolotti F, Cadrobbi P, Crivellaro C, et al. Long‐term outcome of chronic type B hepatitis in patients who acquire hepatitis B virus infection in childhood. Gastroenterology 1990; 99: 805–810 S0016508590003213 [pii]. [DOI] [PubMed] [Google Scholar]
- 37. Marx G, Martin SR, Chicoine JF, Alvarez F. Long‐term follow‐up of chronic hepatitis B virus infection in children of different ethnic origins. Journal of Infectious Diseases 2002; 186: 295–301. DOI:10.1086/341508 JID011277 [pii]. [DOI] [PubMed] [Google Scholar]
- 38. Chang MH, Hsu HY, Hsu HC, Ni YH, Chen JS, Chen DS. The significance of spontaneous hepatitis B e antigen seroconversion in childhood: with special emphasis on the clearance of hepatitis B e antigen before 3 years of age. Hepatology 1995; 22: 1387–1392 S0270913995003855 [pii]. [PubMed] [Google Scholar]
- 39. Iorio R, Giannattasio A, Cirillo F, D' Alessandro L, Vegnente A. Long‐term outcome in children with chronic hepatitis B: a 24‐year observation period. Clinical Infectious Diseases 2007; 45: 943–949. DOI:10.1086/521864 CID51006 [pii]. [DOI] [PubMed] [Google Scholar]
- 40. Kramvis A, Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatology Research 2007; 37: S9–S19. DOI:10.1111/j.1872-034X.2007.00098.x HEP098 [pii]. [DOI] [PubMed] [Google Scholar]
- 41. Malamitsi‐Puchner A, Papacharitonos S, Sotos D, et al. Prevalence study of different hepatitis markers among pregnant Albanian refugees in Greece. European Journal of Epidemiology 1996; 12: 297–301. [DOI] [PubMed] [Google Scholar]
- 42. Ni YH, Huang LM, Chang MH, et al. Two decades of universal hepatitis B vaccination in Taiwan: impact and implication for future strategies. Gastroenterology 2007; 132: 1287–1293. DOI:10.1053/j.gastro.2007.02.055 S0016‐5085(07)00414‐3 [pii]. [DOI] [PubMed] [Google Scholar]
- 43. Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. Journal of Medical Virology 2004; 72: 363–369. DOI:10.1002/jmv.10534. [DOI] [PubMed] [Google Scholar]
- 44. Wen WH, Chang MH, Hsu HY, Ni YH, Chen HL. The development of hepatocellular carcinoma among prospectively followed children with chronic hepatitis B virus infection. Journal of Pediatrics 2004; 144: 397–399. DOI:10.1016/j.jpeds.2003.11.022 S0022‐3476(03)00855‐2 [pii]. [DOI] [PubMed] [Google Scholar]
- 45. Pollicino T, Raimondo G. Occult hepatitis B infection. Journal of Hepatology 2014; 61: 688–689. DOI:10.1016/j.jhep.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 46. Chang MH. Natural history and clinical management of chronic hepatitis B virus infection in children. Hepatology International 2008; 2: 28–36. DOI:10.1007/s12072-008-9050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lok AS, Lai CL. A longitudinal follow‐up of asymptomatic hepatitis B surface antigen‐positive Chinese children. Hepatology 1988; 8: 1130–1133 DOI: 10.1002/hep.1840150304. [DOI] [PubMed] [Google Scholar]
- 48. Hsu HY, Chang MH, Lee CY, Chen JS, Hsu HC, Chen DS. Spontaneous loss of HBsAg in children with chronic hepatitis B virus infection. Hepatology 1992; 15: 382–386 DOI: 10.1002/hep.1840150304. [DOI] [PubMed] [Google Scholar]
- 49. Boxall EH, Sira J, Standish RA, et al. Natural history of hepatitis B in perinatally infected carriers. Archives of Disease in Childhood—Fetal and Neonatal Edition 2004; 89: F456–460. DOI:10.1136/adc.2002.009837 89/5/F456 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bortolotti F, Jara P, Crivellaro C, et al. Outcome of chronic hepatitis B in Caucasian children during a 20‐year observation period. Journal of Hepatology 1998; 29: 184–190. DOI:10.1016/S0168-8278(98)80002-0. [DOI] [PubMed] [Google Scholar]
- 51. Chang MH, Chen PJ, Chen JY, et al. Hepatitis B virus integration in hepatitis B virus‐related hepatocellular carcinoma in childhood. Hepatology 1991; 13: 316–320 DOI: 10.1002/hep.1840130218. [PubMed] [Google Scholar]
- 52. Kramvis A, Kew MC. Relationship of genotypes of hepatitis B virus to mutations, disease progression and response to antiviral therapy. Journal of Viral Hepatitis 2005; 12: 456–464. DOI:10.1111/j.1365-2893.2005.00624.x JVH624 [pii]. [DOI] [PubMed] [Google Scholar]
- 53. Yu H, Yuan Q, Ge SX, et al. Molecular and phylogenetic analyses suggest an additional hepatitis B virus genotype “I”. PloS One 2010; 5: e9297 DOI:10.1371/journal.pone.0009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Norder H, Courouce AM, Coursaget P, et al. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 2004; 47: 289–309. DOI:10.1159/000080872 INT2004047006289 [pii]. [DOI] [PubMed] [Google Scholar]
- 55. Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology 2014; 57: 141–150. DOI:10.1159/000360947 000360947 [pii]. [DOI] [PubMed] [Google Scholar]
- 56. Tatematsu K, Tanaka Y, Kurbanov F, et al. A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. Journal of Virology 2009; 83: 10538–10547. DOI:10.1128/JVI.00462-09 JVI.00462‐09 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Locarnini S, Littlejohn M, Aziz MN, Yuen L. Possible origins and evolution of the hepatitis B virus (HBV). Seminars in Cancer Biology 2013; 23: 561–575. DOI:10.1016/j.semcancer.2013.08.006 S1044‐579X(13)00082‐5 [pii]. [DOI] [PubMed] [Google Scholar]
- 58. Kramvis A, Arakawa K, Yu MC, Nogueira R, Stram DO, Kew MC. Relationship of serological subtype, basic core promoter and precore mutations to genotypes/subgenotypes of hepatitis B virus. Journal of Medical Virology 2008; 80: 27–46. DOI:10.1002/jmv.21049. [DOI] [PubMed] [Google Scholar]
- 59. Kao JH. Hepatitis B viral genotypes: clinical relevance and molecular characteristics. Journal of Gastroenterology and Hepatology 2002; 17: 643–650 DOI: 10.1046/j.1440-1746.2002.02737.x. [DOI] [PubMed] [Google Scholar]
- 60. McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatology International 2009; 3: 334–342. DOI:10.1007/s12072-008-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yousif M, Kramvis A. Genotype D of hepatitis B virus and its subgenotypes: an update. Hepatology Research 2013; 43: 355–364. DOI:10.1111/j.1872-034X.2012.01090.x. [DOI] [PubMed] [Google Scholar]
- 62. Kramvis A, Paraskevis D. Subgenotype A1 of HBV—tracing human migrations in and out of Africa. Antiviral Therapy 2013; 18: 513–521. DOI:10.3851/IMP2657. [DOI] [PubMed] [Google Scholar]
- 63. Owiredu WK, Kramvis A, Kew MC. Hepatitis B virus DNA in serum of healthy black African adults positive for hepatitis B surface antibody alone: possible association with recombination between genotypes A and D. Journal of Medical Virology 2001; 64: 441–454. [DOI] [PubMed] [Google Scholar]
- 64. Bowyer SM, Sim JG. Relationships within and between genotypes of hepatitis B virus at points across the genome: footprints of recombination in certain isolates. The Journal of General Virology 2000; 81: 379–392. [DOI] [PubMed] [Google Scholar]
- 65. Sugauchi F, Orito E, Ichida T, et al. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. Journal of Virology 2002; 76: 5985–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cui C, Shi J, Hui L, et al. The dominant hepatitis B virus genotype identified in Tibet is a C/D hybrid. The Journal of General Virology 2002; 83: 2773–2777. [DOI] [PubMed] [Google Scholar]
- 67. Simmonds P, Midgley S. Recombination in the genesis and evolution of hepatitis B virus genotypes. Journal of Virology 2005; 79: 15467–15476. DOI:10.1128/JVI.79.24.15467-15476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shi W, Carr MJ, Dunford L, Zhu C, Hall WW, Higgins DG. Identification of novel inter‐genotypic recombinants of human hepatitis B viruses by large‐scale phylogenetic analysis. Virology 2012; 427: 51–59. DOI:10.1016/j.virol.2012.01.030 S0042‐6822(12)00077‐3 [pii]. [DOI] [PubMed] [Google Scholar]
- 69. Osiowy C, Giles E, Tanaka Y, Mizokami M, Minuk GY. Molecular evolution of hepatitis B virus over 25 years. Journal of Virology 2006; 80: 10307–10314. DOI:10.1128/JVI.00996-06 80/21/10307 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu X, Margolis HS, Purcell RH, Ebert J, Robertson BH. Identification of hepatitis B virus indigenous to chimpanzees. Proceedings of the National Academy of Sciences of the United States of America 2000; 97: 1661–1664 DOI: 10.1073/pnas.97.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Norder H, Courouce AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology 1994; 198: 489–503. DOI:10.1006/viro.1994.1060 S0042‐6822(84)71060‐9 [pii]. [DOI] [PubMed] [Google Scholar]
- 72. Bartholomeusz A, Schaefer S. Hepatitis B virus genotypes: comparison of genotyping methods. Reviews in Medical Virology 2004; 14: 3–16. [DOI] [PubMed] [Google Scholar]
- 73. Guirgis BS, Abbas RO, Azzazy HM. Hepatitis B virus genotyping: current methods and clinical implications. International Journal of Infectious Diseases 2010; 14: e941–953. DOI:10.1016/j.ijid.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 74. Bell TG, Kramvis A. The study of hepatitis B virus using bioinformatics In Bioinformatics, Abdurakhmanov IY. (ed). InTech—Open Access Publisher, 2016. DOI: 10.5772/63076 Available from:http://www.intechopen.com/ [Google Scholar]
- 75. Chu CM, Yeh CT, Sheen IS, Liaw YF. Subcellular localization of hepatitis B core antigen in relation to hepatocyte regeneration in chronic hepatitis B. Gastroenterology 1995; 109: 1926–1932. DOI:10.1016/0016-5085(95)90760-2. [DOI] [PubMed] [Google Scholar]
- 76. Chu CM, Yeh CT, Lee CS, Sheen IS, Liaw YF. Precore stop mutant in HBeAg‐positive patients with chronic hepatitis B: clinical characteristics and correlation with the course of HBeAg‐to‐anti‐HBe seroconversion. Journal of Clinical Microbiology 2002; 40: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yuen MF, Sablon E, Yuan HJ, et al. Relationship between the development of precore and core promoter mutations and hepatitis B e antigen seroconversion in patients with chronic hepatitis B virus. Journal of Infectious Diseases 2002; 186: 1335–1338. DOI:10.1086/344327 JID020471 [pii]. [DOI] [PubMed] [Google Scholar]
- 78. Buckwold VE, Xu Z, Chen M, Yen TS, Ou JH. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. Journal of Virology 1996; 70: 5845–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ahn SH, Kramvis A, Kawai S, et al. Sequence variation upstream of precore translation initiation codon reduces hepatitis B virus e antigen production. Gastroenterology 2003; 125: 1370–1378 DOI: 10.1016/j.gastro.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 80. Carman WF, Jacyna MR, Hadziyannis S, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet 1989; 2: 588–591. DOI:10.1016/S0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- 81. Chen CY, Crowther C, Kew MC, Kramvis A. A valine to phenylalanine mutation in the precore region of hepatitis B virus causes intracellular retention and impaired secretion of HBe‐antigen. Hepatology Research 2008; 38: 580–592. DOI:10.1111/j.1872-034X.2007.00315.x HEP315 [pii]. [DOI] [PubMed] [Google Scholar]
- 82. Bell TG, Kramvis A. Mutation Reporter Tool: an online tool to interrogate loci of interest, with its utility demonstrated using hepatitis B virus. Virology Journal 2013; 10: 62 DOI:10.1186/1743-422X-10-62 1743‐422X‐10‐62 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li JS, Tong SP, Wen YM, Vitvitski L, Zhang Q, Trepo C. Hepatitis B virus genotype A rarely circulates as an HBe‐minus mutant: possible contribution of a single nucleotide in the precore region. Journal of Virology 1993; 67: 5402–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lok AS, Akarca U, Greene S. Mutations in the pre‐core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre‐genome encapsidation signal. Proceedings of the National Academy of Sciences of the United States of America 1994; 91: 4077–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kramvis A, Kew MC. Molecular characterization of subgenotype A1 (subgroup Aa) of hepatitis B virus. Hepatology Research 2007; 37: S27–32. DOI:10.1111/j.1872-034X.2007.00100.x HEP100 [pii]. [DOI] [PubMed] [Google Scholar]
- 86. Tanaka Y, Hasegawa I, Kato T, et al. A case–control study for differences among hepatitis B virus infections of genotypes A (subtypes Aa and Ae) and D. Hepatology 2004; 40: 747–755. DOI:10.1002/hep.20365. [DOI] [PubMed] [Google Scholar]
- 87. Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology 2002; 122: 1756–1762 DOI: 10.1053/gast.2002.33588. [DOI] [PubMed] [Google Scholar]
- 88. Livingston SE, Simonetti JP, Bulkow LR, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology 2007; 133: 1452–1457. DOI:10.1053/j.gastro.2007.08.010 S0016‐5085(07)01456‐4 [pii]. [DOI] [PubMed] [Google Scholar]
- 89. Candotti D, Opare‐Sem O, Rezvan H, Sarkodie F, Allain JP. Molecular and serological characterization of hepatitis B virus in deferred Ghanaian blood donors with and without elevated alanine aminotransferase. Journal of Viral Hepatitis 2006; 13: 715–724. DOI:10.1111/j.1365-2893.2006.00741.x JVH741 [pii]. [DOI] [PubMed] [Google Scholar]
- 90. Kao JH, Chen PJ, Lai MY, Chen DS. Clinical and virological aspects of blood donors infected with hepatitis B virus genotypes B and C. Journal of Clinical Microbiology 2002; 40: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ott JJ, Stevens GA, Wiersma ST. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC Infectious Diseases 2012; 12: 131 DOI:10.1186/1471-2334-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wong VC, Lee AK, Ip HM. Transmission of hepatitis B antigens from symptom free carrier mothers to the fetus and the infant. British Journal of Obstetrics and Gynaecology 1980; 87: 958–965. [DOI] [PubMed] [Google Scholar]
- 93. Pournik O, Alavian SM, Ghalichi L, Hajibeigi B, Razavi AR, Eslami S. Lower intrafamilial transmission rate of hepatitis B in patients with hepatitis d coinfection: a data‐mining approach. Hepatitis Monthly 2013; 13: e7652 DOI:10.5812/hepatmon.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Madejon A, Romero M, Hernandez A, et al. Hepatitis B and D viruses replication interference: influence of hepatitis B genotype. World Journal of Gastroenterology 2016; 22: 3165–3174. DOI:10.3748/wjg.v22.i11.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ohto H, Lin HH, Kawana T, Etoh T, Tohyama H. Intrauterine transmission of hepatitis B virus is closely related to placental leakage. Journal of Medical Virology 1987; 21: 1–6. [DOI] [PubMed] [Google Scholar]
- 96. Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case–control study. Journal of Medical Virology 2002; 67: 20–26. [DOI] [PubMed] [Google Scholar]
- 97. Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra‐uterine transmission mechanism. World Journal of Gastroenterology 2007; 13: 3625–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li XM, Shi MF, Yang YB, et al. Effect of hepatitis B immunoglobulin on interruption of HBV intrauterine infection. World Journal of Gastroenterology 2004; 10: 3215–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cacciola I, Cerenzia G, Pollicino T, et al. Genomic heterogeneity of hepatitis B virus (HBV) and outcome of perinatal HBV infection. Journal of Hepatology 2002; 36: 426–432 DOI: 10.1016/S0168-8278(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 100. Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver International 2009; 29(Suppl 1): 133–139. DOI:10.1111/j.1478-3231.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 101. Beasley RP, Hwang LY. Postnatal infectivity of hepatitis B surface antigen‐carrier mothers. Journal of Infectious Diseases 1983; 147: 185–190. [DOI] [PubMed] [Google Scholar]
- 102. Lee AK, Ip HM, Wong VC. Mechanisms of maternal–fetal transmission of hepatitis B virus. Journal of Infectious Diseases 1978; 138: 668–671. [DOI] [PubMed] [Google Scholar]
- 103. Towers CV, Asrat T, Rumney P. The presence of hepatitis B surface antigen and deoxyribonucleic acid in amniotic fluid and cord blood. American Journal of Obstetrics and Gynecology 2001; 184: 1514–1518 discussion 1518‐1520. [DOI] [PubMed] [Google Scholar]
- 104. Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti‐e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. New England Journal of Medicine 1976; 294: 746–749. DOI:10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- 105. Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. American Journal of Epidemiology 1977; 105: 94–98. [DOI] [PubMed] [Google Scholar]
- 106. Beasley RP, Hwang LY, Lin CC, et al. Hepatitis B immune globulin (HBIG) efficacy in the interruption of perinatal transmission of hepatitis B virus carrier state. Initial report of a randomised double‐blind placebo‐controlled trial. Lancet 1981; 2: 388–393. DOI:10.1016/S0140-6736(81)90832-1. [DOI] [PubMed] [Google Scholar]
- 107. Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. New England Journal of Medicine 1975; 292: 771–774. [DOI] [PubMed] [Google Scholar]
- 108. Stevens CE, Neurath RA, Beasley RP, Szmuness W. HBeAg and anti‐HBe detection by radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. Journal of Medical Virology 1979; 3: 237–241. [DOI] [PubMed] [Google Scholar]
- 109. Tong MJ, Thursby MW, Lin JH, Weissman JY, McPeak CM. Studies on the maternal–infant transmission of the hepatitis B virus and HBV infection within families. Progress in Medical Virology 1981; 27: 137–147. [PubMed] [Google Scholar]
- 110. Pongpipat D, Suvatte V, Assateerawatts A. Perinatal transmission of hepatitis B virus in Thailand. Asian Pacific Journal of Allergy and Immunology 1985; 3: 191–193. [PubMed] [Google Scholar]
- 111. Edmunds WJ, Medley GF, Nokes DJ, O'Callaghan CJ, Whittle HC, Hall AJ. Epidemiological patterns of hepatitis B virus (HBV) in highly endemic areas. Epidemiology and Infection 1996; 117: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Karayiannis P, Novick DM, Lok AS, Fowler MJ, Monjardino J, Thomas HC. Hepatitis B virus DNA in saliva, urine, and seminal fluid of carriers of hepatitis B e antigen. British Medical Journal (Clinical Research Ed.) 1985; 290: 1853–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Martinson FE, Weigle KA, Royce RA, Weber DJ, Suchindran CM, Lemon SM. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. American Journal of Epidemiology 1998; 147: 478–487. [DOI] [PubMed] [Google Scholar]
- 114. Jombo GT, Egah DZ, Banwat EB. Hepatitis B virus infection in a rural settlement of northern Nigeria. Nigerian Journal of Medicine 2005; 14: 425–428. [PubMed] [Google Scholar]
- 115. Otegbayo JA, Fasola FA, Abja A. Prevalence of hepatitis B surface and e antigens, risk factors for viral acquisition and serum transaminase among blood donors in Ibadan, Nigeria. Tropical Gastroenterology 2003; 24: 196–197. [PubMed] [Google Scholar]
- 116. Ghendon Y. Perinatal transmission of hepatitis B virus in high‐incidence countries. Journal of Virological Methods 1987; 17: 69–79. DOI:10.1016/0166-0934(87)90070-X. [DOI] [PubMed] [Google Scholar]
- 117. Hsu SC, Chang MH, Ni YH, Hsu HY, Lee CY. Horizontal transmission of hepatitis B virus in children. Journal of Pediatric Gastroenterology and Nutrition 1993; 16: 66–69. [DOI] [PubMed] [Google Scholar]
- 118. Dumpis U, Holmes EC, Mendy M, et al. Transmission of hepatitis B virus infection in Gambian families revealed by phylogenetic analysis. Journal of Hepatology 2001; 35: 99–104 S0168827801000642 [pii]. [DOI] [PubMed] [Google Scholar]
- 119. Whittle HC, Bradley AK, McLauchlan K, et al. Hepatitis B virus infection in two Gambian villages. Lancet 1983; 1: 1203–1206. [DOI] [PubMed] [Google Scholar]
- 120. Burnett RJ, Francois G, Kew MC, et al. Hepatitis B virus and human immunodeficiency virus co‐infection in sub‐Saharan Africa: a call for further investigation. Liver International 2005; 25: 201–213. DOI:10.1111/j.1478-3231.2005.01054.x LIV1054 [pii]. [DOI] [PubMed] [Google Scholar]
- 121. Jacobs B, Mayaud P, Changalucha J, et al. Sexual transmission of hepatitis B in Mwanza, Tanzania. Sexually Transmitted Diseases 1997; 24: 121–126. [DOI] [PubMed] [Google Scholar]
- 122. Rosendahl C, Kochen MM, Kretschmer R, Wegscheider K, Kaiser D. Avoidance of perinatal transmission of hepatitis B virus: is passive immunisation always necessary? Lancet 1983; 1: 1127–1129. DOI:10.1016/S0140-6736(83)92866-0. [DOI] [PubMed] [Google Scholar]
- 123. Chan SH, Tan KL, Goh KT, et al. Maternal–child hepatitis B virus transmission in Singapore. International Journal of Epidemiology 1985; 14: 173–177. [DOI] [PubMed] [Google Scholar]
- 124. Tan DS, Zaini Rahman M, Fang R, Collett D, Ooi BG. Hepatitis B markers in non‐icteric medical patients in Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 1986; 17: 214–218. [PubMed] [Google Scholar]
- 125. Zhang SL, Han XB, Yue YF. Relationship between HBV viremia level of pregnant women and intrauterine infection: neated PCR for detection of HBV DNA. World Journal of Gastroenterology 1998; 4: 61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Derso A, Boxall EH, Tarlow MJ, Flewett TH. Transmission of HBsAg from mother to infant in four ethnic groups. British Medical Journal 1978; 1: 949–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zacharakis G, Koskinas J, Kotsiou S, et al. Natural history of chronic hepatitis B virus infection in children of different ethnic origins: a cohort study with up to 12 years' follow‐up in northern Greece. Journal of Pediatric Gastroenterology and Nutrition 2007; 44: 84–91. DOI:10.1097/01.mpg.0000243438.47334.07 14600005176‐200701000‐00018 [pii]. [DOI] [PubMed] [Google Scholar]
- 128. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2: 1099–1102. DOI: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 129. Beasley RP, Hwang LY, Stevens CE, et al. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double‐blind, placebo‐controlled trial. Hepatology 1983; 3: 135–141 DOI: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 130. Marinier E, Barrois V, Larouze B, et al. Lack of perinatal transmission of hepatitis B virus infection in Senegal, West Africa. Journal of Pediatrics 1985; 106: 843–849. [DOI] [PubMed] [Google Scholar]
- 131. Basalamah AH, Serebour F, Kazim E. Materno‐foetal transmission of hepatitis B in Saudi Arabia. Journal of Infection 1984; 8: 200–204. [DOI] [PubMed] [Google Scholar]
- 132. Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. American Journal of Medicine 2004; 116: 829–834. DOI:10.1016/j.amjmed.2003.12.040 S0002934304001469 [pii]. [DOI] [PubMed] [Google Scholar]
- 133. Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen‐positive patients with normal aminotransferase levels at baseline. Journal of Hepatology 2005; 43: 411–417. DOI:10.1016/j.jhep.2005.03.018 S0168‐8278(05)00308‐9 [pii]. [DOI] [PubMed] [Google Scholar]
- 134. Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. Journal of Viral Hepatitis 2007; 14: 147–152. DOI:10.1111/j.1365-2893.2006.00810.x JVH810 [pii]. [DOI] [PubMed] [Google Scholar]
- 135. Botha JF, Ritchie MJ, Dusheiko GM, Mouton HW, Kew MC. Hepatitis B virus carrier state in black children in Ovamboland: role of perinatal and horizontal infection. Lancet 1984; 1: 1210–1212. [DOI] [PubMed] [Google Scholar]
- 136. Candotti D, Danso K, Allain JP. Maternofetal transmission of hepatitis B virus genotype E in Ghana, west Africa. The Journal of General Virology 2007; 88: 2686–2695. DOI:10.1099/vir.0.83102-0 88/10/2686 [pii]. [DOI] [PubMed] [Google Scholar]
- 137. Roingeard P, Diouf A, Sankale JL, et al. Perinatal transmission of hepatitis B virus in Senegal, west Africa. Viral Immunology 1993; 6: 65–73. [DOI] [PubMed] [Google Scholar]
- 138. Menendez C, Sanchez‐Tapias JM, Kahigwa E, et al. Prevalence and mother‐to‐infant transmission of hepatitis viruses B, C, and E in Southern Tanzania. Journal of Medical Virology 1999; 58: 215–220. DOI: 10.1002/(SICI)1096-9071(199907)58:3<215::AID-JMV5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 139. Acquaye JK, Mingle JA. Hepatitis B viral markers in Ghanaian pregnant women. West African Journal of Medicine 1994; 13: 134–137. [PubMed] [Google Scholar]
- 140. Tseng YR, Wu JF, Ni YH, et al. Long‐term effect of maternal HBeAg on delayed HBeAg seroconversion in offspring with chronic hepatitis B infection. Liver International 2011; 31: 1373–1380. DOI:10.1111/j.1478-3231.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 141. Cote PJ, Korba BE, Miller RH, et al. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 2000; 31: 190–200. DOI:10.1002/hep.510310128 S0270913900290794 [pii]. [DOI] [PubMed] [Google Scholar]
- 142. Hadziyannis SJ, Vassilopoulos D. Immunopathogenesis of hepatitis B e antigen negative chronic hepatitis B infection. Antiviral Research 2001; 52: 91–98. DOI:10.1016/S0166-3542(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 143. Hawkins AE, Gilson RJ, Beath SV, et al. Novel application of a point mutation assay: evidence for transmission of hepatitis B viruses with precore mutations and their detection in infants with fulminant hepatitis B. Journal of Medical Virology 1994; 44: 13–21. [DOI] [PubMed] [Google Scholar]
- 144. Hessel L, West DJ. Antibody responses to recombinant hepatitis B vaccines. Vaccine 2002; 20: 2164–2165 DOI: 10.1016/S0264-410X(02)00117-2. [DOI] [PubMed] [Google Scholar]
- 145. Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiologic Reviews 2006; 28: 126–135. DOI:10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- 146. Chen HL, Lin LH, Hu FC, et al. Effects of maternal screening and universal immunization to prevent mother‐to‐infant transmission of HBV. Gastroenterology 2012; 142: 773–781 e772. DOI:10.1053/j.gastro.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 147. Chen HL, Lin LH, Hu FC, et al Effects of maternal screening and universal immunization to prevent mother‐to‐infant transmission of HBV. Gastroenterology; 142: 773–781 e772. DOI: 10.1053/j.gastro.2011.12.035 S0016‐5085(11)01750‐1 [pii] [DOI] [PubMed] [Google Scholar]
- 148. Huang ML, Liao WL, Ho MS. HBV serological markers of vaccinated children in remote areas of Taiwan: emphasis on factors contributing to vaccine failure. Vaccine 2007; 25: 6326–6333. DOI:10.1016/j.vaccine.2007.06.022 S0264‐410X(07)00699‐8 [pii]. [DOI] [PubMed] [Google Scholar]
- 149. Liu CJ, Lo SC, Kao JH, et al. Transmission of occult hepatitis B virus by transfusion to adult and pediatric recipients in Taiwan. Journal of Hepatology 2006; 44: 39–46. DOI:10.1016/j.jhep.2005.06.016 S0168‐8278(05)00449‐6 [pii]. [DOI] [PubMed] [Google Scholar]
- 150. Lu CY, Chiang BL, Chi WK, et al. Waning immunity to plasma‐derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology 2004; 40: 1415–1420. DOI:10.1002/hep.20490. [DOI] [PubMed] [Google Scholar]
- 151. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. Journal of Hepatology 2008; 49: 652–657. DOI:10.1016/j.jhep.2008.07.014 S0168‐8278(08)00479‐0 [pii]. [DOI] [PubMed] [Google Scholar]
- 152. Gjorup IE, Skinhoj P, Bottiger B, Plesner AM. Changing epidemiology of HBV infection in Danish children. Journal of Infection 2003; 47: 231–235 S0163445303000707 [pii]. [DOI] [PubMed] [Google Scholar]
- 153. World Hepatitis Summit 2015. Glasgow, Scotland. 2–4 September. http://www.worldhepatitissummit.com/ [22 January 2016].


