Abstract
Aims
To compare the efficacy and safety of liraglutide versus sitagliptin as add‐on to metformin after 26 weeks of treatment in Chinese patients with type 2 diabetes mellitus (T2DM).
Methods
This 26‐week open‐label, active comparator trial (NCT02008682) randomized patients (aged 18–80 years) with T2DM inadequately controlled with metformin [glycated haemoglobin (HbA1c) 7.0–10.0% (53–86 mmol/mol)] 1 : 1 to once‐daily subcutaneously administered liraglutide 1.8 mg (n = 184) or once‐daily oral sitagliptin 100 mg (n = 184), both as add‐on to metformin. The primary endpoint was change in HbA1c from baseline to week 26.
Results
Liraglutide was superior to sitagliptin in reducing HbA1c from baseline [8.1% (65 mmol/mol)] to 26 weeks, as evidenced by estimated mean HbA1c change of −1.65% (−18.07 mmol/mol) versus −0.98% (−10.72 mmol/mol), respectively [estimated treatment difference for liraglutide vs sitagliptin of −0.67% (95% CI −0.86, −0.48) or −7.35 mmol/mol (95% CI −9.43; −5.26); p < 0.0001]. More patients receiving liraglutide (76.5%) than sitagliptin (52.6%) achieved the HbA1c target of <7.0% (53 mmol/mol) at week 26 [odds ratio 3.65 (95% CI 2.18, 6.12); p < 0.0001]. Reductions in fasting plasma glucose, 7‐point self‐measured plasma glucose and body weight were greater with liraglutide than with sitagliptin (p < 0.0001 for all). More patients experienced nausea (14.8% vs 0.5%), diarrhoea (8.2% vs 2.2%) and decreased appetite (10.9% vs 0.5%) with liraglutide than sitagliptin. Two hypoglycaemic episodes were confirmed for liraglutide and one for sitagliptin; none were severe or nocturnal.
Conclusions
Liraglutide provided better glycaemic control and greater body weight reduction than sitagliptin when administered as add‐on to metformin. More patients had nausea, diarrhoea and decreased appetite with liraglutide versus sitagliptin.
Keywords: Chinese, liraglutide, sitagliptin, type 2 diabetes
Introduction
Diabetes represents a large healthcare burden in China, with the prevalence of type 2 diabetes (T2DM) increasing from 9.7 in 2008 to 11.6% (100 million people) in 2010 1, 2. The focus of T2DM management is to optimize glycaemic control to reduce microvascular complications 3 and potentially macrovascular outcomes 4. Among Chinese adults with diabetes receiving treatment, only 39.7% had adequate glycaemic control 2, indicating sub‐optimal antihyperglycaemic treatment. The choice of glucose‐lowering regimen should be individually tailored and take into account patient characteristics such as age and comorbidities 3. Approximately 50% of Chinese patients with T2DM are classified as overweight [body mass index (BMI) 24.0–27.9 kg/m2) or obese (BMI ≥ 28.0 kg/m2) 5, 6. It is well recognized that overweight and obesity are significantly associated with an increased risk of diabetes in Chinese adults 2. Further, weight loss is associated with improvements in clinical symptoms and cardiovascular disease risk factors in individuals with T2DM 7, 8.
Liraglutide, a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA), and sitagliptin, a dipeptidyl peptidase‐4 (DPP‐4) inhibitor, both incretin‐based therapies, are recommended in global T2DM management guidelines as second‐line treatment when metformin monotherapy is insufficient to maintain glycaemic targets 3. Sitagliptin and liraglutide have different modes of action and are, therefore, expected to have different efficacy and safety profiles. DPP‐4 inhibitors prevent the proteolytic breakdown of endogenous glucagon‐like peptide‐1 (GLP‐1), whereas GLP‐1RAs mimic the effects of endogenous GLP‐1 9. In a head‐to‐head clinical trial conducted in Western countries, liraglutide 1.2 and 1.8 mg were associated with significantly better glycaemic control and greater body weight reduction than sitagliptin 100 mg, both as an add‐on to metformin. Compared with sitagliptin, the estimated mean treatment difference (ETD) at week 26 for glycated haemoglobin (HbA1c)‐lowering and change in body weight for liraglutide 1.8 mg was −0.60% [95% confidence interval (CI) −0.77, −0.43], or −7 mmol/mol (95% CI 8, −5; p < 0.0001), and −2.42 kg (95% CI −3.14, −1.70; p < 0.0001), and for liraglutide the values were 1.2 mg −0.34% (95% CI −0.51, −0.16), or −4 mmol/mol (95% CI −6, −2; p < 0.0001) and −1.90 kg (95% CI −2.61, −1.18; p < 0.0001), respectively 10. The superior effect of liraglutide was sustained over 1 year 11. Post hoc analysis of the 26‐week trial, comparing liraglutide 1.2 and 1.8 mg, showed superiority regarding change in HbA1c and statistically significant improvement in the proportion of patients reaching HbA1c targets of <7.0 and ≤6.5% (53 and 48 mmol/mol) for liraglutide 1.8 mg versus 1.2 mg 10. Although the overall efficacy and safety/tolerability of liraglutide 12 and sitagliptin 13 have been established in Chinese patients with T2DM, there is a lack of data directly comparing the efficacy and safety of these two agents in this population.
We report the results of the LIRA‐DPP‐4 CHINA™ trial, which assessed the efficacy and safety of subcutaneously administered liraglutide 1.8 mg versus orally administered sitagliptin 100 mg, as add‐on to metformin, in Chinese patients with T2DM.
Materials and Methods
Participants
The trial was conducted at 25 sites in China between December 2013 and November 2014. Eligible participants (aged 18–80 years) had T2DM with HbA1c 7.0–10.0% (53–86 mmol/mol) and were treated with metformin monotherapy at a stable dose of ≥1500 mg/day or maximum‐tolerated dose of ≥1000 mg/day for 60 days before screening, and had a BMI ≤ 45.0 kg/m2. Key exclusion criteria included treatment with any antihyperglycaemic agent other than metformin within 60 days before screening, history of pancreatitis, screening calcitonin value ≥50 ng/l, history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, cancer diagnosis in the previous 5 years and impaired renal or hepatic function.
This trial (NCT 02008682) complied with the Declaration of Helsinki and Good Clinical Practice guidelines 14, 15. Independent Ethics Committees approved the trial conduct. All patients gave written consent prior to trial‐related activities.
Trial Design
This 26‐week, open‐label, active‐comparator, two‐armed, parallel‐group, multicentre trial randomized eligible patients 1 : 1 to injectable liraglutide 1.8 mg once daily (Novo Nordisk) or oral sitagliptin 100 mg once daily (Merck), both as add‐on to metformin at stable pre‐trial dose. Randomization was performed using an interactive voice/web response system, with stratification by baseline HbA1c levels of 7.0–8.0% (53–64 mmol/mol) and 8.1–10.0% (65–86 mmol/mol).
The starting dose of subcutaneous liraglutide was 0.6 mg/day, with subsequent weekly escalations of 0.6 mg, according to the approved dose escalation, until the maintenance dose of 1.8 mg/day was reached 16. In the maintenance period, the liraglutide dose could be reduced to 1.2 mg if 1.8 mg was not tolerated, and thereafter increased to 1.8 mg or remain at 1.2 mg at the investigator's discretion.
Liraglutide (once daily) injections and fixed‐dose oral sitagliptin (once daily) could be administered at any time of day, irrespective of meals, but administration time was to remain consistent throughout the trial.
Metformin dose or dosing frequency was not changed during the treatment period.
After randomization, patients unable to tolerate the relevant minimum dose level (liraglutide: 1.2 mg; sitagliptin: 100 mg; metformin: unchanged dose from randomization) were discontinued from the trial product.
Endpoints
The primary endpoint was change in HbA1c from baseline to week 26. Supportive prespecified secondary endpoints included: patients achieving HbA1c <7.0% (<53 mmol/mol) and ≤6.5% (≤48 mmol/mol), patients achieving composite endpoints [HbA1c <7.0% without weight gain, HbA1c <7.0% without confirmed hypoglycaemic episodes, HbA1c <7.0% without weight gain and without confirmed hypoglycaemic episodes, HbA1c <7.0% without weight gain and systolic blood pressure (SBP) <140 mmHg], as well as fasting plasma glucose (FPG), 7‐point self‐measured plasma glucose (SMPG) profile, fasting lipid profiles [total cholesterol, HDL cholesterol, LDL cholesterol, very‐low‐density lipoprotein (VLDL) cholesterol, triglycerides and free fatty acids], body measurements (body weight, BMI, waist circumference and waist‐to‐hip ratio), blood pressure [SBP and diastolic blood pressure (DBP)] and patient‐reported outcomes, assessed using the Diabetes Treatment Satisfaction Questionnaire (DTSQ). Safety endpoints included: confirmed hypoglycaemic episodes, adverse events (AEs), haematology and biochemistry variables (including lipase and amylase), calcitonin and resting pulse. Confirmed hypoglycaemia was defined as severe episodes (requiring third‐party assistance) or biochemically confirmed by a plasma glucose value <3.1 mmol/l (56 mg/dl), with/without symptoms. Nocturnal hypoglycaemia was defined as confirmed episodes occurring between 00:01 and 05:59 am.
Statistical Analyses
Sample size was determined using the assumption of a one‐sided t‐test of size 2.5% and a zero mean treatment difference. Based on previous experience 9, 11, the standard deviation of 1.1% for HbA1c was estimated, leading to the calculation that at least 137 completing patients in each treatment group were required to achieve a power of 85% to show non‐inferiority of liraglutide versus sitagliptin. Based on an assumed drop‐out rate of 25%, the total number of patients planned for randomization was 366, with 183 in each treatment group.
Efficacy endpoints were based on the full analysis set, which included all randomized patients who were exposed to trial products and had any post‐randomization data. Safety endpoints were based on the safety analysis set, which included all patients exposed to trial products. The primary endpoint was analysed using a mixed model for repeated measurements (MMRM) with treatment and HbA1c strata as factors and baseline HbA1c as covariates. Non‐inferiority would be confirmed if the upper bound of the two‐sided 95% CI for the mean HbA1c treatment difference was ≤0.4% (4 mmol/mol) 17 and superiority would be confirmed if the upper limit of the 95% CI was below 0. For the secondary endpoints, continuous variables were analysed using a method similar to that used for the primary endpoint, and the dichotomous variables were analysed using a logistic regression model, with treatment and HbA1c strata as factors and baseline HbA1c as a covariate. Post hoc analyses of weight loss (proportion of patients losing ≥5% of baseline body weight) and composite endpoints [proportion achieving HbA1c <7.0% (<53 mmol/mol) and weight loss ≥5% without confirmed hypoglycaemia], deemed important in the Chinese guidelines on T2DM management 18, were conducted using a logistic regression model. Additionally, post hoc subgroup analyses were performed within treatment groups according to baseline BMI (≥28 and <28 kg/m2) and baseline HbA1c [≤8% (≤64 mmol/mol) and >8%]. Responses by HbA1c were analysed using a MMRM, with treatment, HbA1c subgroup and treatment by HbA1c subgroup as fixed factors and baseline as a covariate, all nested within visit. Responses by BMI group had treatment, stratification groups, BMI subgroup and treatment by BMI subgroup interaction as fixed factors. Unless otherwise specified, safety endpoints were analysed using descriptive statistics only. Statistical analyses were performed using sas software (version 9.3; SAS Institute Inc., Cary, NC, USA).
Results
Efficacy
Of 498 patients screened, 368 were randomly assigned to the treatments, with 183 patients in the liraglutide group and 184 patients in the sitagliptin group exposed to trial product. One patient in the liraglutide group withdrew before exposure and was excluded from the full analysis set (Figure 1). Baseline characteristics were well balanced between the two groups (Table 1). For the liraglutide group, 93.6% of the patients who completed the trial without discontinuation of trial product maintained the 1.8 mg dose until end of trial.
Figure 1.
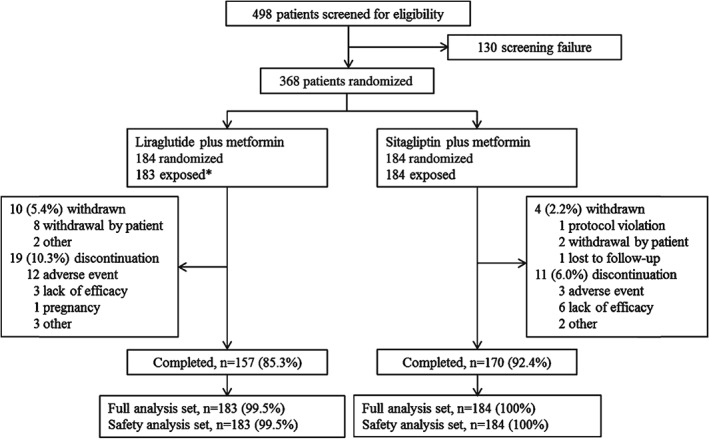
Patient disposition. *One patient withdrew before exposure. Discontinuation: patients stopped trial product but remained in the trial to collect end of trial safety and efficacy information. Withdrawn: patients stopped trial product and left the trial.
Table 1.
Demographic and baseline characteristics.
| Liraglutide n = 183 | Sitagliptin n = 184 | |
|---|---|---|
| Male; female, n (%) | 102 (55.7); 81 (44.3) | 117 (63.6); 67 (36.4) |
| Mean (s.d.) age, years | 51.7 (10.7) | 51.4 (11.0) |
| Mean (s.d.) body weight, kg | 76.2 (13.6) | 75.8 (15.1) |
| Mean (s.d.) BMI, kg/m2 | 27.3 (3.4) | 27.2 (4.0) |
| Mean (s.d.) duration of diabetes, years | 5.3 (4.4) | 5.2 (5.4) |
| Mean (s.d.) HbA1c, % | 8.14 (0.83) | 8.11 (0.78) |
| Mean (s.d.) HbA1c, mmol/mol | 65.5 (9) | 65.1 (9) |
| Mean (s.d.) FPG, mmol/l | 9.26 (2.22) | 9.46 (2.24) |
| Use of concomitant medications*, n (%) | ||
| Angiotension‐converting enzyme inhibitors | 12 (6.6) | 10 (5.4) |
| Angiotensin II antagonist | 1 (0.5) | 1 (0.5) |
| β‐blockers | 12 (6.6) | 10 (5.4) |
| Statins | 26 (14.2) | 18 (9.8) |
BMI, body mass index; HbA1c, glycated haemoglobin; FPG, fasting plasma glucose; s.d., standard deviation.
Concomitant medication ongoing at screening. Data are number (% of total participants in the treatment group) for gender and use of concomitant medications, and mean (s.d) for other baseline parameters.
At week 26, mean reductions in HbA1c from baseline were −1.65% (−18.07 mmol/mol) for liraglutide and −0.98% (−10.72 mmol/mol) for sitagliptin (Figure 2A, B). The ETD for liraglutide versus sitagliptin was −0.67% (95% CI −0.86, −0.48), or −7.35 mmol/mol (95% CI −9.43; −5.26; p < 0.0001), confirming the superiority of liraglutide. More patients achieved HbA1c <7.0% (<53 mmol/mol) or ≤6.5% (≤48 mmol/mol) and the composite endpoints with liraglutide than with sitagliptin (Figure 3A).
Figure 2.
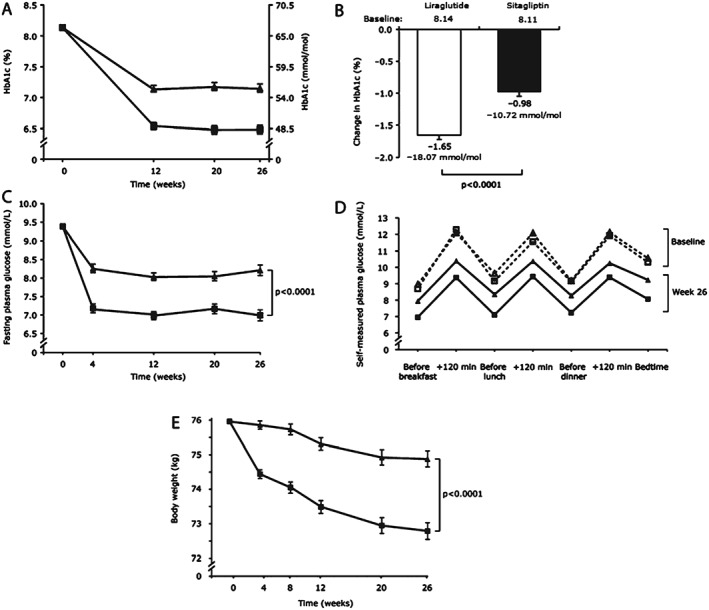
Efficacy endpoints from baseline to week 26. (A) HbA1c estimated means [±standard error of mean (s.e.m.)]. (B) Change in glycated haemoglobin (HbA1c). (C) Fasting plasma glucose estimated means (±s.e.m.). (D) Seven‐point self‐measured plasma glucose profiles. Dashed line: baseline; solid line: week 26. (E) Body weight estimated means (±s.e.m.). For (A), (C), (D) and (E): squares, liraglutide; triangles, sitagliptin.
Figure 3.
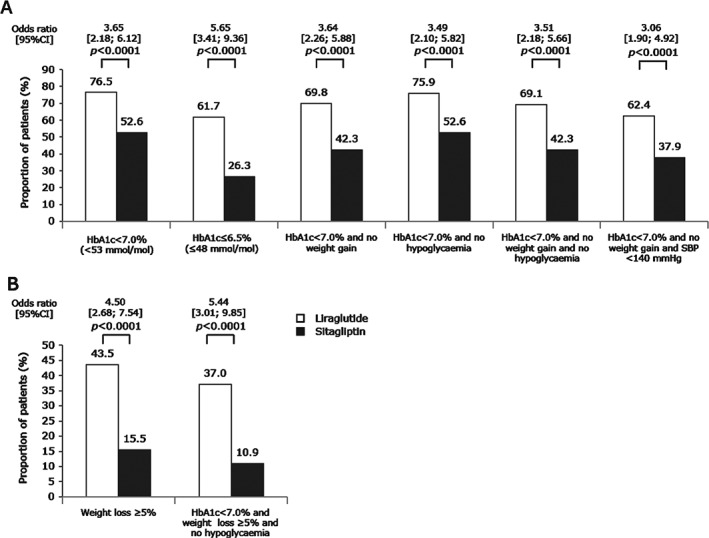
Proportion of patients achieving treatment targets and composite endpoints after 26 weeks of treatment. (A) Glycated haemoglobin (HbA1c) <7.0% (53 mmol/mol) and ≤6.5% (48 mmol/mol) the composite endpoints. (B) Post hoc weight loss ≤5% and composite endpoints. CI, confidence interval; SBP, systolic blood pressure. Hypoglycaemia refers to confirmed hypoglycaemia. p values refer to odds ratios.
Both treatments reduced FPG levels after 26 weeks, but reduction was greater with liraglutide (−2.39 mmol/l) than with sitagliptin (−1.17 mmol/l), resulting in an ETD at week 26 of −1.22 mmol/l (95% CI −1.63, −0.81; p < 0.0001) in favour of liraglutide (Figure 2C).
At week 26, the 7‐point SMPG profile was improved at all seven time points for both treatments (Figure 2D). Improvements were greater with liraglutide than sitagliptin, as evidenced by the change in the mean of the SMPG profile of −2.28 mmol/l versus −1.33 mmol/l [ETD −0.95 mmol/l (95% CI −1.29, −0.61; p < 0.0001)].
Body weight reduction at week 26 was greater with liraglutide than with sitagliptin [−3.17 kg vs −1.08 kg, respectively; ETD −2.10 kg (95% CI −2.76, −1.43); p < 0.0001 (Figure 2E)]. Liraglutide was also associated with greater reductions in BMI [−1.17 kg/m2 vs −0.40 kg/m2; ETD −0.77 kg/m2 (95% CI −1.00, −0.53); p < 0.0001] and waist circumference [−2.64 cm vs −1.11 cm, ETD −1.53 cm (95% CI −2.38, −0.67); p = 0.0005] (Table S1, Supporting Information). Waist‐to‐hip ratio was almost unchanged for both groups.
In post hoc analyses of additional endpoints, more liraglutide‐treated patients achieved the composite endpoint of HbA1c <7.0% (<53 mmol/mol) and weight loss ≥5% without confirmed hypoglycaemia [37.0% vs 10.9%, OR 5.44 (95% CI 3.01, 9.85); p < 0.0001 (Figure 3B)].
Both treatments decreased SBP and DBP [SBP change −4.31 mmHg vs −2.76 mmHg and DBP change −1.37 mmHg vs −0.96 mmHg, for the liraglutide and sitagliptin groups, respectively; p > 0.05 for both (Table S1, Supporting Information)].
There was no statistically significant difference between the two groups for any of the lipid variables (Table S1, Supporting Information).
Results of post hoc subgroup analyses demonstrated statistically significant greater mean reductions in HbA1c and body weight from baseline with liraglutide versus sitagliptin, regardless of HbA1c and BMI at baseline (Table S2, Supporting Information). Patient‐reported outcomes, as evaluated using the DTSQ, showed a slightly improved overall treatment satisfaction score in both treatment groups; no statistically significant difference was found between the groups [mean change from baseline 0.70 (liraglutide) vs. 0.76 (sitagliptin)].
Safety and Tolerability
Overall, both treatments were well tolerated. More patients reported AEs with liraglutide than with sitagliptin (55.7% vs 34.2%, respectively; Table 2). Serious AEs (SAEs) were reported in three patients (1.6%) with liraglutide versus six patients (3.3%) with sitagliptin. SAEs showed no consistent pattern regarding system organ class or preferred terms of the events, and most SAEs in both groups occurred as single events (Table 2). Two neoplasms (thyroid cancer and malignant thymoma) were reported in the sitagliptin group. No deaths occurred during the trial. More patients were discontinued from trial product as a result of AEs with liraglutide than with sitagliptin (6.6% vs 1.6%). The proportion of patients with AEs assessed by the investigators to be possibly/probably related to trial products was higher in the liraglutide group (43.2%) versus the sitagliptin group (13.0%). AEs were mostly mild or moderate in both groups, and the majority of patients reporting AEs had recovered by end of trial. The difference in incidence of AEs was primarily attributable to more patients reporting gastrointestinal disorders and metabolism and nutrition disorders in the liraglutide group versus the sitagliptin group (Table 2). The following AEs were reported in a higher proportion of liraglutide‐treated patients versus sitagliptin‐treated patients: nausea (14.8% vs 0.5%), diarrhoea (8.2% vs 2.2%) and decreased appetite (10.9% vs 0.5%). Most cases of nausea and diarrhoea occurred during the initial weeks of liraglutide treatment (Figure S1, Supporting Information). Increased lipase levels were reported in 11 liraglutide‐treated patients (6.0%) versus 8 sitagliptin‐treated patients (4.3%).
Table 2.
Treatment‐emergent adverse events.
| Liraglutide n = 183 | Sitagliptin n = 184 | |
|---|---|---|
| Overall, n (%) | 102 (55.7) | 63 (34.2) |
| Serious adverse events, n (%) | ||
| Overall | 3 (1.6) | 6 (3.3) |
| Gastric ulcer | 0 | 1 (0.5) |
| Haemorrhoids | 0 | 1 (0.5) |
| Thyroid cancer | 0 | 1 (0.5) |
| Thymoma malignant | 0 | 1 (0.5) |
| Atrial fibrillation | 0 | 1 (0.5) |
| Sudden hearing loss | 1 (0.5) | 0 |
| Bronchitis | 1 (0.5) | 0 |
| Diabetic ketoacidosis | 1 (0.5) | 0 |
| Cerebral infarction | 0 | 1 (0.5) |
| Adverse events in ≥5% patients, n (%) | ||
| Gastrointestinal disorders | ||
| Nausea | 27 (14.8) | 1 (0.5) |
| Diarrhoea | 15 (8.2) | 4 (2.2) |
| Metabolism and nutrition disorders, n (%) | ||
| Decreased appetite | 20 (10.9) | 1 (0.5) |
| Investigations | ||
| Increased lipase | 11 (6.0) | 8 (4.3) |
No episodes of severe hypoglycaemia were reported. Two patients reported two episodes of confirmed hypoglycaemia (liraglutide group), and one patient reported one episode of confirmed hypoglycaemia (sitagliptin group); none of these episodes was nocturnal.
Serum amylase levels increased slightly in both groups; no statistically significant difference was found between the treatments [ratio to baseline 1.10 (liraglutide) and 1.06 (sitagliptin); estimated treatment ratio (ETR) 1.04 (95% CI 0.98, 1.09; p = 0.1733)]. The increase in lipase levels was greater with liraglutide than sitagliptin [ratio to baseline 1.38 vs 1.17; ETR 1.19 (95% CI 1.09, 1.29); p < 0.0001 (Table S1, Supporting Information)]. No pancreatitis cases or suspicion of pancreatitis was reported. For haematology or biochemistry variables, changes from baseline to week 26 were small and no clinically relevant differences were observed between the two treatment groups. After 26 weeks, no increase in serum calcitonin level was noted with either treatment. At week 26, mean resting pulse had increased slightly from baseline with liraglutide (2.00 beats/min), but remained almost unchanged with sitagliptin (−0.38 beats/min; Table S1, Supporting Information). The treatment difference in resting pulse was statistically significant (ETD 2.37; 95% CI 0.69, 4.06; p = 0.0059).
Discussion
This head‐to‐head comparison of liraglutide versus sitagliptin in Chinese patients with T2DM, inadequately controlled on metformin monotherapy, was undertaken to obtain local comparative data on efficacy and safety of incretin‐based therapies. Results of a meta‐analysis comparing the HbA1c‐lowering efficacy of GLP‐1RAs between Asian and non‐Asian patients with T2DM suggest that GLP‐1RAs lower HbA1c to a greater extent in Asian‐dominant studies than in non‐Asian‐dominant cohorts 19. In contrast to the multi‐ethnic populations of the Liraglutide Effect and Action in Diabetes (LEAD) one to six trials and the previous head‐to‐head comparison of liraglutide and sitagliptin (LIRA‐DPP‐4), the present trial's population was exclusively Chinese. Furthermore, at baseline, the population of this trial had lower mean HbA1c and lower mean BMI than patients treated with liraglutide 1.8 mg in the LEAD 1–6 and LIRA‐DPP‐4 populations at baseline [HbA1c 8.1% (65 mmol/mol) vs 8.2–8.5% (66–69 mmol/mol) and 8.4% (68 mmol/mol); BMI 27.3 kg/m2 vs 30.0–33.5 kg/m2 and 33.1 kg/m2, respectively]; however, the mean duration of diabetes was within the range seen in the LEAD and LIRA‐DPP‐4 trials (5.3 years vs 5.3–9.2 and 6.4 years, respectively) 10, 20, 21, 22, 23, 24, 25. In the present trial, liraglutide was associated with better glycaemic control, as evidenced by larger HbA1c reduction, greater percentage of patients reaching HbA1c targets, larger reduction in FPG and mean SMPG profile, and greater improvements in body measurements (body weight, BMI, waist circumference), compared with sitagliptin. The reductions in blood glucose and body weight observed with liraglutide or sitagliptin in the present trial were consistent with previous results observed with these agents in Chinese patients with T2DM 12, 13, 26, 27.
The reduction of cardiovascular risk factors is an important consideration in T2DM management. In the present trial, both liraglutide and sitagliptin had a favourable effect on risk factors such as SBP and body weight, with the latter being reduced to a greater extent with liraglutide than sitagliptin. Excessive body weight and high BMI are associated with various metabolic abnormalities such as worsened insulin resistance, adding difficulties in controlling glycaemia and increasing the risk of diabetes complications 28, 29. In contrast to traditional antihyperglycaemic agents, such as insulin and sulphonylureas, liraglutide has been shown to promote weight loss, with a reduction of ∼2–3 kg 12, 30, 31. Weight loss in the present trial was 3.17 kg with liraglutide, and approximately half of liraglutide‐treated patients lost at least 5% of their body weight.
A composite endpoint of patients achieving HbA1c <7.0% (<53 mmol/mol) without weight gain or hypoglycaemic episodes was achieved by more liraglutide‐treated patients compared with the sitagliptin group. Because of the low number of confirmed hypoglycaemic events reported in this trial (liraglutide group, n = 2; sitagliptin group, n = 1), this result was largely driven by more liraglutide‐treated patients achieving HbA1c <7.0% and more patients not exhibiting weight gain. Similarly, more liraglutide‐treated patients reached a composite endpoint of HbA1c <7.0% and weight loss ≥5% without hypoglycaemia, compared with those treated with sitagliptin.
The improved treatment effects with liraglutide versus sitagliptin did not show any dependence on HbA1c or BMI subgroup (p > 0.05), as demonstrated by post hoc subgroup analyses.
Overall, both liraglutide and sitagliptin were well tolerated. Consistent with previous studies, the most commonly reported AEs, which were more frequent in liraglutide‐treated patients, were gastrointestinal disorders 20, 21, 22, 23, 24, 25 (nausea and diarrhoea), as well as metabolism and nutrition disorders (decreased appetite). Gastrointestinal AEs are a known side effect of GLP‐1RAs and are typically mild and transient, disappearing after the initial few weeks to month of treatment 32 and are mitigated by stepwise dose escalation, as indicated in the labelling 16. Occurrence of hypoglycaemia was low in the present trial, in line with the glucose‐dependent mechanism of action of both GLP‐1RAs and DPP‐4 inhibitors 9. As previously reported with liraglutide and other GLP‐1RAs 10, 33, 34, 35, liraglutide treatment resulted in an increased resting pulse in patients in the present trial. Although the clinical relevance of this is unclear, results from localization studies suggest the mechanism may be partly explained by GLP‐1‐mediated stimulation of GLP‐1 receptor on sinoatrial myocytes 36. The ongoing cardiovascular outcomes trial (LEADER) is evaluating the long‐term effects of liraglutide on cardiovascular safety in patients with T2DM at high risk of cardiovascular disease 37.
Up to 25% of patients with T2DM have elevated serum amylase or lipase 38. Additionally, incretin‐based therapies have been associated with increased serum amylase or lipase 35, 38, 39, 40. The clinical mechanism by which this increase occurs is unknown. In the present trial, there was a greater serum lipase increase in liraglutide‐treated patients than in sitagliptin‐treated patients. No pancreatitis or suspicion of pancreatitis was observed here, consistent with previous findings that increased lipase levels are not indicative of pancreatitis 38.
In the present trial, patients were allowed to reduce the liraglutide dose from 1.8 to 1.2 mg if required to because of tolerability issues; however, 93.6% of liraglutide‐treated patients completed the trial on the 1.8 mg/day dose. The efficacy achieved in the present trial is consistent with that previously reported for liraglutide 1.8 mg/day 10 and no new safety signals were encountered, thereby confirming the documented favourable risk–benefit profile of liraglutide 1.8 mg/day for treatment of T2DM in Chinese patients 12.
The open‐label trial design was considered a limitation that may introduce bias. Furthermore, although the 26‐week treatment duration was considered sufficient to assess changes in glycaemic control variables, it was not sufficient to assess whether the observed cardiometabolic improvements could be maintained in the long term. However, as the results in this trial were similar to those conducted in Western populations 10, a similar long‐term effect could be expected. While adequate glycaemic control may be achieved with 1.2 mg liraglutide 20, 21, 22, 23, the majority (93.6%) of patients in the liraglutide arm of this trial were treated with 1.8 mg liraglutide; this may be considered a limitation of the trial.
Liraglutide and sitagliptin are both recommended as possible second‐line treatment options for the management of hyperglycaemia in T2DM after metformin failure by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) 3. Sitagliptin is also recommended by the Chinese Diabetes Society as a second‐line treatment, in accordance with its labelling in China; however, GLP‐1RAs are only recommended as third‐line therapy in the Chinese guideline for treatment of T2DM 18, partially because of a paucity of data in Chinese patients 41. In line with the ADA/EASD recommendation, the results from the present trial suggest that liraglutide might be used as a second‐line treatment, providing good glycaemic control and body weight reduction, and being a generally well‐tolerated treatment option in Chinese patients.
Conflict of Interest
H. B.‐T. and Y. S. are Novo Nordisk employees. Y. M. has received lecture fees and research funding from Sanofi, Novo Nordisk, Eli Lilly, Novartis and Bayer. The other authors declare no conflicts of interests.
The study sponsor participated in the study design, data collection, review and analysis.
All authors were involved in the trial operation, data collection, and preparation, review and final approval of the manuscript.
Supporting information
Table S1. Changes in the secondary endpoints.
Table S2. Change from baseline glycated haemoglobin (HbA1c) and body weight by body mass index and HbA1c groups.
Figure S1. Proportion of patients with nausea by time.
Acknowledgements
This trial was funded by Novo Nordisk. The authors thank LIRA‐DPP‐4 CHINA™ investigators and patients for their participation, and also Cai Tang of Novo Nordisk and Aisling O'Keeffe of Watermeadow Medical, UK, for writing assistance.
References
- 1. Yang W, Lu J, Weng J et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Wang L, He J et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. Hayward RA, Reaven PD, Wiitala WL et al. Follow‐up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372: 2197–2206. Erratum in: N Engl J Med 2015; 373: 198.. [DOI] [PubMed] [Google Scholar]
- 5. Ji LN, Lu JM, Guo XH et al. Glycemic control among patients in China with type 2 diabetes mellitus receiving oral drugs or injectables. BMC Public Health 2013; 13: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooperative Meta‐analysis Group of the Working Group on Obesity in China . Predictive value of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut‐off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002; 15: 83–95. [PubMed] [Google Scholar]
- 7. Wing RR, Lang W, Wadden TA et al., Look AHEAD Research Group . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vilsbøll T, Garber AJ. Non‐glycaemic effects mediated via GLP‐1 receptor agonists and the potential for exploiting these for therapeutic benefit: focus on liraglutide. Diabetes Obes Metab 2012; 14(Suppl. 2): 41–49. [DOI] [PubMed] [Google Scholar]
- 9. Nauck M. Incretin therapies–highlighting common features and differences in the modes of action of GLP‐1 receptor agonists and DPP‐4 inhibitors. Diabetes Obes Metab 2016; 18: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pratley RE, Nauck M, Bailey T et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet 2010; 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 11. Pratley R, Nauck M, Bailey T et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel‐group, open‐label trial. Int J Clin Pract 2011; 65: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang W, Chen L, Ji Q et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16‐week, randomized, double‐blind, active control trial(*). Diabetes Obes Metab 2011; 13: 81–88. [DOI] [PubMed] [Google Scholar]
- 13. Yang W, Guan Y, Shentu Y et al. The addition of sitagliptin to ongoing metformin therapy significantly improves glycemic control in Chinese patients with type 2 diabetes. J Diabetes 2012; 4: 227–237. [DOI] [PubMed] [Google Scholar]
- 14. World Medical Association . Declaration of Helsinki‐Ethical principles for medical research involving human subjects. Last Amended by the 59th WMA General Assembly, Seoul, 2008. [Google Scholar]
- 15. International Conference on Harmonisation . ICH harmonised tripartite guideline. Good clinical practice, 1 May 1996. Available from URL: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed December 2015.
- 16. EMC . Victoza Summary of Product Characteristics. Available from URL: https://www.medicines.org.uk/emc/medicine/21986. Accessed 03 May 2016.
- 17. Federal Drug Agency (FDA) . FDA guidance for industry, diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention, 2008. Available from URL: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm071624.pdf. Accessed 03 May 2016.
- 18. Chinese Diabetes Society . Chinese guideline for the management of type 2 diabetes mellitus (2013 edition). Chin J Diabetes Mellitus 2014; 6: 447–498. Accessed 03 May 2016. [Google Scholar]
- 19. Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 900–909. [DOI] [PubMed] [Google Scholar]
- 20. Marre M, Shaw J, Brändle M et al. LEAD‐1 SU study group. Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nauck M, Frid A, Hermansen K et al. LEAD‐2 study group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garber A, Henry R, Ratner R et al. LEAD‐3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 23. Zinman B, Gerich J, Buse JB et al. LEAD‐4 study investigators. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009; 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell‐Jones D, Vaag A, Schmitz O et al. Liraglutide effect and action in diabetes 5 (LEAD‐5) met + SU study group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met + SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buse JB, Rosenstock J, Sesti G et al. LEAD‐6 study group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 26. Craddy P, Palin HJ, Johnson KI. Comparative effectiveness of dipeptidylpeptidase‐4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther 2014; 5: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shyangdan DS, Royle PL, Clar C, Sharma P, Waugh NR, Snaith A. Glucagon‐like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 10: CD006423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bloomgarden ZT. Diabetes and obesity: part 1. Diabetes Care 2007; 30: 3145–3151. [DOI] [PubMed] [Google Scholar]
- 29. Bloomgarden ZT. Diabetes and obesity: part 2. Diabetes Care 2008; 31: 176–182. [DOI] [PubMed] [Google Scholar]
- 30. Niswender K, Pi‐Sunyer X, Buse J et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab 2013; 15: 42–54. [DOI] [PubMed] [Google Scholar]
- 31. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 32. Blonde L, Russell‐Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1‐5 studies. Diabetes Obes Metab 2009; 11(Suppl. 3): 26–34. [DOI] [PubMed] [Google Scholar]
- 33. Pratley RE, Nauck MA, Barnett AH et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2: 289–297. [DOI] [PubMed] [Google Scholar]
- 34. Meier JJ, Rosenstock J, Hincelin‐Méry A et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care 2015; 38: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 35. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 36. Pyke C, Heller RS, Kirk RK et al. GLP‐1 provoked severe hypoglycemia in an individual with type 2 diabetes and a benign insulinoma. Diabetes Care 2014; 37: e177–e178. [DOI] [PubMed] [Google Scholar]
- 37. Marso SP, Poulter NR, Nissen SE et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J 2013; 166: 823–830. [DOI] [PubMed] [Google Scholar]
- 38. Steinberg WM, Nauck MA, Zinman B et al. LEADER 3–lipase and amylase activity in subjects with type 2 diabetes: baseline data from over 9000 subjects in the LEADER Trial. Pancreas 2014; 43: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide‐1 receptor agonists or dipeptidyl‐peptidase‐4 inhibitors in the outpatient setting. Endocr Pract 2012; 18: 472–477. [DOI] [PubMed] [Google Scholar]
- 40. Tokuyama H, Kawamura H, Fujimoto M et al. A low‐grade increase of serum pancreatic exocrine enzyme levels by dipeptidyl peptidase‐4 inhibitor in patients with type 2 diabetes. Diabetes Res Clin Pract 2013; 100: e66–e69. [DOI] [PubMed] [Google Scholar]
- 41. Yang W, Weng J. Early therapy for type 2 diabetes in China. Lancet Diabetes Endocrinol 2014; 2: 992–1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Changes in the secondary endpoints.
Table S2. Change from baseline glycated haemoglobin (HbA1c) and body weight by body mass index and HbA1c groups.
Figure S1. Proportion of patients with nausea by time.


